Effects of Defoliation at Fruit Set on Vine Physiology and Berry Composition in Cabernet Sauvignon Grapevines
Abstract
1. Introduction
2. Results
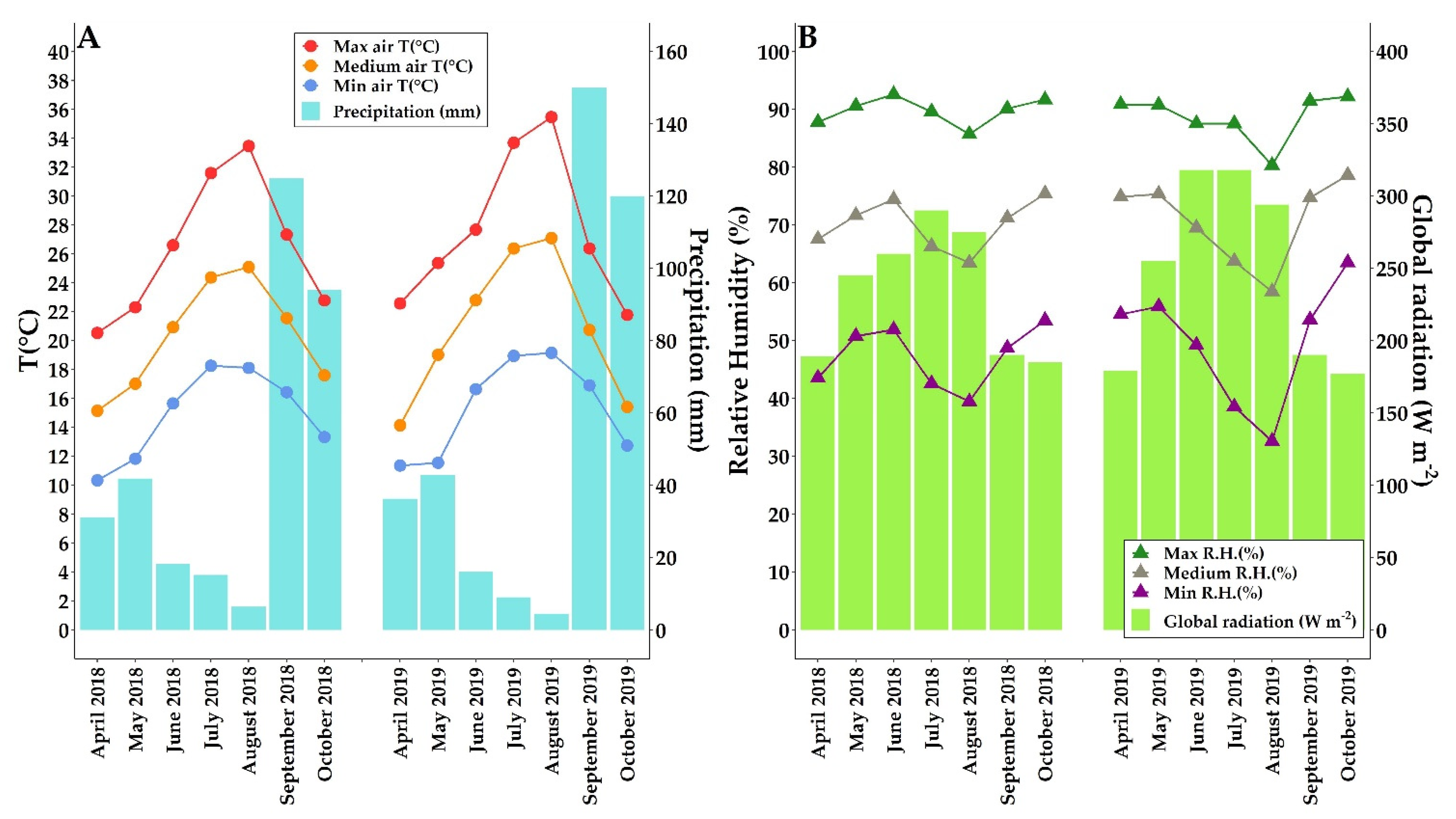
2.1. Climatic Conditions
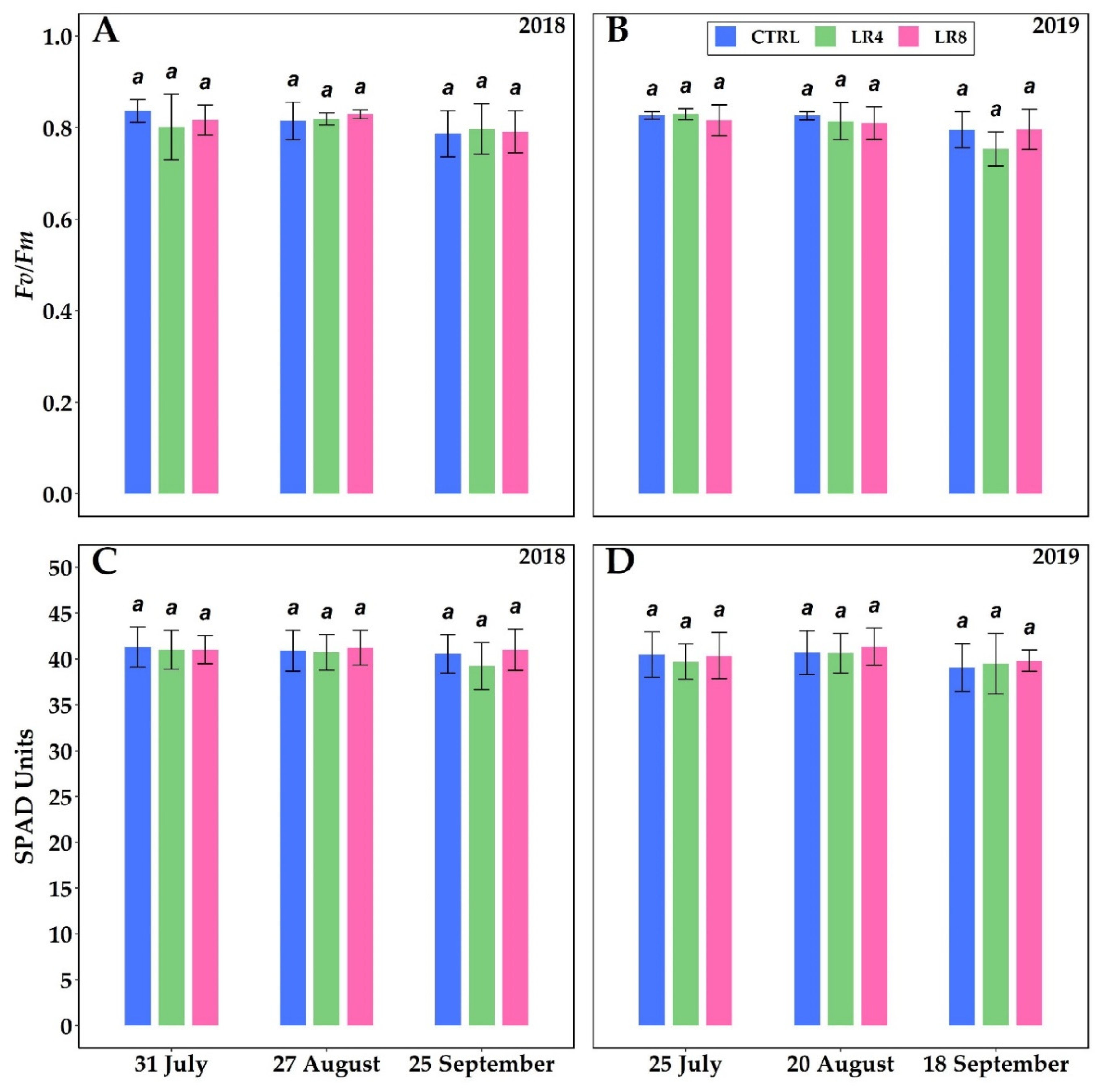
2.2. Leaf Gas Exchanges, Leaf Area, Leaf Water Potential, Leaf Chlorophyll a Fluorescence and Content
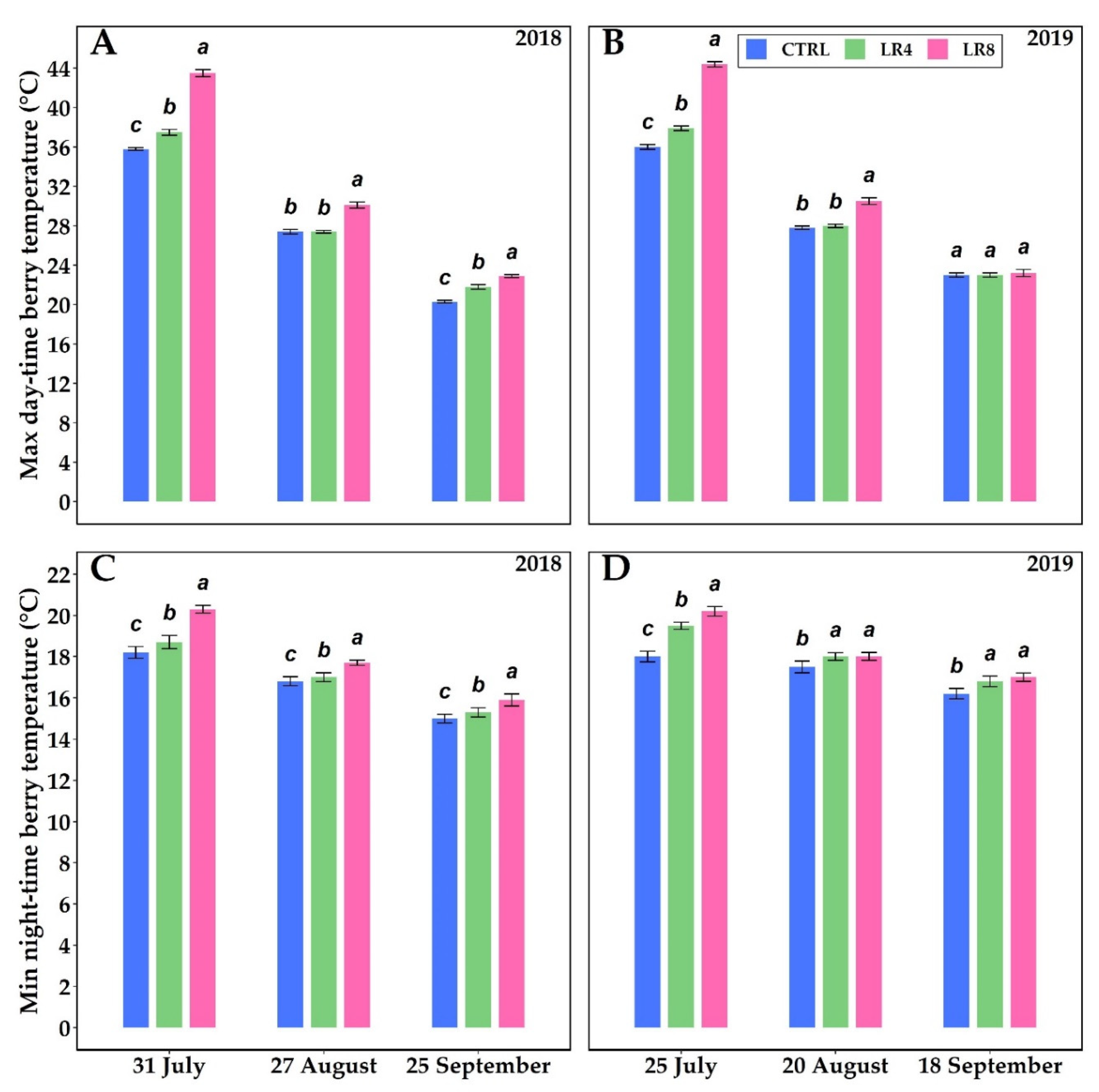
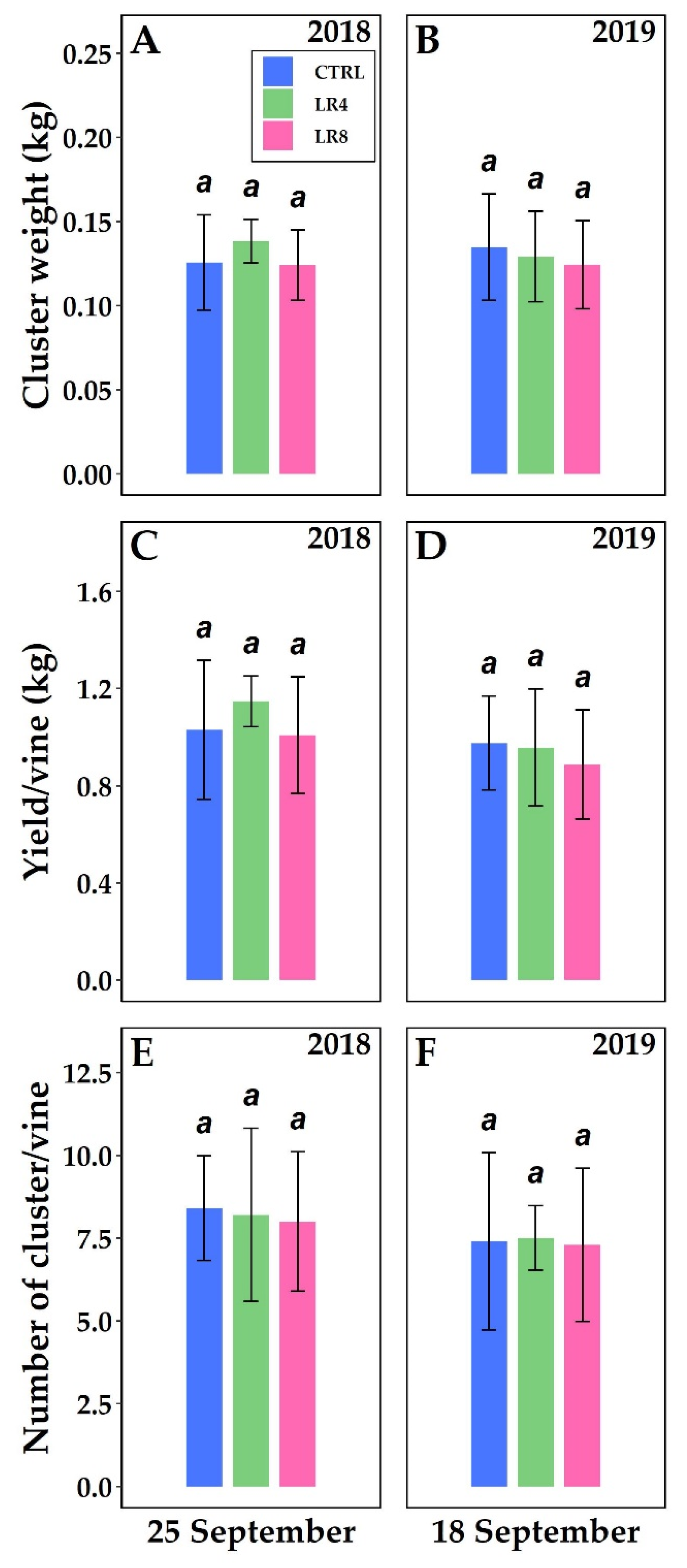
2.3. Berry Composition and Temperature
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Settings
4.2. Leaf Gas Exchanges, Leaf Area, Leaf Water Potential, Leaf Chlorophyll a Fluorescence and Content
4.3. Berry Composition and Temperature
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabbatini, P.; Howell, G.S. Effects of early defoliation on yield, fruit composition, and harvest season cluster rot complex of grapevines. HortScience 2010, 45, 1804–1808. [Google Scholar] [CrossRef]
- Bavaresco, L.; Gatti, M.; Pezzutto, S.; Fregoni, M.; Mattivi, F. Effect of leaf removal on grape yield, berry composition, and stilbene concentration. Am. J. Enol. Vitic. 2008, 59, 292–298. [Google Scholar]
- Hunter, J.J.; De Villiers, O.T.; Watts, J.E. The effect of partial defoliation on quality characteristics of Vitis vinifera L. cv. Cabernet Sauvignon grapes. II. Skin color, skin sugar, and wine quality. Am. J. Enol. Vitic. 1991, 42, 13–18. [Google Scholar]
- Smart, R.E.; Smith, S.M.; Winchester, R.V. Light quality and quantity effects on fruit ripening for Cabernet Sauvignon. Am. J. Enol. Vitic. 1988, 39, 250–258. [Google Scholar]
- Diago, M.P.; Ayestaran, B.; Guadalupe, Z.; Poni, S.; Tardaguila, J. Impact of prebloom and fruit set basal leaf removal on the flavonol and anthocyanin composition of Tempranillo grapes. Am. J. Enol. Vitic. 2012, 63, 367–376. [Google Scholar] [CrossRef]
- Diago, M.P.; Ayestaran, B.; Guadalupe, Z.; Garrido, A.; Tardaguila, J. Phenolic composition of Tempranillo wines following early defoliation of the vines. J. Sci. Food Agric. 2012, 92, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Intrigliolo, D.S.; Llacer, E.; Revert, J.; Esteve, M.D.; Climent, M.D.; Palau, D.; Gómez, I. Early defoliation reduces cluster compactness and improves grape composition in Mandó, an autochthonous cultivar of Vitis vinifera from southeastern Spain. HortScience 2014, 167, 71–75. [Google Scholar] [CrossRef]
- Risco, D.; Pérez, D.; Yeves, A.; Castel, J.R.; Intrigliolo, D.S. Early defoliation in a temperate warm and semi-arid Tempranillo vineyard: Vine performance and grape composition. Aust. J. Grape Wine Res. 2014, 20, 111–122. [Google Scholar] [CrossRef]
- Ćirković, D.; Matijašević, S.; Deletić, N.; Ćirković, B.; Gašić, U.; Sredojević, M.; Jovanović, Z.; Djurić, V.; Tešić, Ž. The Effect of Early and Late Defoliation on Phenolic Composition and Antioxidant Properties of Prokupac Variety Grape Berries (Vitis vinifera L.). Agronomy 2019, 9, 822. [Google Scholar] [CrossRef]
- Howell, G.S.; Mansfield, T.K.; Wolpert, J.A. Influence of training system, pruning severity, and thinning on yield, vine size, and fruit quality of Vidal blanc grapevines. Am. J. Enol. Vitic. 1987, 38, 105–112. [Google Scholar]
- Poni, S.; Casalini, L.; Bernizzoni, F.; Civardi, S.; Intrieri, C. Effects of early defoliation on shoot photosynthesis, yield components, and grape composition. Am. J. Enol. Viticult. 2006, 57, 397–407. [Google Scholar]
- Poni, S.; Bernizzoni, F. A three-year study on the impact of pre-flowering leaf removal on berry growth components and grape composition in cv. Barbera vines. J. Int. Sci. Vigne Vin. 2010, 44, 21–30. [Google Scholar]
- Cataldo, E.; Salvi, L.; Sbraci, S.; Storchi, P.; Mattii, G.B. Sustainable Viticulture: Effects of Soil Management in Vitis vinifera. Agronomy 2020, 10, 1949. [Google Scholar] [CrossRef]
- Kliewer, W.M. Effect of time and severity of defoliation on growth and composition of Thompson Seedless grapes. Am. J. Enol. Vitic. 1970, 21, 37–47. [Google Scholar]
- Intrieri, C.; Filippetti, I.; Allegro, G.; Centinari, M.; Poni, S. Early defoliation (hand vs. mechanical) for improved crop control and grape composition in Sangiovese (Vitis vinifera L.). Aust. J. Grape Wine Res. 2008, 14, 25–32. [Google Scholar] [CrossRef]
- Poni, S.; Bernizzoni, F.; Briola, G.; Cenni, A. Effects ofearly leaf removal on cluster morphology, shoot efficiency andgrape quality in two Vitis vinifera cultivars. Acta Hortic. 2005, 689, 217–225. [Google Scholar] [CrossRef]
- Gatti, M.; Bernizzoni, F.; Civardi, S.; Poni, S. Effects of cluster thinning and preflowering leaf removal on growth and grape composition in cv. Sangiovese. Am. J. Enol. Vitic. 2012, 63, 325–332. [Google Scholar] [CrossRef]
- Diago, M.P.; Vilanova, M.; Tardaguila, J. Effects of timing of manual and mechanical early defoliation on the aroma of Vitis vinifera L. Tempranillo wine. Am. J. Enol. Vitic. 2010, 61, 382–391. [Google Scholar]
- Lee, J.; Skinkis, P. Oregon Pinot noir grape anthocyanin enhancement by early leaf removal. Food Chem. 2013, 139, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Cook, M.G.; Yacco, R.S.; Watrelot, A.A.; Gambetta, G.; Kennedy, J.A.; Kurtural, S.K. Effects of leaf removal and applied water on flavonoid accumulation in grapevine (Vitis vinifera L. cv. Merlot) berry in a hot climate. J. Agric. Food Chem. 2016, 64, 8118–8127. [Google Scholar] [CrossRef] [PubMed]
- May, P.; Shaulis, N.J.; Antcliff, A.J. The effect of controlled defoliation in the Sultana vine. Am. J. Enol. Vitic. 1969, 20, 237–250. [Google Scholar]
- Gómez, I.; Revert, J.; Esteve, M.D.; Climent, M.D.; Martínez, A.; Jiménez, J.; Intrigliolo, D.S. Effects of early defoliation in grape yield and quality in “Mando”, an autochthon cultivar of south-east Spain. Acta Hortic. 2012, 931, 365–370. [Google Scholar] [CrossRef]
- Kotseridis, Y.; Georgiadou, A.; Tikos, P.; Kallithraka, S.; Koundouras, S. Effects of severity of post-flowering leaf removal on berry growth and composition of three red Vitis vinifera L. cultivars grown under semiarid conditions. J. Agric. Food Chem. 2012, 60, 6000–6010. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.G.; Wardle, D.A.; Naylor, A.P. Impact of training system, vine spacing, and basal leaf removal on Riesling. Vine performance, berry composition, canopy microclimate, and vineyard labor requirements. Am. J. Enol. Vitic. 1966, 47, 63–76. [Google Scholar]
- Zoecklein, B.W.; Wolf, T.K.; Duncan, N.W.; Judge, J.M.; Cook, M.K. Effects of fruit zone leaf removal on yield, fruit composition, and fruit rot incidence of Chardonnay and White Riesling (Vitis vinifera L.) grapes. Am. J. Enol. Vitic. 1992, 43, 139–148. [Google Scholar]
- Verdenal, T.; Zufferey, V.; Dienes-Nagy, A.; Gindro, K.; Belcher, S.; Lorenzini, F.; Rosti, J.; Koestel, C.; Spring, J.L.; Viret, O. Pre-flowering defoliation affects berry structure and enhances wine sensory parameters. OENO ONE 2017, 51. [Google Scholar] [CrossRef]
- Verdenal, T.; Zufferey, V.; Dienes-Nagy, A.; Bourdin, G.; Gindro, K.; Viret, O.; Spring, J.L. Timing and intensity of grapevine defoliation: An extensive overview on five cultivars in Switzerland. Am. J. Enol. Vitic. 2019, 70, 427–434. [Google Scholar] [CrossRef]
- Percival, D.C.; Sullivan, J.A.; Fisher, K.H. Effect of cluster exposure, berry contact and cultivar on cuticular membrane formation and occurrence of bunch rot (Botrytis cinerea PERS.: FR.) with 3 Vitis vinifera L. cultivars. Vitis 1993, 32, 87–97. [Google Scholar]
- Kunz, B.; Cahill, D.; Mohr, P.; Osmond, M.; Vonarx, J. Plant responses to UV radiation and links to pathogen resistance. Int. Rev. Cytol. 2006, 255, 1–40. [Google Scholar]
- Coombe, B.G. Fruit set and development in seeded grape varieties as affected by defoliation, topping, girdling, and other treatments. Am. J. Enol. Vitic. 1959, 10, 85–100. [Google Scholar]
- Belancic, A.; Agosin, E.; Ibacache, A.; Bordeu, E.; Baumes, R.; Razungles, A.; Bayonove, C. Influence of sun exposure on the aromatic composition of Chilean Muscat grape cultivars Moscatel de Alejandria and Moscatel rosada. Am. J. Enol. Vitic. 1997, 48, 181–186. [Google Scholar]
- Kozina, B.; Karoglan, M.; Herjavec, S.; Jeromel, A.; Orlic, S. Influence of basal leaf removal on the chemical composition of Sauvignon Blanc and Riesling wines. J. Food Agric. Environ. 2008, 6, 28–33. [Google Scholar]
- Radeka, S.; Herjavec, S.; Peršurić, Đ.; Lukić, I.; Sladonja, B. Effect of different maceration treatments on free and bound varietal aroma compounds in wine of Vitis vinifera L. cv. Malvazija istarska bijela. Food Technol. Biotechnol. 2008, 46, 86–92. [Google Scholar]
- Bubola, M.; Peršuri’c, D.J.; Gani’c, K.K.; Cossetto, M. Influence of timing and intensity of basal leaf removal on aromatic composition of cv. istrian Malvasia wines. In Abstracts Book III International Symposium; Malvasias: Canary Islands, Spain, 2009; pp. 64–65. [Google Scholar]
- Perez, J.R.; Kliewer, W.M. Influence of light regime and nitrate fertilization on nitrate reductase activity and concentrations of nitrate and arginine in tissues of three cultivars of grape vines. Am. J. Enol. Vitic. 1982, 33, 86–93. [Google Scholar]
- Roubelakis-Angelakis, K.A.; Kliewer, W.M. Effects of exogenous factors on phenylalanine ammonia-lyase activity and accumulation of anthocyanins and total phenolics in grape berries. Am. J. Enol. Vitic. 1986, 37, 275–280. [Google Scholar]
- Dokoozlian, N.K.; Kliewer, W.M. Influence of light on grape berry growth and composition varies during fruit development. J. Am. Soc. Hortic 1996, 121, 869–874. [Google Scholar] [CrossRef]
- Mori, K.; Gotto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperatures. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Stoll, M.; Lafontaine, M.; Schultz, H.R. Possibilities to reduce the velocity of berry maturation through various leaf area to fruit ratio modifications in Vitis vinifera L. Riesling. Progrès Agric. Vitic. 2010, 127, 68–71. [Google Scholar]
- Parker, A.K.; Hofmann, R.W.; Van Leeuwen, C.; McLachlan, A.R.G.; Trought, M.C.T. Manipulating the leaf area to fruit mass ratio alters the synchrony of total soluble solids accumulation and titratable acidity of grape berries. Aust. J. Grape Wine Res. 2015, 21, 266–276. [Google Scholar] [CrossRef]
- Parker, A.K.; Raw, V.; Martin, D.; Haycock, S.; Sherman, E.; Trought, M.C.T. Reduced grapevine canopy size post-flowering via mechanical trimming alters ripening and yield of ‘Pinot noir’. Vitis 2016, 55, 1–9. [Google Scholar]
- Poni, S.; Gatti, M.; Bernizzoni, F.; Civardi, S.; Bobeica, N.; Magnanini, E.; Palliotti, A. Late leaf removal aimed at delaying ripening in cv. Sangiovese: Physiological assessment and vine performance. Aust. J. Grape Wine Res. 2013, 19, 378–387. [Google Scholar] [CrossRef]
- Filippetti, I.; Movahed, N.; Allegro, G.; Valentini, G.; Pastore, C.; Colucci, E.; Intrieri, C. Effect of post-veraison source limitation on the accumulation of sugar, anthocyanins and seed tannins in Vitis vinifera cv. Sangiovese berries. Aust. J. Grape Wine Res. 2014, 21, 1–10. [Google Scholar]
- Tardaguila, J.; Petrie, P.R.; Poni, S.; Diago, M.P.; Martinez de Toda, F. Effects of mechanical thinning on yield and fruit composition of Tempranillo and Grenache grapes trained to a vertical shoot-positioned canopy. Am. J. Enol. Vitic. 2008, 59, 412–417. [Google Scholar]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Smart, R.E.; Dick, J.K.; Gravett, I.M.; Fisher, B.M. Canopy management to improve grape yield and wine quality-principles and practices. S. Afr. J. Enol. Vitic. 1990, 11, 3–17. [Google Scholar] [CrossRef]
- Vasconcelos, M.C.; Castagnoli, S. Leaf canopy structure and vine performance. Am. J. Enol. Vitic. 2000, 51, 390–396. [Google Scholar]
- Hunter, J.J.; Visser, J.H. The effect of partial defoliation, leaf position and developmental stage of the vine on the photosynthetic activity of Vitis vinifera L. cv Cabernet Sauvignon. S. Afr. J. Enol. Vitic. 1988, 9, 9–15. [Google Scholar] [CrossRef]
- Petrie, P.R.; Trought, M.C.T.; Howell, G.S. Influence of leaf ageing, leaf area and crop load on photosynthesis, stomatal conductance and senescence of grapevine (Vitis vinifera L. cv. Pinot Noir) leaves. Vitis 2000, 39, 31–36. [Google Scholar]
- Petrie, P.R.; Trought, M.C.; Howell, G.S.; Buchan, G.D. The effect of leaf removal and canopy height on whole-vine gas exchange and fruit development of Vitis vinifera L. Sauvignon Blanc. Funct. Plant Biol. 2003, 30, 711–717. [Google Scholar] [CrossRef]
- Soar, C.J.; Dry, P.R.; Loveys, B.R. Scion photosynthesis and leaf gas exchange in Vitis vinifera L. cv. Shiraz: Mediation of rootstock effects via xylem sap ABA. Aust. J. Grape Wine Res. 2006, 12, 82–96. [Google Scholar] [CrossRef]
- Flexas, J.; Barón, M.; Bota, J.; Ducruet, J.M.; Gallé, A.; Galmés, J.; Jiménez, M.; Pou, A.; Ribas-Carbó, M.; Sajnani, C.; et al. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris). J. Exp. Bot. 2009, 60, 2361–2377. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Giorio, P.; Nuzzo, V. Leaf area, light environment, and gas exchange in Montepulciano grapevines trained to Tendone trellising system. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2012, 146, 322–333. [Google Scholar]
- Candolfi-Vasconcelos, M.C.; Koblet, W. Influences of partial defoliation on gas exchange parameters and chlorophyll content of field-grown grapevines: Mechanisms and limitations of the compensation capacity. Vitis 1991, 30, 129–141. [Google Scholar]
- Glanz-Idan, N.; Wolf, S. Upregulation of photosynthesis in mineral nutrition-deficient tomato plants by reduced source-to-sink ratio. Plant Signal. Behav. 2020, 15, 1712543. [Google Scholar] [CrossRef]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-taking plants: Anisohydric behavior as a stress-resistance trait. Plant. Signal. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef]
- Matese, A.; Baraldi, R.; Berton, A.; Cesaraccio, C.; Di Gennaro, S.F.; Duce, P.; Facini, O.; Mameli, M.G.; Piga, A.; Zaldei, A. Estimation of water stress in grapevines using proximal and remote sensing methods. Remote Sens. 2018, 10, 114. [Google Scholar] [CrossRef]
- Mitra, S.; Irshad, M.; Debnath, B.; Lu, X.; Li, M.; Dash, C.K.; Qiu, D. Effect of vineyard soil variability on chlorophyll fluorescence, yield and quality of table grape as influenced by soil moisture, grown under double cropping system in protected condition. PeerJ 2018, 6, e5592. [Google Scholar] [CrossRef]
- Bergqvist, J.; Dokoozlian, N.; Ebisuda, N. Sunlight exposure and temperature effects on berry growth and composition of Cabernet Sauvignon and Grenache in the Central San Joaquin Valley of California. Am. J. Enol. Vitic. 2001, 52, 1–7. [Google Scholar]
- Pastore, C.; Zenoni, S.; Fasoli, M.; Pezzotti, M.; Tornielli, G.B.; Filippetti, I. Selective defoliation affects plant growth, fruit transcriptional ripening program and flavonoid metabolism in grapevine. BMC Plant Biol. 2013, 13, 1–16. [Google Scholar] [CrossRef]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality: A review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- Holzapfel, B.P.; Smith, J.P.; Field, S.K.; Hardie, W.J. Dynamics of carbohydrate reserves in cultivated grapevines and vine growth. Hortic. Rev. 2010, 37, 143–211. [Google Scholar]
- Frioni, T.; Acimovic, D.; Tombesi, S.; Sivilotti, P.; Palliotti, A.; Poni, S.; Sabbatini, P. Changes in within-shoot carbon partitioning in Pinot Noir grapevines subjected to early basal leaf removal. Front. Plant Sci. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Ruffner, H.P.; Volschenk, C.G.; Le Roux, D.J. Partial defoliation of Vitis vinifera L. cv. Cabernet Sauvignon/99Richter: Effect on root growth, canopy efficiency, grape compo-sition, and wine quality. Am. J. Enol. Vitic. 1995, 46, 306–314. [Google Scholar]
- Main, G.L.; Morris, J.R. Leaf-removal effects on Cynthiana yield, juice composition, and wine composition. Am. J. Enol. Vitic. 2004, 55, 147–152. [Google Scholar]
- Tardaguila, J.; de Toda, F.M.; Poni, S.; Diago, M.P. Impact of early leaf removal on yield and fruit and wine composition of Vitis vinifera L. Graciano and Carignan. Am. J. Enol. Vitic. 2010, 61, 372–381. [Google Scholar]
- Carbonell-Bejerano, P.; Diago, M.-P.; Martínez-Abaigar, J.; Martínez-Zapater, J.M.; Tardáguila, J.; Núñez-Olivera, E. Solar ultraviolet radiation is necessary to enhance grapevine fruit ripening transcriptional and phenolic responses. BMC Plant Biol. 2014, 14, 183. [Google Scholar] [CrossRef]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar]
- Movahed, N.; Pastore, C.; Cellini, A.; Allegro, G.; Valentini, G.; Zenoni, S.; Cavallini, E.; D’Incà, E.; Tornielli, G.B.; Filippetti, I. The grapevine VviPrx31 peroxidase as a candidate gene involved in anthocyanin degradation in ripening berries under high temperature. J. Plant Res. 2016, 129, 513–526. [Google Scholar] [CrossRef]
- Arrizabalaga, M.; Morales, F.; Oyarzun, M.; Delrot, S.; Gomès, E.; Irigoyen, J.J.; Hilbert, G.; Pascual, I. Tempranillo clones differ in the response of berry sugar and anthocyanin accumulation to elevated temperature. Plant Sci. 2018, 267, 74–83. [Google Scholar] [CrossRef]
- Mucalo, A.; Budić-Leto, I.; Lukšić, K.; Maletić, E.; Zdunić, G. Early Defoliation Techniques Enhance Yield Components, Grape and Wine Composition of cv. Trnjak (Vitis vinifera L.) in Dalmatian Hinterland Wine Region. Plants 2021, 10, 551. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, K.W.; Lorenz, D.H. Phänologische Entwick-lungs-stadien der Rebe. Nachrichtenblatt des Deutschen Pflanzen- schutzdienstes, Braunschweig 1977, 29, 119–120. [Google Scholar]
- Cataldo, E.; Salvi, L.; Mattii, G.B. Effects of irrigation on ecophysiology, sugar content and thiol precursors (3-S-cysteinylhexan-1-ol and 3-S-glutathionylhexan-1-ol) on Vitis vinifera cv. Sauvignon Blanc. Plant Physiol. Biochem. 2021, 164, 247–259. [Google Scholar] [CrossRef]
- Lopes, M.A.; Pinto, P. Easy and accurate estimation of grapevine leaf area with simple mathematical models. Vitis 2005, 44, 55–61. [Google Scholar]
- De Miguel, P.S.; Junquera, P.; De la Fuente, M.; Jimenez, L.; Linares, R.; Trujillo, P.B.; Gutiérrez, J.R.L.G. Estimation of vineyard leaf area by linear regression. Span. J. Agric. Res. 2011, 202–212. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence: A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Sommer, S.; Cohen, S.D. Comparison of different extraction methods to predict anthocyanin concentration and color characteristics of red wines. Fermentation 2018, 4, 39. [Google Scholar] [CrossRef]
- Yamane, T.; Jeong, S.T.; Goto-Yamamoto, N.; Koshita, Y.; Kobayashi, S. Effects of temperature on anthocyanin biosynthesis in grape berry skins. Am. J. Enol. Vitic. 2006, 57, 54–59. [Google Scholar]
- Fernandes de Oliveira, A.; Mercenaro, L.; Del Caro, A.; Pretti, L.; Nieddu, G. Distinctive Anthocyanin Accumulation Responses to Temperature and Natural UV Radiation of Two Field-Grown Vitis vinifera L. Cultivars. Molecules 2015, 20, 2061–2080. [Google Scholar] [CrossRef]
- Allaire, J. RStudio: Integrated Development Environment for R; Citeseer: Boston, MA, USA, 2012; Volume 770, p. 394. [Google Scholar]
| Total Leaf Area (2018) | Total Leaf Area (2019) | |||||
|---|---|---|---|---|---|---|
| Stage | CTRL | LR4 | LR8 | CTRL | LR4 | LR8 |
| Fruit set | 1.15 ± 0.46 a | 0.77 ± 0.21 b | 0.46 ± 0.12 c | 1.00 ± 0.38 a | 0.77 ± 0.17 b | 0.42 ± 0.10 c |
| Full veraison | 1.24 ± 0.40 a | 0.82 ± 0.12 b | 0.58 ± 0.21 b | 1.11 ± 0.32 a | 0.86 ± 0.11 b | 0.54 ± 0.17 c |
| Mid-maturation | 1.41 ± 0.49 a | 0.99 ± 0.28 b | 0.87 ± 0.34 b | 1.31 ± 0.26 a | 1.00 ± 0.31 a | 0.74 ± 0.23 b |
| Full maturation | 1.62 ± 0.34 a | 1.19 ± 0.43 b | 0.78 ± 0.35 c | 1.55 ± 0.30 a | 1.13 ± 0.32 ab | 0.82 ± 0.28 b |
| Pn (µmol CO2 m2 s−1) | gs (mmol H2O m2 s−1) | |||||
| Stage | CTRL | LR4 | LR8 | CTRL | LR4 | LR8 |
| 31 July 2018 | 11.05 ± 4.11 b | 14.68 ± 3.96 a | 15.11 ± 2.87 a | 126.50 ± 41.78 b | 198.00 ± 31.63 a | 187.70 ± 24.43 a |
| 27 August 2018 | 8.24 ± 2.08 b | 12.51 ± 2.62 a | 13.85 ± 3.85 a | 114.87 ± 16.08 a | 117.70 ± 26.43 a | 115.35 ± 32.73 a |
| 25 September 2018 | 7.98 ± 1.99 b | 9.46 ± 2.76 a | 9.16 ± 3.80 a | 99.49 ± 22.45 b | 120.11 ± 34.17 a | 124.10 ± 24.53 a |
| 25 July 2019 | 10.65 ± 3.42 b | 15.42 ± 1.58 a | 15.55 ± 1.57 a | 116.25 ± 35.66 b | 145.10 ± 33.85 b | 209.80 ± 29.30 a |
| 20 August 2019 | 7.58 ± 2.68 b | 11.93 ± 2.56 a | 12.53 ± 3.37 a | 99.60 ± 20.41 b | 115.55 ± 32.17 a | 102.88 ± 29.15 ab |
| 18 September 2019 | 5.82 ± 2.33 b | 7.61 ± 2.56 a | 7.64 ± 3.75 a | 92.30 ± 22.70 a | 91.40 ± 31.75 a | 95.65 ± 33.08 a |
| eWUE (µmol CO2/mmol H2O) | Ψleaf (MPa) | |||||
| Stage | CTRL | LR4 | LR8 | CTRL | LR4 | LR8 |
| 31 July 2018 | 2.64 ± 0.54 b | 2.26 ± 0.31 ab | 1.58 ± 0.60 a | −1.04 ± 1.41 a | −1.07 ± 1.22 a | −1.03 ± 1.39 a |
| 27 August 2018 | 2.00 ± 0.37 a | 1.82 ± 0.64 a | 1.69 ± 0.68 a | −1.27 ± 1.71 a | −1.28 ± 1.81 a | −1.36 ± 0.99 a |
| 25 September 2018 | 2.89 ± 0.32 b | 2.02 ± 0.55 a | 2.11 ± 0.84 a | −1.25 ± 1.36 a | −1.26 ± 1.52 a | −1.20 ± 1.33 a |
| 25 July 2019 | 2.76 ± 0.78 b | 1.70 ± 0.82 a | 1.68 ± 0.46 a | −1.07 ± 0.70 a | −1.05 ± 1.08 a | −1.06 ± 0.98 a |
| 20 August 2019 | 1.65 ± 0.54 a | 1.49 ± 0.23 a | 1.45 ± 0.46 a | −1.28 ± 1.79 a | −1.30 ± 1.95 a | −1.37 ± 1.13 a |
| 18 September 2019 | 2.53 ± 0.75 b | 1.77 ± 0.83 ab | 1.41 ± 0.59 a | −1.29 ± 1.77 a | −1.29 ± 1.61 a | −1.21 ± 1.68 a |
| Sugar Content (°Brix) | TA (mg L−1 Tartaric Acid) | |||||
| Stage | CTRL | LR4 | LR8 | CTRL | LR4 | LR8 |
| 31 July 2018 | 12.92 ± 0.09 a | 13.81 ± 0.28 a | 12.7 ± 0.35 a | 13.84 ± 0.07 a | 13.68 ± 0.05 a | 14.52 ± 0.09 a |
| 27 August 2018 | 22.00 ± 0.12 a | 21.89 ± 0.18 a | 20.81 ± 0.20 a | 6.90 ± 0.10 b | 7.30 ± 0.07 ab | 7.74 ± 0.03 a |
| 25 September 2018 | 26.16 ± 0.09 a | 26.88 ± 0.15 a | 25.19 ± 0.05 b | 5.98 ± 0.03 ab | 5.86 ± 0.04 b | 6.41 ± 0.06 a |
| 25 July 2019 | 13.10 ± 0.06 b | 16.29 ± 0.10 a | 13.08 ± 0.07 b | 13.42 ± 0.08 a | 13.00 ± 0.06 a | 13.82 ± 0.07 a |
| 20 August 2019 | 22.50 ± 0.07 a | 22.59 ± 0.03 a | 21.26 ± 0.15 a | 6.36 ± 0.04 b | 6.70 ± 0.08 ab | 7.54 ± 0.010 a |
| 18 September 2019 | 26.90 ± 0.22 b | 27.75 ± 0.21 a | 25.98 ± 0.19 c | 5.43 ± 0.07 b | 5.53 ± 0.09 b | 6.02 ± 0.06 a |
| pH | Berry Weight (g) | |||||
| Stage | CTRL | LR4 | LR8 | CTRL | LR4 | LR8 |
| 31 July 2018 | 2.60 ± 0.05 a | 2.75 ± 0.02 a | 2.55 ± 0.05 a | 0.88 ± 0.02 a | 0.81 ± 0.04 a | 0.82 ± 0.02 a |
| 27 August 2018 | 3.28 ± 0.08 a | 3.22 ± 0.04 a | 3.10 ± 0.05 a | 1.12 ± 0.10 a | 1.00 ± 0.08 ab | 0.90 ± 0.05 b |
| 25 September 2018 | 3.53 ± 0.03 a | 3.54 ± 0.02 a | 3.22 ± 0.04 b | 1.18 ± 0.05 a | 1.16 ± 0.02 a | 1.13 ± 0.03 a |
| 25 July 2019 | 3.04 ± 0.08 a | 3.02 ± 0.03 a | 3.02 ± 0.07 a | 0.85 ± 0.04 a | 0.75 ± 0.03 a | 0.70 ± 0.04 b |
| 20 August 2019 | 3.30 ± 0.02 a | 3.28 ± 0.03 a | 3.21 ± 0.01 a | 1.03 ± 0.05 a | 0.92 ± 0.05 a | 0.98 ± 0.03 a |
| 18 September 2019 | 3.70 ± 0.05 a | 3.64 ± 0.03 a | 3.05 ± 0.02 b | 1.15 ± 0.08 a | 1.12 ± 0.05 a | 1.12 ± 0.03 a |
| Tot. Anth. (mg L−1) | Extr. Anth. (mg L−1) | |||||
| Stage | CTRL | LR4 | LR8 | CTRL | LR4 | LR8 |
| 31 July 2018 | 751.41 ± 27.68 b | 880.25 ± 16.44 a | 738.50 ± 10.68 b | 370.40 ± 9.21 b | 393.73 ± 13.86 a | 344.15 ± 9.41 c |
| 27 August 2018 | 1450.75 ± 24.87 b | 1550.50 ± 21.22 a | 1312.50 ± 17.89 c | 638.75 ± 15.87 b | 653.75 ± 11.04 a | 595.00 ± 14.16 c |
| 25 September 18 | 1690.15 ± 23.55 b | 1830.12 ± 19.48 a | 1490.75 ± 14.32 c | 737.15 ± 7.60 b | 850.15 ± 10.09 a | 645.25 ± 13.91 c |
| 25 July 2019 | 938.23 ± 11.74 b | 1030.20 ± 8.86 a | 910.51 ± 7.53 b | 561.22 ± 7.60 b | 743.20 ± 15.58 a | 505.34 ± 14.78 c |
| 20 August 2019 | 1605.20 ± 10.00 b | 2030.77 ± 12.62 a | 1459.50 ± 12.62 c | 1241.27 ± 9.44 b | 1470.50 ± 7.66 a | 1025.31 ± 11.73 c |
| 18 September 19 | 1711.00 ± 23.14 b | 2227.50 ± 7.08 a | 1437.30 ± 21.98 c | 782.51 ± 16.12 b | 1087.78 ± 9.12 a | 695.37 ± 10.07 c |
| Tot. Polyp. (mg L−1) | Extr. Polyp. (mg L−1) | |||||
| Stage | CTRL | LR4 | LR8 | CTRL | LR4 | LR8 |
| 31 July 2018 | 3164.54 ± 46.76 b | 3247.10 ± 43.16 a | 3195.27 ± 36.89 b | 2832.80 ± 39.67 a | 2889.67 ± 45.98 a | 2876.90 ± 42.76 a |
| 27 August 2018 | 3395.87 ± 31.72 b | 3439.08 ± 33.05 ab | 3457.15 ± 29.34 a | 2920.60 ± 45.00 a | 2974.97 ± 34.16 a | 2901.56 ± 67.81 a |
| 25 September 18 | 3367.29 ± 49.15 a | 3155.70 ± 54.25 b | 3389.34 ± 44.21 a | 2889.45 ± 32.57 a | 2950.12 ± 54.15 a | 2745.98 ± 64.75 b |
| 25 July 2019 | 4021.65 ± 24.43 a | 4005.21 ± 37.78 a | 4051.81 ± 37.60 a | 3682.59 ± 29.62 a | 3614.36 ± 55.08 a | 3669.18 ± 63.12 a |
| 20 August 2019 | 3996.14 ± 43.22 a | 4013.25 ± 47.25 a | 3848.61 ± 34.31 b | 3600.45 ± 42.00 a | 3609.67 ± 24.17 a | 3565.45 ± 35.90 b |
| 18 September 19 | 3576.17 ± 38.27 a | 3520.16 ± 32.25 a | 3598.28 ± 34.46 a | 3369.11 ± 57.51 b | 3481.57 ± 55.15 a | 3306.21 ± 45.32 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Mattii, G.B. Effects of Defoliation at Fruit Set on Vine Physiology and Berry Composition in Cabernet Sauvignon Grapevines. Plants 2021, 10, 1183. https://doi.org/10.3390/plants10061183
Cataldo E, Salvi L, Paoli F, Fucile M, Mattii GB. Effects of Defoliation at Fruit Set on Vine Physiology and Berry Composition in Cabernet Sauvignon Grapevines. Plants. 2021; 10(6):1183. https://doi.org/10.3390/plants10061183
Chicago/Turabian StyleCataldo, Eleonora, Linda Salvi, Francesca Paoli, Maddalena Fucile, and Giovan Battista Mattii. 2021. "Effects of Defoliation at Fruit Set on Vine Physiology and Berry Composition in Cabernet Sauvignon Grapevines" Plants 10, no. 6: 1183. https://doi.org/10.3390/plants10061183
APA StyleCataldo, E., Salvi, L., Paoli, F., Fucile, M., & Mattii, G. B. (2021). Effects of Defoliation at Fruit Set on Vine Physiology and Berry Composition in Cabernet Sauvignon Grapevines. Plants, 10(6), 1183. https://doi.org/10.3390/plants10061183








