Calligonum polygonoides L. as Novel Source of Bioactive Compounds in Hot Arid Regions: Evaluation of Phytochemical Composition and Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
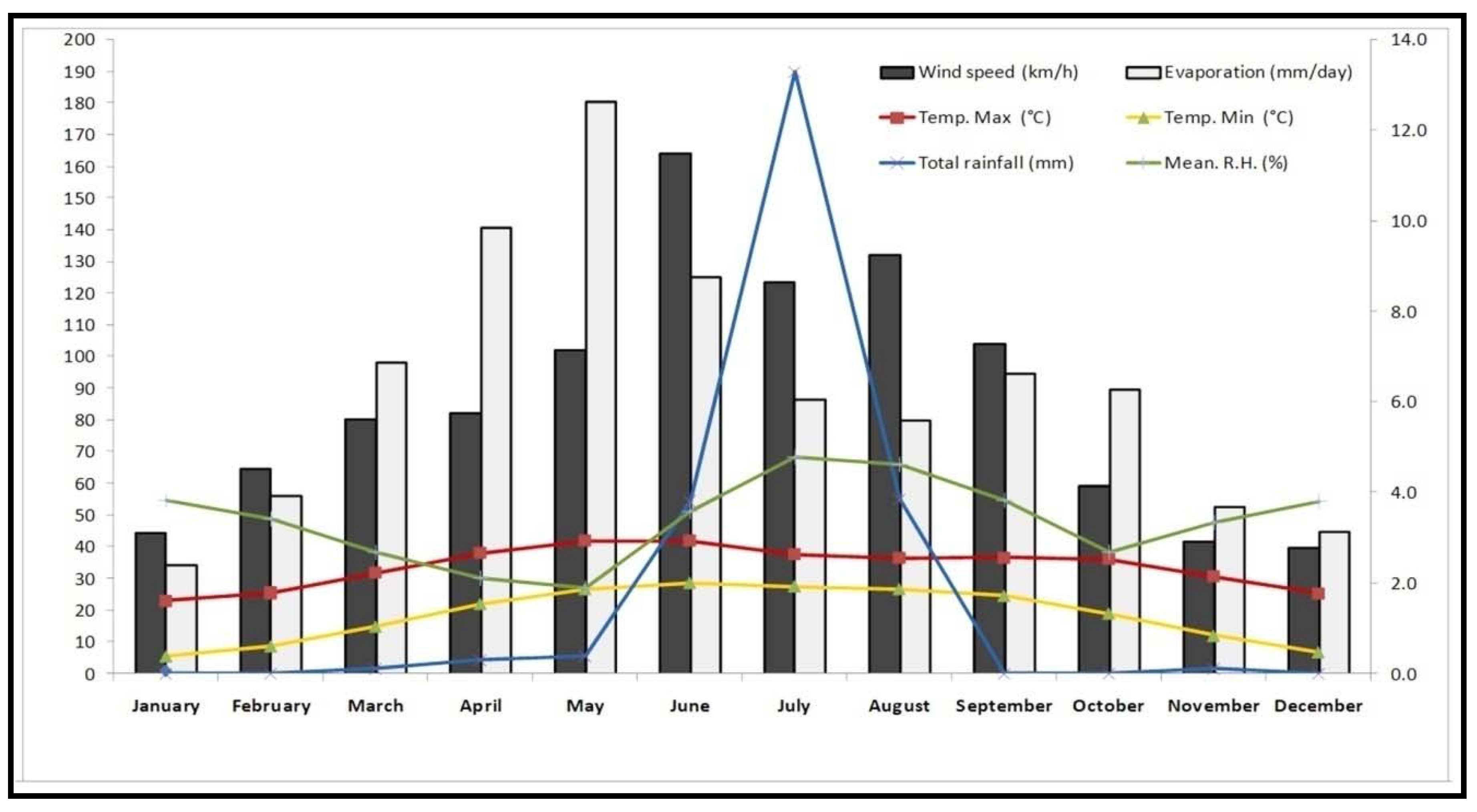
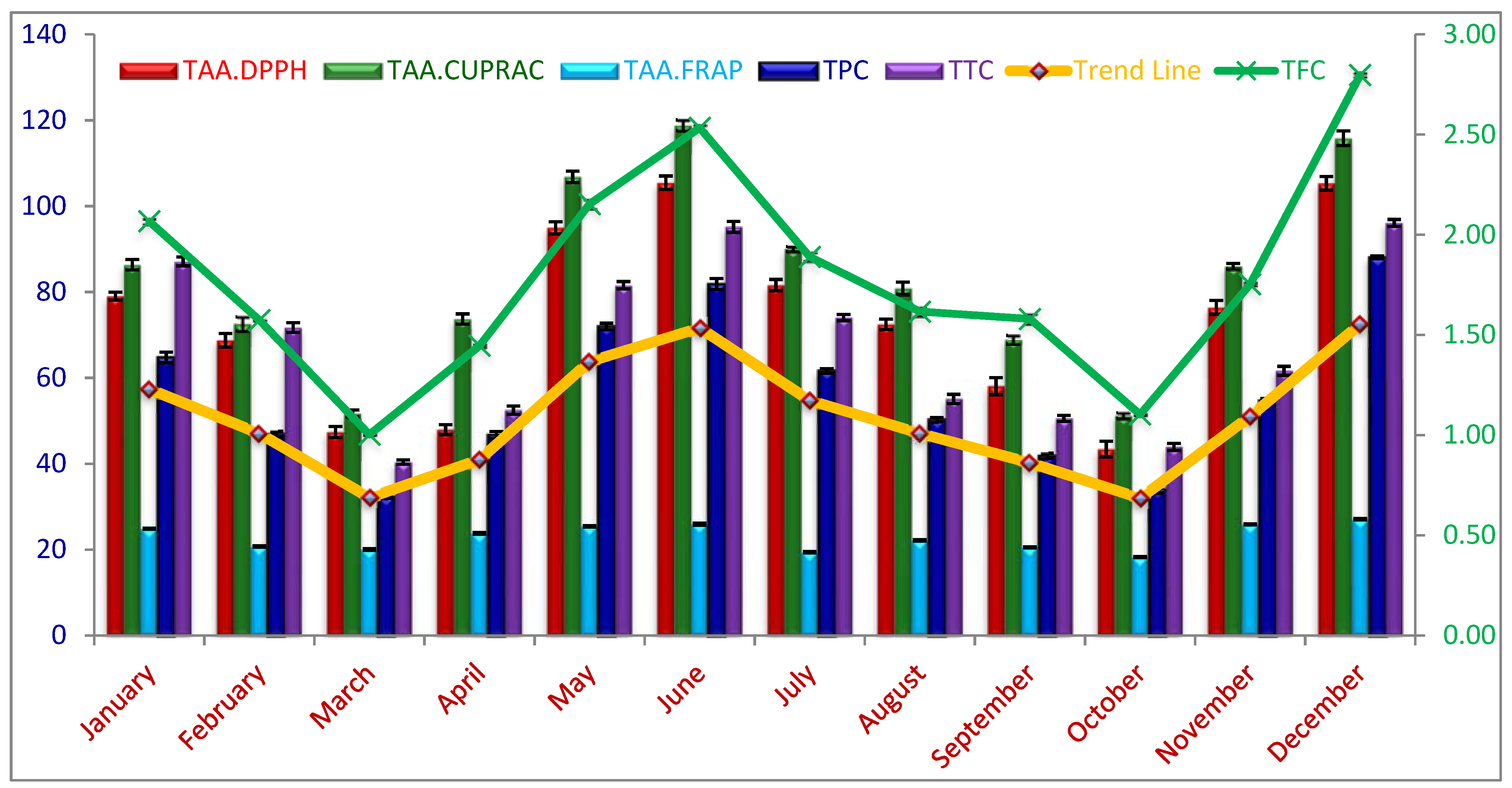
2.1. Seasonal Variations of Total Phenolic Content (TPC), Flavonoids (TFC), and Tannin Content (TTC)
2.2. Seasonal Variations of Total Antioxidant Activity
2.3. Principal Component Analysis (PCA)
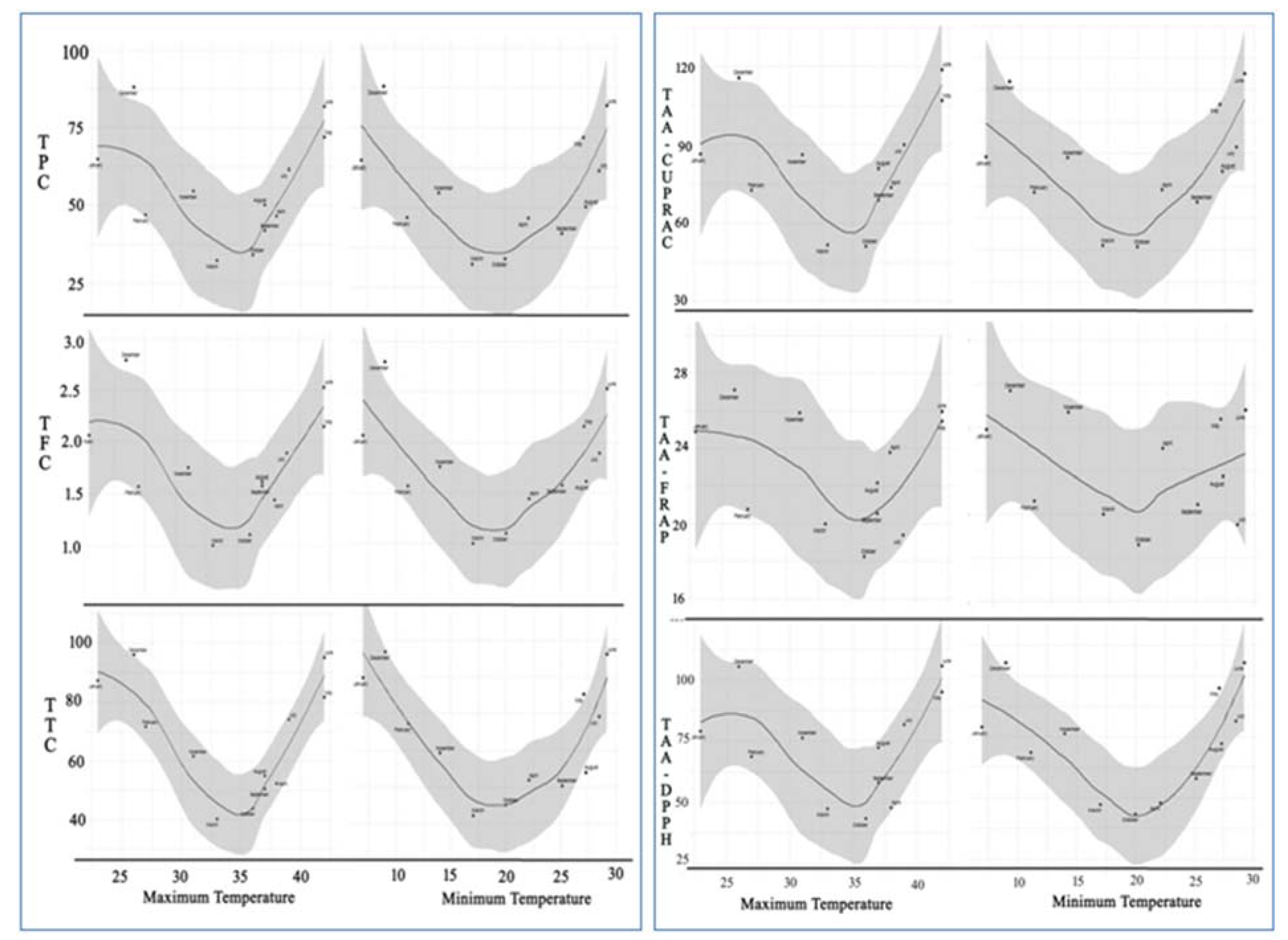
2.4. Loess Regression Analysis against Maximum and Minimum Temperature
2.5. Correlation between Contents of Phenolic Compounds and Antioxidant Activity
3. Discussion
4. Material and Methods
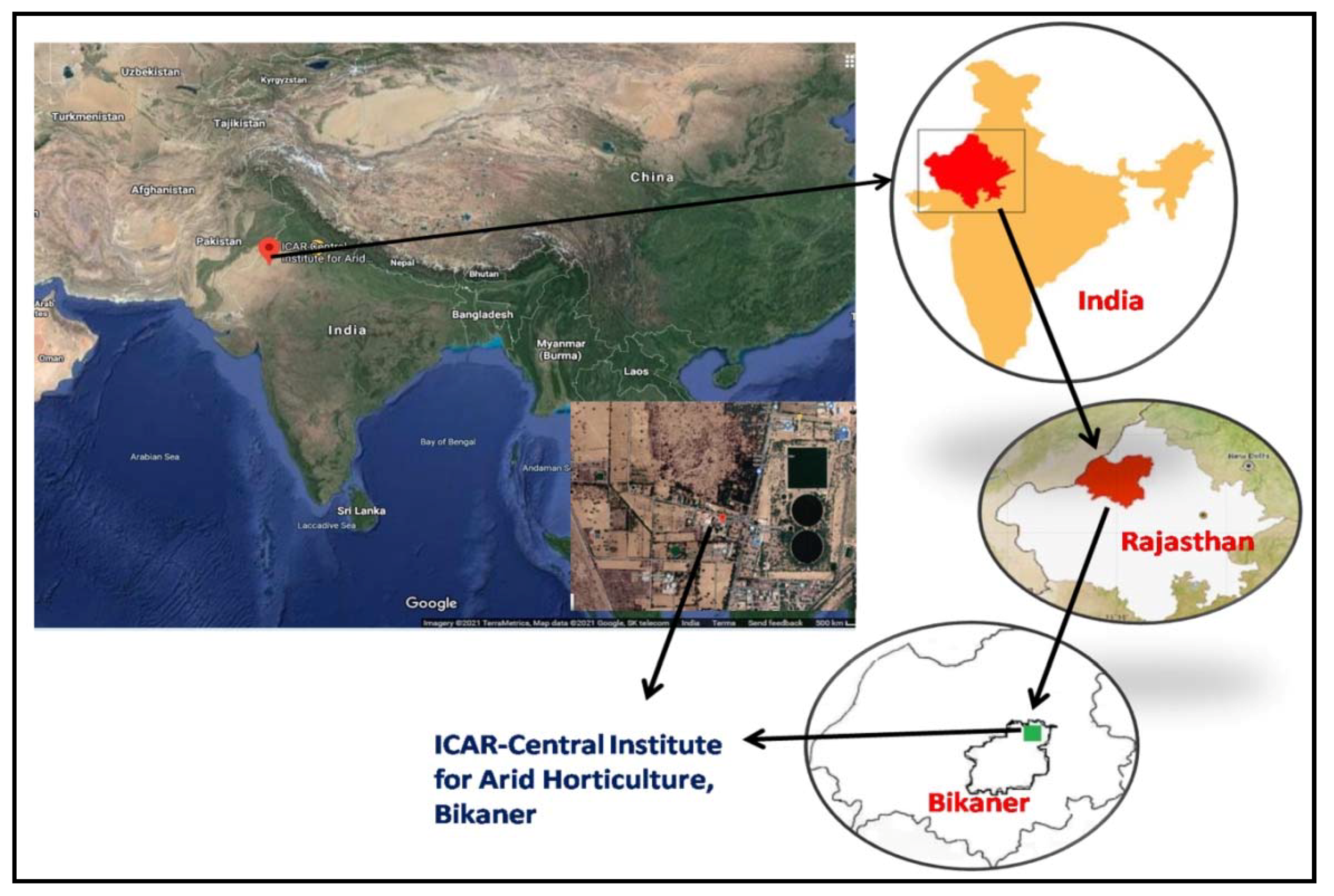
4.1. Experimental Site and Plant Material
4.2. Chemicals
4.3. Sample Extraction
4.4. Total Phenolic Content (TPC)
4.5. Total Flavonoids Content (TFC)
4.6. Tannin Content (TTC)
4.7. Total Antioxidant Activity (TAA)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jalili, A.; Sadeghzade, A. Comparative phenolic profile of persian walnut (Juglans regia L.) leaves cultivars grown in Iran. Afr. J. Biochem. Res. 2012, 6, 33–38. [Google Scholar]
- Figueroa, F.; Marhuenda, J.; Cerda, B.; Zafrilla, P.; Martinez-Cacha, A.; Tejada, L.; Villaño, D.; Mulero, J. HPLC-DAD determination and availability of phenolic compounds in 10 genotypes of walnuts. Int. J. Food Prop. 2016, 20, 1–33. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L.; Pereira, J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) Green Husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef]
- Contini, M.; Baccelloni, S.; Massantini, R.; Anelli, G. Extraction of natural antioxidants from hazelnut (Corylus Avellana L.) shell and skin wastes by long maceration at room temperature. Food Chem. 2008, 110, 659–669. [Google Scholar] [CrossRef]
- Khan, T.I. Conservation of biodiversity in Western India. Environment 1997, 17, 283–287. [Google Scholar]
- Samadia, D.K.; Berwal, M.K.; Gurjar, P.S.; Verma, A.K.; Chaodhary, H.R. Phog Production Techniques Standardized for Horticultural Promotion; ICAR-CIAH: Beechhwal Rural, India, 2020; Bikaner News Letter: July–December. [Google Scholar]
- Bhandari, M.M. Flora of the Indian Desert; Scientific Publishers: Jodhpur, Rajasthan, 1978; pp. 331–332. [Google Scholar]
- Berwal, M.K.; Haldhar, S.M.; Chet Ram Saroj, P.L. Phenolic compositions, total phenolic, flavonoids and antioxidant capacity of an unexploited herb Calligonum polygonoides L. from Thar Desert. J. Environ. Biol. 2021, 42. (accepted). [Google Scholar]
- Samejo, M.Q.; Memon, S.M.I.; Bhanger, K.; Khan, M. Preliminary phytochemical screening of Calligonum polygonoides Linn. J. Pharma. Res. 2011, 4, 4402–4403. [Google Scholar]
- Khan, A.; Khan, R.A.; Ahmed, M.; Mustaq, N. In-vitro antioxidant, antifungal and cytotoxic activity of methanolic extract of Calligonum polygonoides. Bangladesh J. Pharmaco. 2015, 10, 316–320. [Google Scholar] [CrossRef]
- Gomes, S.M.C.; Fernandes, I.P.G.; Shekhawat, N.S.; Kumbhat, S.; Oliveira-Brett, A.M. Calligonum polygonoides linnaeus extract: HPLC-EC and total antioxidant capacity evaluation. Electroanalysis 2015, 27, 293–301. [Google Scholar] [CrossRef]
- Yawer, M.A.; Ahmed, E.; Malik, A.; Ashraf, M.; Rasool, M.A.; Afza, N. New lipoxygenase inhibiting constituents from Calligonum polygonoides. Chem. Biodiver. 2007, 7, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Berwal, M.K.; Haldhar, S.M.; Chet Ram Gora, G.S.; Singh, D.; Samadia, D.K. GC-MS/MS based phytochemical screening revealed the therapeutic potential of Calligonum polygonoides L. flower bud against chronic diseases. Pharmacog. Mag. 2021, accepted. [Google Scholar]
- Gomez-Martineza, M.; Ascacio-Valdesa, J.A.; Flores-Gallegosa, A.C.; Gonzalez-Dominguezb, J.; Gomez-Martinezb, S.; Aguilara, C.N.; Morlett-Chaveza, J.A.; Rodriguez-Herreraa, R. Location and tissue effects on phytochemical composition and in vitro antioxidant activity of Moringa oleifera. Ind. Crop. Prod. 2020, 151, 112439. [Google Scholar] [CrossRef]
- Kumar, M.; Saurabh, V.; Tomar, M.; Hasan, M.; Changan, S.; Sasi, M.; Maheshwari, C.; Prajapati, U.; Singh, S.; Prajapat, R.K.; et al. Mango (Mangifera indica L.) leaves: Nutritional composition, phytochemical profile, and health-promoting bioactivities. Antioxidants 2021, 10, 299. [Google Scholar] [CrossRef]
- Kumar, M.; Changan, S.; Tomar, M.; Prajapati, U.; Saurabh, V.; Hasan, M.; Sasi, M.; Maheshwari, C.; Singh, S.; Dhumal, S.; et al. Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Biological Activities. Biomolecules 2021, 11, 614. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Grasso, S.; Arrutia, F.; Choudhary, J.; Singh, S.; Verma, P.; Mahapatra, A.; Patil, S.; et al. Cottonseed: A sustainable contributor to global protein requirements. Trends Food Sci. Technol. 2021, 111, 100–113. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Tiwari, S.; Punia, S.; Tak, Y.; Amarowicz, R.; Bhoite, A.G.; Singh, S.; Joshi, S.; Panesar, P.S.; et al. Recent trends in extraction of plant bioactives using green technologies: A review. Food Chem. 2021, 129431. [Google Scholar] [CrossRef]
- Kumar, M.; Potkule, J.; Patil, S.; Saxena, S.; Patil, P.G.; Mageshwaran, V.; Punia, S.; Varghese, E.; Mahapatra, A.; Ashtaputre, N.; et al. Extraction of ultra-low gossypol protein from cottonseed: Characterization based on antioxidant activity, structural morphology and functional group analysis. LWT 2021, 140, 110692. [Google Scholar] [CrossRef]
- Kumar, M. Paruthi Paal, a nutrient-rich healthy drink from cottonseed: An Indian delicacy. J. Ethn. Foods 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Cao, Y.N.; Deng, B.; Fang, S.Z.; Shang, X.L.; Fu, X.X.; Yang, W.X. Genotypic variation in tree growth and selected flavonoids in leaves of Cyclocarya paliurus. South. For. 2018, 80, 67–74. [Google Scholar] [CrossRef]
- Amaral, J.S.; Seabra, R.; Andrade, P.B.; Valentao, P.; Pereira, J.A.; Ferreres, F. Phenolic profile in the quality control of walnut (Juglans regia L.) leaves. Food Chem. 2004, 88, 373–379. [Google Scholar] [CrossRef]
- Phatak, R.S.; Hendre, A.S. Total antioxidant capacity (TAC) of fresh leaves of Kalanchoe pinnata. J. Pharmacog. Phytochem. 2014, 2, 32–35. [Google Scholar]
- Ferreira, M.L.; Domingos, M. Seasonal characterization of antioxidant responses in plants of Ipomoea nil cv. Scarlet O’Hara. Braz. J. Biol. 2012, 72, 831–837. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, S.; Fu, X.; Shang, X.; Yang, W. Seasonal variation in phenolic compounds and antioxidant activity in leaves of Cyclocarya paliurus (Batal.) Iljinskaja. Forests 2019, 10, 624. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, W.; Xue, L.; Xuejun, P. Seasonal variations of phenolic profiles and antioxidant activity of walnut (Juglans sigillata Dode) green husks. Int. J. Food Prop. 2007, 20, S2635–S2646. [Google Scholar] [CrossRef]
- Zlotek, U.; Mikulska, S.; Nagajek, M.; Swieca, M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Agullo, A.; Pereira, E.; Freire, M.S.; Valentao, P.; Andrade, P.B.; Gonzalez-Alvarez, J.; Pereira, J. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) Green Husks Extracts. Ind. Crops Prod. 2013, 42, 126–132. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Cliford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Noratto, G.D.; Bertoldi, M.C.; Krenek, K.; Talcott, S.T.; Stringheta, P.C.; Mertens-Talcott, S.U. Anticarcinogenic e_ects of polyphenolics from mango (Mangifera indica) varieties. J. Agric. Food Chem. 2010, 58, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Chang, Y.Y.; Hsu, C.L.; Liu, C.W.; Lin, Y.L.; Lin, Y.H.; Liu, K.C.; Chen, Y.C. Antiobesity and hypolipidemic effects of polyphenol-rich longan (Dimocarpus longans Lour.) flower water extract in hypercaloric-dietary rats. J. Agric. Food Chem. 2010, 58, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tomar, M.; Amarowicz, R.; Saurabh, V.; Nair, M.S.; Maheshwari, C.; Sasi, M.; Prajapati, U.; Hasan, M.; Singh, S.; et al. Guava (Psidium guajava L.) leaves: Nutritional composition, phytochemical profile, and health-promoting bioactivities. Foods 2021, 10, 752. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Amarowicz, R.; Kaur, C. Evaluation of cellulolytic enzyme-assisted microwave extraction of Punica granatum peel phenolics and antioxidant Activity. Plant Foods Hum. Nutr. 2020, 75, 614–620. [Google Scholar] [CrossRef]
- Punia, S.; Kumar, M. Litchi (Litchi chinenis) seed: Nutritional profile, bioactivities, and its industrial applications. Trends Food Sci. Technol. 2021, 108, 58–70. [Google Scholar] [CrossRef]
- Kumar, M.; Potkule, J.; Tomar, M.; Punia, S.; Singh, S.; Patil, S.; Singh, S.; Ilakiya, T.; Kaur, C.; Kennedy, J.F. Jackfruit seed slimy sheath, a novel source of pectin: Studies on antioxidant activity, functional group, and structural morphology. Carbohydr. Polym. Technol. Appl. 2021, 2, 100054. [Google Scholar]
- Nishad, J.; Dutta, A.; Saha, S.; Rudra, S.G.; Varghese, E.; Sharma, R.R.; Tomar, M.; Kumar, M.; Kaur, C. Ultrasound-assisted development of stable grapefruit peel polyphenolic nano-emulsion: Optimization and application in improving oxidative stability of mustard oil. Food Chem. 2021, 1–11. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Grasso, S.; Purewal, S.S.; Kaur, M.; Siroha, A.K.; Kumar, K.; Kumar, V.; Kumar, M. Aspergillus oryzae fermented rice bran: A byproduct with enhanced bioactive compounds and antioxidant potential. Foods 2021, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Ruhmann, S.; Leser, C.; Bannert, M.; Treutter, D. Relationship between growth, secondary metabolism, and resistance of apple. Plant. Biol. 2002, 4, 137–143. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defense mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Dong, T.T.; Cui, X.M.; Song, Z.H.; Zhao, K.J.; Ji, Z.N.; Lo, C.K.; Tsim, K.W. Chemical assessment of roots of Panax notoginseng in China: Regional and seasonal variations in its active constituents. J. Agric. Food Chem. 2003, 51, 4617–4623. [Google Scholar] [CrossRef]
- Treutter, D. Biosynthesis of phenolic compounds and its regulation in apple. Plant. Growth Regul. 2001, 34, 71–89. [Google Scholar] [CrossRef]
- Deng, B.; Shang, X.L.; Fang, S.Z.; Li, Q.Q.; Fu, X.X.; Su, J. Integrated effects of light intensity and fertilization on growth and flavonoid accumulation in Cyclocarya paliurus. J. Agric. Food Chem. 2010, 60, 6286–6292. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Henbest, R.G.C.; Davis, F.J.; Battey, N.H.; Hadley, P.; Wagstafe, A. UV irradiance as a major influence on growth, development and secondary products of commercial importance in Lollo Rosso lettuce ‘Revolution’ grown under polyethylene films. Environ. Exp. Bot. 2008, 63, 232–239. [Google Scholar] [CrossRef]
- Crespo-Sempere, A.; Selma-Lazaro, C.; Palumbo, J.D.; González-Candelas, L.; Martinez-Culebras, P.V. Effect of oxidant stressors and phenolic antioxidants on the ochratoxigenic fungus Aspergillus carbonarius. J. Sci. Food Agric. 2016, 96, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Edreva, A. The importance of non-photosynthetic pigments and cinnamic acid derivatives in the photoprotection. Agric. Ecosyst. Environ. 2005, 106, 135–146. [Google Scholar] [CrossRef]
- Margna, U.; Margna, E.; Vainjarv, T. Influence of nitrogen nutrition on the utilization of L-phenylalanine for building flavonoids in buckwheat seedling tissues. J. Plant. Physiol. 1989, 134, 697–702. [Google Scholar] [CrossRef]
- Margna, U. Control at the level of substrate supply-an alternative in the regulation of phenylpropanoid accumulation in plant cells. Phytochemistry 1977, 16, 419–426. [Google Scholar] [CrossRef]
- Phillips, R.; Henshaw, G.G. The regulation of synthesis of phenolics in stationary phase cell cultures of Acer pseudoplatanus L. J. Exp. Bot. 1977, 28, 785–794. [Google Scholar] [CrossRef]
- Ma, M.; Hong, C.L.; An, S.Q.; Li, B. Seasonal, spatial, and interspecific variation in quercetin in Apocynum venetum and Poacynum hendersonii, Chinese traditional herbal teas. J. Agric. Food Chem. 2003, 51, 2390–2393. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Wang, J.; Lu, J. Extraction, distribution and characterisation of phenolic compounds and oil in grapeseeds. Food Chem. 2010, 122, 688–694. [Google Scholar] [CrossRef]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef]
- Rebaya, R.; Belghith, S.I.; Baghdikian, B.; Leddet, V.M.; Mabrouki, F.; Olivier, E.; Cherif, J.K.; Ayadi, M.T. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae). J. App. Pharma. Sci. 2010, 5, 052–057. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing ability of plasma (FRAP) a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- R Core Team. A language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 24 April 2021).
- Friedman, J.; Hastie, T.; Tibshirani, R. Additive logistic regression: A statistical view of boosting (with discussion). Annal. Stat. 2000, 28, 337–407. [Google Scholar] [CrossRef]
- Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-3. 2020. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 24 April 2021).

| Sampling Month | TPC (mg.GAE.g−1) | TFC (mg.CtE.g−1) | TTC (mg.catechin.E.g−1) | Total Antioxidant Activity (mg.AAE.g−1) | ||
|---|---|---|---|---|---|---|
| CUPRAC | FRAP | DPPH | ||||
| January | 64.75 d | 2.07 d | 87.13 b | 86.38 cd | 24.88 c | 79.06 c |
| February | 46.91 f | 1.57 g | 71.73 d | 72.59 e | 20.77 f | 68.75 de |
| March | 32.28 h | 1.00 j | 52.48 f | 51.72 f | 20.02 fg | 47.39 g |
| April | 46.70 f | 1.45 h | 40.40 g | 73.70 e | 23.77 d | 47.95 g |
| May | 71.97 c | 2.15 c | 81.57 c | 106.82 b | 25.46b c | 94.93 b |
| June | 81.84 b | 2.53 b | 95.17 a | 118.84 a | 25.97 b | 105.46 a |
| July | 61.61 d | 1.89 e | 74.05 d | 90.00 c | 19.42 g | 81.65 c |
| August | 50.26 f | 1.62 g | 55.12 f | 80.89 d | 22.18 e | 72.46 e |
| September | 41.87 g | 1.58 g | 50.56 f | 68.88 e | 20.56 f | 58.09 f |
| October | 34.05 h | 1.10 i | 43.94 g | 51.22 f | 18.26 h | 43.42 h |
| November | 54.66 e | 1.75 f | 61.64 e | 86.02 cd | 25.88 b | 76.42 de |
| December | 88.08 a | 2.80 a | 96.09 a | 115.81 a | 27.11 a | 105.30 a |
| TPC | TFC | TTC | TAA.CUPRAC | TAA.FRAP | TAA.DPPH | |
|---|---|---|---|---|---|---|
| TPC | 1 | 0.99 ** | 0.95 ** | 0.98 ** | 0.79 ** | 0.96 ** |
| TFC | 1 | 0.94 ** | 0.97 ** | 0.78 ** | 0.95 ** | |
| TTC | 1 | 0.91 ** | 0.70 * | 0.93 ** | ||
| TAA.CUPRAC | 1 | 0.80 ** | 0.97 ** | |||
| TAA.FRAP | 1 | 0.73 * | ||||
| TAA.DPPH | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berwal, M.K.; Haldhar, S.M.; Ram, C.; Shil, S.; Kumar, R.; Gora, J.S.; Singh, D.; Samadia, D.K.; Kumar, M.; Mekhemar, M. Calligonum polygonoides L. as Novel Source of Bioactive Compounds in Hot Arid Regions: Evaluation of Phytochemical Composition and Antioxidant Activity. Plants 2021, 10, 1156. https://doi.org/10.3390/plants10061156
Berwal MK, Haldhar SM, Ram C, Shil S, Kumar R, Gora JS, Singh D, Samadia DK, Kumar M, Mekhemar M. Calligonum polygonoides L. as Novel Source of Bioactive Compounds in Hot Arid Regions: Evaluation of Phytochemical Composition and Antioxidant Activity. Plants. 2021; 10(6):1156. https://doi.org/10.3390/plants10061156
Chicago/Turabian StyleBerwal, Mukesh K., Shravan M. Haldhar, Chet Ram, Sandip Shil, Ramesh Kumar, Jagan S. Gora, Dhurendra Singh, Dilip K. Samadia, Manoj Kumar, and Mohamed Mekhemar. 2021. "Calligonum polygonoides L. as Novel Source of Bioactive Compounds in Hot Arid Regions: Evaluation of Phytochemical Composition and Antioxidant Activity" Plants 10, no. 6: 1156. https://doi.org/10.3390/plants10061156
APA StyleBerwal, M. K., Haldhar, S. M., Ram, C., Shil, S., Kumar, R., Gora, J. S., Singh, D., Samadia, D. K., Kumar, M., & Mekhemar, M. (2021). Calligonum polygonoides L. as Novel Source of Bioactive Compounds in Hot Arid Regions: Evaluation of Phytochemical Composition and Antioxidant Activity. Plants, 10(6), 1156. https://doi.org/10.3390/plants10061156











