Abstract
Mango peel, a byproduct from the mango processing industry, is a potential source of food-grade mango peel pectin (MPP). Nonetheless, the influence of fruit physical characteristics and phytochemicals of peels on their correspondent pectin level has never been examined, particularly when high-quality food additives are of commercial need. Subsequently, the ultimate aim of the present study was to comprehend their relationship using chemometric data analyses as part of raw material sourcing criteria. Principal component analysis (PCA) advised that mangoes of ‘mahachanok’ and ‘nam dok mai’ could be distinguished from ‘chok anan’ and ‘kaew’ on the basis of physiology, peel morphology, and phytochemical characteristics. Only pectin extracted from mango var. ‘chok anan’ was classified as low-methoxyl type (Mox value ~4%). Using the partial least-squares (PLS) regression, the multivariate correlation between the fruit and peel properties and the degree of esterification (DE) value was reported at R2 > 0.9 and Q2 > 0.8. The coefficient factors illustrated that yields of byproducts such as seed and total biomass negatively influenced DE values, while they were positively correlated with crude fiber and xylose contents of the peels. Overall, it is interesting to highlight that, regardless of the differences in fruit varieties, the amount of biomass and peel proximate properties can be proficiently applied to establish classification of desirable properties of the industrial MPP.
1. Introduction
Mango, the king of fruits with high nutritive value, is extensively cultivated in the tropical and subtropical regions [1]. It is one of the most important commercial fruit crops worldwide in terms of production, processing, and consumption [2,3]. In Thailand, approximately 300,000 tons of local varieties such as ‘mahachanok’, ‘chok anan’, ‘nam dok mai’, and ‘kaew’ mangoes, especially those of ripe fruits, are used for food processing [4]. The commercially processed products include preserved canned fruits, frozen slices, purée, juices, nectar, and various dehydrated products [5,6]. During processing, it is estimated that almost 200,000 tons of food loss is generated, and mango peels account for as much as 24% of those volumes [7]. More importantly, poor management of this industrial loss could have a great impact on the environment; therefore, attempts have been made in order to add value to these byproducts such as biomass from mango processing [8,9,10,11].
Mango peel is a potential source of dietary fiber, and it contains 5–11% pectin depending on fruit varieties and the extraction methods [12,13,14,15]. Additionally, it comprises various classes of polyphenols, carotenoids, and vitamins with excellent antioxidative and functional properties [16,17]. Therefore, this byproduct is a promising target for commercial valorization [18,19]. Previous reports indicated that the peel contains high contents of carbohydrates (80%), crude fiber (8%), and pectin (13%), as well as reasonable quantities of proteins (4%) and fats (2%) [20,21].
Pectin is a structural heteropolysaccharide found in the primary cell walls that provides mechanical strength and flexibility via interaction with other cell-wall components [22]. High contents of pectin can be found in almost all parts of fruits depending on the varieties and maturity stages [23,24,25,26,27]. The major constituent of pectin is poly (1,4)-α-d-galacturonan as a backbone with the carboxyl groups presenting in either free acid or methyl ester forms [28]. Pectin can be categorized into two classes according to the proportion of the esterified groups into low-methoxyl pectin (LMP) (DE < 50%) and high-methoxyl pectin (HMP) (DE > 50%). The latter is an excellent emulsifier and stabilizer which can be used as a gelling agent and thickening agent [29,30,31]. It is also used as a fat replacer and health-promoting functional food ingredient [10,32]. Additionally, pectin can be added to pharmaceutical products such as bioactive components, drug and gene delivery compounds, tissue engineering products, and wound healing patches [33].
To recover pectin from plant resources, microwave-assisted extraction (MAE) is more effective for the extraction of high-quality pectin than conventional heating [34,35,36,37]. Such a technique has been implemented in the recovery of pectin from dietary-rich biomasses such as banana peels [38] and orange peels [39], and it has shown greater success when applied to mango peels [11,12,40]. It is believed that the quality of raw materials is foremost responsible for the extractable quality of the pectin. Nevertheless, the relationship between the physicochemical properties of fruit and peel along and the chemical qualities of the pectin has never been reported, especially to develop an index for raw material sourcing. With this rationale, the objectives of the present study were first to evaluate the physiological and physicochemical characteristics along with the proximate values of mango peels from commercially available mango varieties. Then, their relationships with the chemical qualities of the MAE-extracted mango peel pectin (MPP) were evaluated. The research outcomes will be beneficial for setting up the selection criteria of the biological materials for MPP production on a substantial scale.
2. Results and Discussion
2.1. Physical Characteristics of Mango Fruit Varieties
The physical characteristics of the mango fruits are illustrated in Table 1, including color (L*, a*, b*), arithmetic mean diameter (Da), geometric mean diameter (Dg), aspect ratio (Ra), sphericity (Φ), surface area (S), peel-to-fruit ratio, and the percentage of peel, flesh, and seed.

Table 1.
Physical characteristics of different mango varieties.
Lightness (L*), redness (a*), and yellowness (b*) are considered the most informative parameters for quality assessment of agricultural produce due to its uniform distribution and close relationship with sensorial perception by humans [41,42]. The CIE color space has been used to determine maturity index and the ripening process of mangoes [43,44]. According to the results, L* values varied among different varieties ‘mahachanok’ (68.83), ‘chok anan’ (69.98), and ‘kaew’ (67.68), while ‘nam dok mai’ showed the highest luminosity (72.26). Lightness alteration of the fruit skin has been linked to an advancement of the ripening stage and deterioration of mango fruits [45,46,47]. The a* value is also a good indicator of the ripening process of mango in association with the degradation of chlorophyll, coupled with the loss of greenness in fruit [48]. The value of a* was the largest in ‘nam dok mai’ (6.74) and the lowest in ‘mahachanok’ (3.28). The increase in yellowness as depicted by b* values is attributed to an increase in carotenoid content [49]. The yellowness of mango was the greatest in ‘chok anan’ (43.09) and the least in ‘nam dok mai’ (36.63). Color change in ripe fruit is caused by a reduction in chlorophyll, leading to the synthesis of different types of anthocyanins within the vacuoles [50]. Along with that, carotenoids such as β-carotene, xanthophyll esters, xanthophylls, and lycopene accumulate during the process of ripening in the plastids [51].
The average values of geometric mean diameter (Dg) ranged from 78.66–85.50 with the highest and the lowest values being that of ‘mahachanok’ and ‘kaew’, respectively. The geometric mean diameter represents the central tendency of the primary dimension and is normally used as parameter when designing fruit sorting machines [52,53]. Our result showed that the Dg values were greater than reported by Osadare et al. [54]. The arithmetic mean diameter (Da) is also an important indicator in determining the quality attribute of a particular mango variety according to size and maturity stage [55]. The result showed that size of the ripe mangoes of different varieties varied between 81.76 and 96.30 mm. Both Dg and Da are also regarded as physical parameters for fruit grading [56]. The aspect ratio (Ra) is related to the width-to-length ratio determination, which indicates an ellipsoid shape during the process of fruit development [57]. The higher value in ‘chok anan’ (66.98) and ‘kaew’ (63.32) denotes that the shapes of these mango varieties were round, similar to ‘cogshall’ mango which is triaxial ellipsoid in shape [57]. The sphericity that provides an indication of the tendency of shape varied from 258.13–297.95. The high sphericity of ‘chok anan’ showed its tendency of the morphology toward a sphere. The lowest aspect ratio and sphericity in ‘mahachanok’ indicated its inclination toward an elongated oblong shape. It is worth highlighting that both parameters intercorrelate with each other, and greater values of Ra and sphericity denote more advanced ripening stages of the fruits [58]. Additionally, a higher fruit ripeness leads to a greater content of pectin from fruit peel [59].
The surface areas (S) of mango fruits varied from 180.13–213.31 cm2. The highest area was obtained from ‘mahachanok’ (213.31 cm2), followed by ‘nam dok mai’ (208.09 cm2), ‘chok anan’ (188.70 cm2), and ‘kaew’ (180.13 cm2). Meanwhile, ‘mahachanok’ also illustrated the highest ratio of peel to total fruit weight (16.64%), whereas ‘chok anan’ and ‘nam dok mai’ had the lowest ratio at approximately 14%. Both surface area and peel-to-fruit ratio may be associated with pectin content due to the naturally heterogeneous polysaccharide present in plant cell walls, especially between the middle lamella [60]. However, the composition of pectin in plant cell walls varies as a function of the type and the variety [61]. In term of processing, ‘nam dok mai’ gave the largest yield of flesh (73%) and the least biomass (~26%), followed by ‘kaew’, while ‘mahachanok’ and ‘chok anan’ were much less economical for processing. The most frequently used varieties for processing are ‘sampee’ and ‘kaew’ for candies, ‘mahachanok’ for juice, and ‘chok anan’ for rehydrated mango products, in which heat treatment is commonly incorporated [12]. In comparison with yield compositions in other varieties, Abdualrahm [62] and Anila and Radha [63] reported that peel, seed, and flesh weights of different mango varieties in India were in the ranges of 10–22%, 7–20%, and 58–81%, respectively. On the other hand, nine Hispanic mango varieties consisted of 6–12% peel weight, 4–12% seed weight, and 75–86% flesh weight [64]. We were particularly interested in the valorization of mango peel, and it seems that ‘mahachanok’ is a preferred variety with the highest peel-to-fruit ratio and total biomass. A similar study of Sommano et al. [11] also found that ‘mahachanok’ gave the maximum yield of 6.0% of peel-to-fruit ratio, and a substantial amount of pectin could be obtained.
Additionally, to analyze the overall influence of fruit physical properties and mango varieties, we used a chemometric PCA as presented in Figure 1. All physical properties were combined to reduce the size of the analyzed samples. The first two dimensions of the PCA accounted for a total of 71.54% across the PCA score plot (PC1 = 40.43% and PC2 = 31.11% of the variance). The PCA plot illustrated that ‘chok anan’ and ‘kaew’ were somewhat identical in terms of their physiology, whereas ‘nam dok mai’ and ‘mahachanok’ were highly distinctive from others.
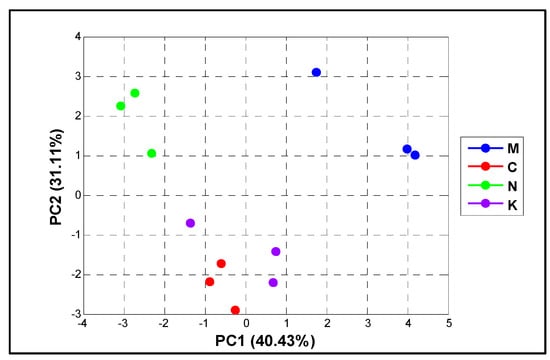
Figure 1.
The chemometric PCA score plot based on the physiological characteristics and mango varieties (‘mahachanok’; M, ‘chok anan’; C, ‘nam dok mai’; N, ‘kaew’; K).
2.2. Physicochemical Characteristics of Mango Peel
2.2.1. FT-IR
FT-IR was used to identify the functional groups of the phytochemical compositions of mango peel (Figure 2). The FT-IR region ranged from 600 to 4000 cm−1. This demonstrates the similarity of the absorbance patterns in peel from different varieties. The broad and intense peak at around 3400 cm−1 represents stretching of O–H group due to inter- and intramolecular hydrogen bonding of polymeric compounds such as alcohols, phenols, and carboxylic acids, as in pectin, cellulose, and lignin [65]. The small peak at 2900 cm−1 indicates C–H stretching of the CH2 groups [66,67]. The small absorption at around 1730 cm−1 shows the characteristic of esterified pectin, arising from the ester carbonyl stretching band [68]. The region at wavenumbers between 1500 and 1800 cm−1 is associated with the assessment of the degree of methylation [69]. The region between 900 and 1200 cm−1 is accordingly referred to as the ‘fingerprint’ for carbohydrates, especially in terms of sugar composition [70]. The peaks relate to the characteristics of pectin polysaccharides (polygalacturonic acid) identified at 962, 1024, 1099, 1156, and 1223 cm−1, which were assigned to C–O bending, C–C stretching, C–O stretching, C–H stretching, and C–O stretching, respectively [71]. The peaks at 1370 cm−1 could be the symmetric stretching of –COO− of pectin [72]. The FT-IR patterns consequently verify that the mango peel was composed of pectin.
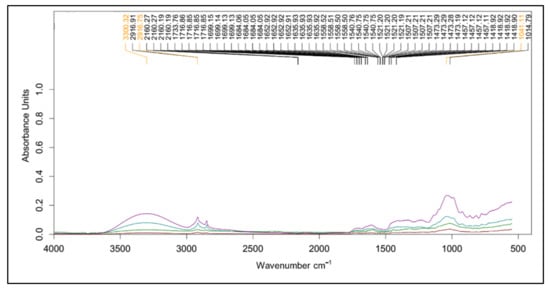
Figure 2.
The FT-IR spectra of mango peels var. ‘mahachanok’ (―), ‘chok anan’ (―), ‘nam dok mai’ (―), and ‘kaew’ (―) from 600 to 4000 cm−1 (x-axis) in terms of absorbance units (y-axis).
2.2.2. Scanning Electron Microscopy (SEM) and Light Microscopy (LM)
The mango peel structure was characterized using SEM and LM as illustrated in Figure 3. SEM images showed that cell packings of ‘mahachanok’ (a) and ‘nam dok mai’ (c) were similar, and cells had an angular polyhedral shape with a flat cell compartment, as well as great intercellular space. On the other hand, ‘chok anan’ (b) cells were spherical, with a large compartment and less intercellular space. The cellular profile of ‘kaew’(d) was slightly irregular, with a flat compartment and no obvious intercellular space. According to LM observations, the intercellular space characteristics (I) of all mango varieties were associated with the SEM visualization. The variety of ‘chok anan’ exhibited a larger size and more space, while ‘kaew’ showed a small space and fewer cells. In addition, the color of cell components stained with toluidine blue O could define the different chemical compositions of each peel variety. Toluidine blue O is a cationic dye that binds to negatively charged groups and provides different colors, including a pinkish purple color when reacting with carboxylated polysaccharides such as pectin, a green, greenish blue, or bright blue color when reacting with aromatic substances such as lignin and tannins, and a purplish or greenish blue color when reacting with nucleic acids [73,74]. From the LM results, it could be implied that all varieties were composed of pectin, lignin, or tannin and nucleic acids because of their cell color. However, ‘chok anan’ and ‘kaew’ presented a high intensity of pinkish purple color as compared to the others. Therefore, they possibly contained a high content of pectin.
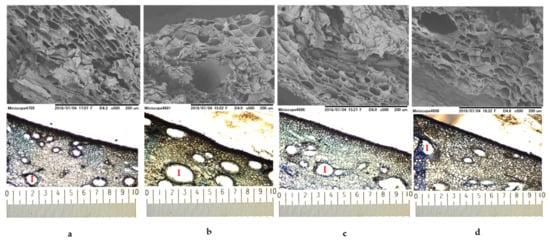
Figure 3.
The SEM and LM images of mango peels var. ‘mahachanok’ (a), ‘chok anan’ (b), ‘nam dok mai’ (c), and ‘kaew’ (d). The images were viewed at ×500 and ×50 (0.1 mm/div).
To describe and compare the mango peel anatomy of each variety, we then quantified cell structure compositions, as shown in Table 2. The greatest value of epidermis thickness was obtained in ‘chok anan’, followed by ‘mahachanok’, ‘nam dok mai’, ‘kaew’. While ‘mahachanok’ showed the highest cell density and number of intercellular spaces, the lowest of these values were seen in ‘kaew’. However, ‘mahachanok’ gave the lowest size of intercellular space. The size of the cell compartment of ‘chok anan’ and ‘nam dok mai’ was somewhat similar, whereas ‘mahachanok’ had the smallest. The decrease in the number of cell layers was associated with peel firmness, which declined along with the advancement in the ripening stage [75]. During ripening in most fruits, parenchyma cell walls are considerably modified, altering their mechanical properties, and the walls of some cells collapse while some cells fuse with others [76]. Cell-wall and middle lamella modifications (dissolution and depolymerization of pectin, hemicellulose, and cellulose) leading to fruit softening result from the action of cell-wall-modifying enzymes, including polygalacturonase, pectin methylesterase, pectate lyase, β-galactosidase, and cellulase [77,78,79]. Accordingly, the differences in anatomical components in each mango peel variety were possibly due to the variation of maturity stage. Similar results were also found in the peel of ‘hom thong’ banana at different maturation stages [75]. Noteworthily, soluble pectin is a general indicator of fruit ripening. To add to this point, the ripening process provides an increase in the content of pectin loosely bound to the cell wall [80,81], which occurs in parallel with a decrease in the amount of covalently bound pectin [82,83,84]. This implies that the ripening stage and the extraction ability of pectin from the cell are intercorrelated.

Table 2.
Anatomical components of mango peels from different varieties using SEM and LM.
2.2.3. Proximate and Sugar Compositions
The proximate analysis and sugar types of dried mango peels are shown in Table 3. Peels of all mango varieties contained ~59–69% moisture content, >9% carbohydrate, moderate contents of crude fiber, crude protein, and crude fat, and a low content of ash. The moisture content of fruit peel is an indicator of fruit ripeness, and ripe fruit typically consists of higher moisture content than raw fruit [85]. The moisture content values in previous reports were higher than those observed in this study, which might be due to the different ripening stages [86,87].

Table 3.
Proximate and sugar analyses of different varieties of mango peels.
Carbohydrate contents of all samples were slightly variable. The contents of ‘nam dok mai’, ‘chok anan’, ‘mahachanok’, and ‘kaew’ were 11.45%, 11.23%, 10.53%, and 8.93%, respectively. Carbohydrate was the most abundant macronutrient in mango peel. This was in conformity with other studies (15–30%) [62,63,64]. Major carbohydrate compositions in ripened mango fruit are sugars (glucose, fructose, and sucrose) and others such as starch and pectin [88]. Pectin is a structural carbohydrate abundant in mango fruit and is considered as an important gelling sugar. When fruit is unripe, pectin is accumulated, whereas, during ripening, the pectin molecular weight decreases [88,89]. This is attributed to the hydrolysis activity of pectin enzymes at this stage [90].
There was a significant difference in crude protein content in peels of all varieties, ranging from 7.03–8.06%. The highest yield was seen in ‘kaew’, while ‘nam dok mai’ gave the lowest yield. When compared with other studies, the protein content in our research was much higher [62,63,91]. The content of protein in the peel may be correlated with pectin modification during the maturity stage [84]. The reason is that pectin is naturally solubilized and sequentially disassembled because of the loss of neutral sugars from the side-chain via depolymerization during the ripening stage [92,93,94,95]. The incidence involves pectolytic enzymes such as polygalacturonase, pectin methylesterase, and galactosidase. As a consequence, the pectin molecular weight decreases, which is in line with the concomitant loss of neutral sugars (arabinose and galactose), associated with the softening of mango [96,97,98]. Nevertheless, the extension of these changes varies greatly among different species [80,81].
Crude fat contents of ‘mahachanok’, ‘nam dok mai’, ‘kaew’, and ‘chok anan’ were 2.48%, 1.86%, 1.68%, and 1.51%, respectively. The content of crude fat was rather low when compared to other components. Studies on different varieties of mango peel reported values of fat content between 4% and 5% [99,100]. The fat content in mango peel was analyzed and reported in the form of fatty acid by Maldonado-Celis et al. [98] and Saleem Dar et al. [89]. They observed that the fatty-acid content increased during the ripening stage. Bandyopadhyay and Gholap [101] also found that the ratio of palmitic–palmitoleic acid in ripe mango could be applied as an index of aroma and flavor of mangoes. The contents of crude fiber in ‘kaew’, ‘nam dok mai’, ‘mahachanok’, and ‘chok anan’ were 20.53%, 19.90%, 12.44%, and 10.92%, respectively. It is worth noting that the contents of crude fiber in var. ‘kaew’ and ‘nam dok mai’ accounted for about one-fifth of the total dried sample weight. Therefore, both varieties could be used as an ingredient in food products with supplemented dietary fiber in order to achieve higher profitable utilization. Nonetheless, the fiber contents of mango peel in this study were greater than the quantity reported in ‘amarpali’ (8.4%) and ‘dasheri’ (6.7%) by Tokas et al. [87], as well as in ‘nyala’ (4.5%), ‘edelfursan’ (4.2%), and ‘kaboom’ (4.4%) by Abdualrahm [62]. The ash content of the mango variety ‘mahachanok’ was greatest (0.54%), while others were not statistically different (0.24–0.27%). Ash consists of the important nutritional ingredients, especially minerals, as well as both micro and macronutrients, which are very important for the normal physiological functions of the human system [102].
The major sugar compositions of all mango peels were fructose and xylose. The contents of both sugars in each variety were not apparently distinct. Meanwhile, glucose and sucrose were not detected (Table 3). Fructose is the main monosaccharide during the pre-climacteric phase, while xylose, derived from hemicellulose, is the second most common sugar in nature and accounts for 18–30% of lignocellulose hydrolysate sugars [103]. It comes as no surprise that we detected a large quantity of xylose from peel byproduct. In general, mango flesh is predominantly composed of sucrose, fructose, and glucose in the order of highest to lowest content [88]. Kumar et al. [104] also found that the extracted sugars obtained from mango peel were mostly glucose, sucrose, and fructose. Nevertheless, the sugar types in mango peels are probably correlated with the neutral sugars attached on the side-chain of the pectin structure [96,97,98].
The relationship of proximate compositions of peel and the mango varieties was determined using PCA, whereby the first two dimensions of the PCA accounted for a total of 65.56% of variance across the PCA score plot (PC1 = 37.27% and PC2 = 28.29% of the variance). As presented in Figure 4a, the four varieties could be evidently classified on the basis of their chemical components since the score values of each variety were significantly different. Accordingly, the chemometric PCA of the phytochemical was appropriate for variety classification of the mango.
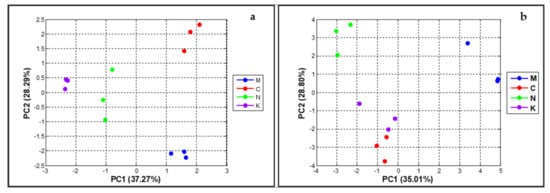
Figure 4.
The chemometric PCA score plots of proximate compositions (a) and of physiological characteristics and proximate compositions (b) (‘mahachanok’; M, ‘chok anan’; C, ‘nam dok mai’; N, ‘kaew’; K). The representative points of each variety that are far apart indicate that the characteristics of fruit and peel of the mangoes are significantly different.
We were also interested in the relationship of the combinations of fruit physiology and peel characteristics and the mango varieties (Figure 4b). The first two dimensions of the PCA described a total of 63.81% of the variance across the PCA score plot (PC1 = 35.01% and PC2 = 28.80% of the variance). The PCA pattern was greatly analogous to Figure 1, describing that ‘chok anan’ and ‘kaew’ could not be remarkably separated because of the slight difference in their score values, whereas ‘nam dok mai’ and ‘mahachanok’ were clearly clustered from other varieties. From these results, it can be assumed that chemical properties hold greater potential for the categorization of mango varieties when compared with physiological characteristics. Therefore, the different varieties of mango were composed of distinctive proximate compositions in their peels.
2.3. Chemical Characteristics of Mango Peel Pectin
The Eq.W is an index of free galacturonic acid content in the pectin. Absolute pectic acid is composed entirely of polygalacturonic acid [105]. without any methyl ester groups [106]. The Eq.W of pectin from these mango varieties could be categorized into two levels. The highest level was 1000–2000 mg/mol from peels of ‘mahachanok’, ‘chok anan’, and ‘kaew’, while the peel of ‘nam dok mai’ showed the lowest level at about 600 mg/mol (Table 4). The values are comparable with citrus pectin, which illustrated ranges of Eq.W between 635.63 and 2219.39 mg/mol depending on the extraction method [71]. The larger Eq.W could be due to higher partial degradation of pectin side-chain leading to pectin purification and free acid being obtained [106,107]. The partial degradation of pectin is probably due to pectolytic enzymes (polygalacturonase, pectin methylesterase and galactosidase), leading to a decrease in pectin molecular weight with attendant loss of neutral sugars, together correlated with more ripeness in several mango varieties [96,97,108]. Subsequently, it is possible that a greater ripeness of mango fruit leads to higher values of Eq.W.

Table 4.
Chemical characteristics of mango peel from different varieties.
Meanwhile, the Mox could be categorized into 3 levels: the high level (Mox 20.0–40.0%) including ‘mahachanok’ and ‘keaw’, the moderate level (Mox 10.0–20.0%) including ‘nam dok mai’, and the low level (Mox <10.0%) including ‘chok anan’. Mox content is an essential indicator of pectin setting time, related to its distribution ability in water and gel formation ability [109,110,111]. Commercially, a high-Mox pectin (generally at 8–11% Mox) can form gels at a high sugar content (>65% sugar), while a low-methoxyl pectin (LMP) with less than 7% Mox can form gels at a lower sugar content [112]. Depending on the DE, pectin can be divided into two groups: pectin with DE higher than 50%, known as high-methoxyl pectin (HMP), and DE lower than 50%, known as low-methoxyl pectin [113]. The DE of extracted pectin from various mango varieties ranged between 56.88% and 92.93%, indicating that all mango peel pectin was of HMP type. Although, the pectin obtained from mango peel var. ‘chok anan’ was composed of a DE content higher than 50%, the Mox value was fairly low (3.99%). Therefore, the pectin extracted from ‘chok anan’ could be classified as an LMP, which can be used to supplement a low-sugar diet.
2.4. Chemometric Studies of Fruit Physiological and Peel Proximate Compositions with Pectin Qualities
To examine the relationship of fruit physiological and peel physicochemical properties with the qualities of pectin, PLS models were established. It should be noted here that the data were standardized prior to the PLS modeling to ensure that each parameter equally influenced the estimation of the models. The correlation graphs between the observed parameters and the predicted pectin quality are presented in Table S1 (Supplementary Materials). The values of R2, Q2, and their standard errors are summarized in Table 5. The PLS models were of inordinate predictive performance (R2 > 0.7), while the Q2 values of the trained samples were low in all cases. Tandee et al. [114] described that predictive errors using the test sampling mode should be slightly greater than those of the auto-predictive mode, which is in line with our analysis. As shown in Table 5, the relationship between the physiological properties and Eq.W depicted high Q2 scores, while the others performed poorly. The relationship between the proximate properties and the DE value showed the lowest score of Q2, whereas the combined properties with all parameters were considerably acceptable with the exception of the Mox value. This could be due to the variation of the mango varieties (Figure 4b); however, our study did not determine the influence of the variety variation. Looking at the predictive models based on the highest Q2 scores presented, we were able to pick up a strong relationship of the physiological and phytochemical properties with the DE value. To comprehend the influence of the analyzed parameters in each model of interest; we used PLS regression coefficients and selected the top three parameters illustrating the highest coefficient values (Figure 5).

Table 5.
R2 and Q2 values with their error scores obtained from the correlation graph of the expected and predicted pectin quality values with fruit physiological properties and nutritional compositions of peel using the PLS model.
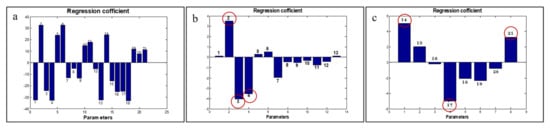
Figure 5.
The corresponding PLS values of the impact of physiological and proximate characteristics on DE (a); the corresponding PLS values of the impact of physiological properties on DE (b); the corresponding PLS values of the impact of proximate compositions on DE (c). Physiological properties of mango fruit were %peel (1), %flesh (2), %seed (3), %total biomass (4), L* (5), a* (6), b*(7), Dg (8), Da (9), Ra (10), Φ (11), surface area (12), and peel-to-fruit ratio (13); the proximate compositions were crude fiber (14), crude protein (15), crude lipid (16), moisture content (17), ash (18), carbohydrate (19), fructose (20), and xylose (21). The bar graphs of each parameter indicate positive and negative impacts on DE value.
Based on the coefficient values, the biomass yield parameters such as flesh (No. 2), seed (No. 3), total biomass (No. 4) (Figure 5b), crude fiber (No. 14), moisture content (No. 17), and xylose (No. 21) among the proximate properties (Figure 5c) had a strong influence on the model prediction of DE. It could be described that a higher flesh yield of mango fruit led to a greater DE value of the extracted pectin. On the other hand, fruit with high percentage of seed and total biomass had a tendency to give pectin of low-methoxyl type. Fruit biomass is an indicator used to determine the ripening stage of fruit. Peter et al. [115] reported that the ripening stages of ‘dodo’ mango fruit had a positive correlation to the flesh yield, whereas the seed and peel volumes were slightly changed. Therefore, it is likely that the quality of the extractable pectin depends upon fruit maturity. According to the phytochemical properties, the contents of crude fiber and xylose in mango peel resulted in a larger value of DE, whereas moisture content had the inverse effect. Mostly during ripening, the decrease in moisture content of mango peel happens during the diffusion of moisture from the flesh to the peel, along with carbohydrate hydrolyzation and alteration of crude fiber. These incidents are associated with an increase in the amount of the soluble pectin [115,116,117].
3. Materials and Methods
3.1. Physical Characteristics of Mango Fruit Varieties
3.1.1. Collection of Mango Samples
Four mangoes varieties (‘mahachanok’, ‘chok anan’, ‘nam dok mai’, and ‘kaew’) were harvested at a commercial ripening stage with their specific gravities in the range of 1.01–1.02, as described by Wongkaew et al. [6]. The mangoes were obtained from the orchard of Maejo University located in Sansai district, Chiang Mai, Thailand.
3.1.2. CIE Color Spacing
The color measurement was repeated six times at different positions over the fruit surface using a handheld color spectrophotometer (NS800, 3nh, China). Before each measurement, the instrument was calibrated using a white ceramic tile. The measurement was assessed using the CIE Lab system, where L* denotes lightness on a 0–100 scale from black to white, a∗ denotes (+) red or (−) green, and b∗ denotes (+) yellow or (−) blue.
3.1.3. Physical Properties
Linear dimensions, including length (L), width (W), and thickness (T), were measured using a digital vernier caliper with an accuracy of 0.01 mm. The physical properties were calculated according to the following equations [118, 119, 120]:
3.2. Physicochemical Characteristics of Mango Peel
3.2.1. Preparation of Mango Peel Powder
Peel was removed from the ripe mangoes prior to cutting into small pieces, washing with tap water, blanching with hot water at 95 °C for 10 min, draining, and leaving to cool at room temperature. It was then dried at 60 ± 1 °C until a moisture content of 4–6% was reached [10,121]. The dried peel was ground to fine powder using the high-speed mode of a food processor and passed through a sieve, resulting in a final mass of particles smaller than 0.6 mm in diameter [122,123].
3.2.2. Fourier-Transform Infrared Spectrophotometry (FT-IR)
The FT-IR spectra were acquired using a compact infrared spectrometer (Alpha II Bruker, Bruker Corporation, Billerica, MA, USA) equipped with a deuterated triglycine sulfate (DTGS) detector. Each powder sample was scanned by placing the sample on the platinum ATR with a durable magnetic diamond interface. The spectrum was verified in transparent mode from 500 to 4000 cm−1, with a resolution of 4.0 cm−1 [12]. Each IR spectrum was validated with reference standards.
3.2.3. Scanning Electron Microscopy (SEM)
Fresh mango peel was cut into 1 × 1 × 0.2 cm pieces and fixed with a mixed solution of formaldehyde and glacial acetic acid in a ratio of 1:1 at a temperature of 4 °C for 12 h. Subsequently, the fragments were dehydrated in an ethanol series and dried using a freeze-dryer. Mango peel was attached onto a specimen stub with a double-sided tape and sputter-coated with platinum [12,66]. The images were viewed at magnifications of ×500 using SEM (JELO JSM-5910, JEOL Ltd., Japan) with an accelerating voltage of 10 kV.
3.2.4. Light Microscopy (LM)
Similarly sized mango peels were fixed and dehydrated according to the protocol of SEM preparation. Afterward, the materials were fixed and embedded in paraffin at 60 °C for 12 h. Sections (about 1 mm thick) were cut with a ultramicrotome and fixed to microscope slides. Sections were stained with toluidine blue O solution in 0.1 M phosphate buffer (pH 6.8). The samples were observed using an inverted light microscope according to the modified method of Rongkaumpan et al. [124].
3.2.5. Proximate Compositions
Air-dried mango peel samples were used for proximate analyses with the exception of the moisture content, which was analyzed from fresh mango peels. The proximate composition analyses were carried out according to the methods of Association of Official Analytical Chemists (2000) [125]. Total carbohydrate contents were calculated using the following equation:
% Carbohydrate = 100 − (% moisture content + % crude protein + % ash + % crude fat + % crude fiber).
3.2.6. Sugar Compositions
Two grams of the peel powder samples were extracted with 20 mL of 80% methanol for 30 min in a shaker at room temperature. The extracts were filtered through filter paper (Whatman No. 1), and the residue was re-extracted under the same condition. The combined filtrate was evaporated in a rotary evaporator at a temperature below 45 °C. The extracts obtained after evaporation of methanol were used for the analyses of sugar content via HPLC. The mixture was separated in Shimadzu® Prominence™ LC-20A System, Japan, with a reversed-phase HPLC column on RezexTM RHM Monosaccharide H+ (8%) (Phenomenex Inc., Torrance, CA, USA), LC column 300 × 7.8 mm column, using degassed water as mobile phase at flow rate of 0.6 mL/min. Pure samples of d-(+)-arabinose, d-(+)-xylose, d-(+)-glucose, d-(+)-fructose, and d-(+)-sucrose were used as standards [126].
3.3. Chemical Characteristics of Mango Peel Pectin
3.3.1. Extraction of Pectin from Mango Peel Using Microwave Technique
Twenty grams of mango peel powder was suspended in 600 mL of diluted acidic solution (distilled H2O adjusted to pH 1.5 with 2 M HCl) and soaked for 20 min at room temperature. The slurry was heated in a microwave oven (ME711K-XST, Samsung, Thailand) with an optimal output power (700 watts for 3 min) followed by cooling to room temperature [12]. The solution was filtered and pressed manually using a nylon cloth. The filtrates were centrifuged at 5000× g for 20 min to eliminate any remaining coarse particles. Pectin was precipitated from the supernatant by adding the same volumes of ethanol (95%), before being mixed and stored in a refrigerator at 4 °C for 30 min. The separation was achieved by vacuum filtration. The obtained pectin was dried in a hot-air oven at 40 °C until constant weight was reached [127]. The yield of pectin was calculated from the following equation [123]:
where M0 (g) is the weight of dried pectin, and M (g) is the weight of dried mango peel powder.
3.3.2. Mango Peel Pectin Characterizations
Equivalent Weight (Eq.W)
The equivalent weight (Eq.W) was determined using the method of Sommano et al. [11]. Briefly, 0.5 g of dried pectin was dissolved in 100 mL of distilled water at 25 °C and stirred for 2 h until completely dissolved. One gram of sodium chloride was added and titrated with 0.1 M of sodium hydroxide (NaOH) using five drops of phenol red as an indicator. Eq.W was calculated using the following equation:
Methoxyl Content (Mox) and Degree of Esterification (DE)
The methods of Pinheiro et al. [128] were followed. Dried pectin (0.2 g) was stirred in CO2-free distilled water (20 mL) until fully dissolved. One gram of NaCl was added to the solution, prior to titrating with 0.1 N NaOH in the presence of phenolphthalein. The volume was recorded as the initial titer (V1). Then, 0.1 N NaOH solution (10 mL) was added to a neutralized polygalacturonic acid sample after determination of the free carboxyl groups. The solution was mixed thoroughly until the color of the solution became purple. A few drops of the indicator (0.25 N HCl) were added, and the mixture was titrated with 0.1 N NaOH until the color turned from yellow to pink. The volume was noted as V2. Mox and DE were then calculated using the following equations:
where S is the mass of dried pectin (g), Nis the NaOH concentration (N), V1 is the volume of NaOH used (mL), V2 is the volume of NaOH used (mL), and E is equivalent weight of methoxyl = 31.
3.4. Statistical Analysis
The analyses of physical and chemical properties in this experiment were carried out at least in biological and technical triplicates. Data was analyzed using one-way analysis of variance and Duncan’s test. Differences in values were considered significantly different when the p-value was <0.05. All statistical analysis was performed using IBM SPSS program v. 23.0 (Armonk, New York, NY, USA). Principal component analysis (PCA), partial least-squares regression (PLS), and PLS coefficient evaluations was conducted to comprehend the influence of mango varieties on the physiological and physicochemical characteristics using in-house MATLAB scripts (MATLAB V10.0, The Math Works Inc., Natick). Relationships between the parameters of interest and chemical qualities of pectin were fitted using PLS models, where fruit and peel characteristics were used as predictive parameters, while pectin qualities were used as responses. Standardization (STD) was used for data preprocessing to equalize the effect of each variable’s contribution to the model evaluation [129].
4. Conclusions
Chemometric analysis is able to elucidate the differences in mango varieties according to their physiological attributes and peel proximate compositions. In terms of MPP recovery, the percentages of flesh, peel, and total biomass, as well as contents of crude fiber, moisture, and xylose in the peels, can be used to justify the pectin type and its associated DE value. Future directions from our study can target the development of a nondestructive tool for biomass sourcing in the recovery process of high-quality pectin production.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants10061148/s1: Table S1: Correlation graph of PLS model.
Author Contributions
Conceptualization, S.R.S.; methodology, M.W. and S.R.S.; validation, M.W. and B.T.; formal analysis, M.W., S.K., N.P., C.T., and F.M.B.; investigation, M.W., S.K., and N.P.; data curation, M.W. and S.R.S.; writing—original draft preparation, M.W., B.T., F.M.B., and S.R.S.; writing—review and editing, S.R.S., M.W., B.T., and R.C.; visualization, K.S., R.C., and S.R.S.; supervision, S.R.S., K.S., and R.C.; funding acquisition, T.P., B.C., and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was partially supported by Chiang Mai University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dick, E.; N’DaAdopo, A.; Camara, B.; Moudioh, E. Influence of maturity stage of mango at harvest on its ripening quality. Fruits 2009, 64, 13–18. [Google Scholar] [CrossRef]
- Evans, E.; Ballen, F.; Siddiq, M. Mango Production, Global Trade, Consumption Trends, and Postharvest Processing and Nutrition; John Wiley & Sons: Chichester, UK, 2017; pp. 1–16. [Google Scholar]
- Tharanathan, R.N.; Hosakote, Y.; Prabha, T.N. Mango (Mangifera indica L.), “The King of Fruits”—An Overview. Food Rev. Int. 2006, 22, 95–123. [Google Scholar] [CrossRef]
- Maneenpun, S.; Yunchalad, M. Developing processed mango products for international markets. Acta Hortic. 2004, 645, 93–105. [Google Scholar] [CrossRef]
- Siafunda, M. Study of marketing and processing of mango enterprise with a view toreduce wastage among local mango producers in Zambia. Texila Int. J. Manag. 2019, 1–7. [Google Scholar] [CrossRef]
- Wongkaew, M.; Sangta, J.; Chansakaow, S.; Jantanasakulwong, K.; Rachtanapun, P.; Sommano, S.R. Volatile profiles from over-ripe purée of Thai mango varieties and their physiochemical properties during heat processing. PLoS ONE 2021, 16, e0248657. [Google Scholar] [CrossRef]
- Rojas, R.; Alvarez-Pérez, O.B.; Contreras-Esquivel, J.C.; Vicente, A.; Flores, A.; Sandoval, J.; Aguilar, C.N. Valorisation of mango peels: Extraction of pectin and antioxidant and antifungal polyphenols. Waste Biomass Valoriz. 2020, 11, 89–98. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Masavang, S.; Mahe, J.; Sommano, S.; Ruksiriwanich, W.; Brachais, C.-H.; Chambin, O.; Jantrawut, P. Mango (cv. Nam Dokmai) peel as a source of pectin and its potential use as a film-forming polymer. Food Hydrocoll. 2020, 102, 105611. [Google Scholar] [CrossRef]
- Min, B.; Lim, J.; Ko, S.; Lee, K.-G.; Lee, S.H.; Lee, S. Environmentally friendly preparation of pectins from agricultural byproducts and their structural/rheological characterization. Bioresour. Technol. 2011, 102, 3855–3860. [Google Scholar] [CrossRef]
- Vieira, W.A.; Michereff, S.; Morais, M.; Hyde, K.; Câmara, M. Endophytic species of Colletotrichum associated with mango in northeastern Brazil. Fungal Divers. 2014, 67. [Google Scholar] [CrossRef]
- Sommano, S.; Ounamornmas, P.; Nisoa, M.; Sriwattana, S. Bioactive functionality of pectin from peels of seven Thai mango cultivars. Acta Hortic. 2018, 423–428. [Google Scholar] [CrossRef]
- Sommano, S.; Ounamornmas, P.; Nisoa, M.; Sriwattana, S.; Page, P.; Colelli, G. Characterisation and physiochemical properties of mango peel pectin extracted by conventional and phase control microwave-assisted extractions. Int. Food Res. J. 2018, 25, 2657–2665. [Google Scholar]
- Wongkaew, M.; Sommano, S.; Tangpao, T.; Rachtanapun, P.; Jantanasakulwong, K. Mango peel pectin by microwave-assisted extraction and its use as fat replacement in dried Chinese sausage. Foods 2020, 9, 450. [Google Scholar] [CrossRef]
- Ajila, C.M.; Prasada Rao, U.J.S. Mango peel dietary fibre: Composition and associated bound phenolics. J. Funct. Foods 2013, 5, 444–450. [Google Scholar] [CrossRef]
- De Lourdes Garcia-Magana, M.; Garcia, H.S.; Bello-Perez, L.A.; Sayago-Ayerdi, S.G.; de Oca, M.M. Functional properties and dietary fiber characterization of mango processing by-products (Mangifera indica L., cv Ataulfo and Tommy Atkins). Plant Foods Hum. Nutr. 2013, 68, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Perkins-Veazie, P. Influences of harvest date and location on the levels of beta-carotene, ascorbic acid, total phenols, the in vitro antioxidant capacity, and phenolic profiles of five commercial varieties of mango (Mangifera indica L.). J. Agric. Food Chem. 2009, 57, 10825–10830. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Berardini, N.; Carle, R. Identification of flavonol and xanthone glycosides from mango (Mangifera indica L. Cv. “Tommy Atkins”) peels by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2003, 51, 5006–5011. [Google Scholar] [CrossRef]
- Nagel, A.; Sirisakulwat, S.; Carle, R.; Neidhart, S. An acetate-hydroxide gradient for the quantitation of the neutral sugar and uronic acid profile of pectins by HPAEC-PAD without postcolumn pH adjustment. J. Agric. Food Chem. 2014, 62, 2037–2048. [Google Scholar] [CrossRef]
- Panouillé, M.; Ralet, M.C.; Bonnin, E.; Thibault, J.F. 16—Recovery and reuse of trimmings and pulps from fruit and vegetable processing. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Waldron, K., Ed.; Woodhead Publishing: Cambridge, UK, 2007; pp. 417–447. [Google Scholar]
- Ajila, C.M.; Bhat, S.G.; Prasada Rao, U.J.S. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Beerh, O.P.; Raghuramaiah, B.; Krishnamurthy, G.; Giridhar, N. Utilization of mango waste: Recovery of juice from waste pulp and peel. J. Food Sci. Technol. 1976, 13, 138–141. [Google Scholar]
- Singthong, J.; Cui, S.; Ningsanond, S.; Goff, H. Structural characterization, degree of esterification and some gelling properties of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohydr. Polym. 2004, 58, 391–400. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Functional properties and characterization of dietary fiber from Mangifera pajang Kort. fruit pulp. J. Agric. Food Chem. 2011, 59, 3980–3985. [Google Scholar] [CrossRef] [PubMed]
- Nguyễn, H.; Savage, G. The effects of temperature and pH on the extraction of oxalate and pectin from green kiwifruit (Actinidia deliciosa L.), golden kiwifruit (Actinidia chinensis L.), kiwiberry (Actinidia arguta) and persimmon (Diospyros kaki). Int. J. Food Sci. Technol. 2012, 48, 794–800. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- El Bulk, R.E.; Babiker, E.F.E.; El Tinay, A.H. Changes in chemical composition of guava fruits during development and ripening. Food Chem. 1997, 59, 395–399. [Google Scholar] [CrossRef]
- Zhou, H.C.; Li, G.; Zhao, X.; Li, L.J. Comparative analysis of polygalacturonase in the fruit of strawberry cultivars. Genet. Mol. Res. 2015, 14, 12776–12787. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Schols, H.A.; Voragen, A.G.J. Complex Pectins: Structure elucidation using enzymes. In Progress in Biotechnology; Visser, J., Voragen, A.G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 14, pp. 3–19. [Google Scholar]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Valorisation of fruit by-products: Production characterization of pectins from fruit peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Robledo, V.R.; Vázquez, L.I.C. Pectin—Extraction, Purification, Characterization and Applications; IntechOpen: London, UK, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Fishman, M.L.; Chau, H.K.; Hoagland, P.D.; Hotchkiss, A.T. Microwave-assisted extraction of lime pectin. Food Hydrocoll. 2006, 20, 1170–1177. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Guolin, H.; Jeffrey, S.; Kai, Z.; Xiaolan, H. Application of ionic liquids in the microwave-assisted extraction of pectin from lemon peels. J. Anal. Methods Chem. 2012, 2012, 302059. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. J. Food Eng. 2007, 78, 693–700. [Google Scholar] [CrossRef]
- Swamy, G.J.; Muthukumarappan, K. Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chem. 2017, 220, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef]
- Matharu, A.; Houghton, J.; Lucas-Torres, C.; Moreno, A. Acid-free microwave-assisted hydrothermal extraction of pectin and porous cellulose from mango peel waste—Towards a zero waste mango biorefinery. Green Chem. 2016, 18. [Google Scholar] [CrossRef]
- Sarıçoban, C.; Özalp, B.; Yılmaz, M.T.; Özen, G.; Karakaya, M.; Akbulut, M. Characteristics of meat emulsion systems as influenced by different levels of lemon albedo. Meat Sci. 2008, 80, 599–606. [Google Scholar] [CrossRef] [PubMed]
- León, K.; Mery, D.; Pedreschi, F.; León, J. Color measurement in L∗a∗b∗ units from RGB digital images. Food Res. Int. 2006, 39, 1084–1091. [Google Scholar] [CrossRef]
- Jha, S.N.; Chopra, S.; Kingsly, A.R.P. Modeling of color values for nondestructive evaluation of maturity of mango. J. Food Eng. 2007, 78, 22–26. [Google Scholar] [CrossRef]
- Malevski, Y.; Brito, L.; Peleg, M.; Silberg, M. External color as maturity index of mango. J. Food Sci. 2006, 42, 1316–1318. [Google Scholar] [CrossRef]
- Nambi, E.; Kulandasamy, T.; Jesudas, M. Scientific classification of ripening period and development of colourgrade chart for Indian mangoes (Mangifera indica L.) using multivariate cluster analysis. Sci. Hortic. 2015. [Google Scholar] [CrossRef]
- Liang, D.; Lin, F.; Yang, G.; Yue, X.; Zhang, Q.; Zhang, Z.; Chen, H. Advantages of immersion freezing for quality preservation of litchi fruit during frozen storage. LWT Food Sci. Technol. 2015, 60, 948–956. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.-H.; Ding, Y.; Nie, Y.; Xiao, H.-W.; Zhu, Z.; Tang, X.-M. Effects of state/phase transitions on the quality attributes of mango (Mangifera indica L.) during frozen storage. Int. J. Food Sci. Technol. 2017, 52, 239–246. [Google Scholar] [CrossRef]
- Ribeiro, S.; Queiroz, J.; Queiroz, M.; Campos, F.; Pinheiro-Sant’Ana, H. Antioxidant in mango (Mangifera indica L.) pulp. Plant Foods Hum. Nutr. 2007, 62, 13–17. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.D.J.; Yahia, E.M.; Gardea, A.A. Changes in external and internal color during postharvest ripening of ‘Manila’ and ‘Ataulfo’ mango fruit and relationship with carotenoid content determined by liquid chromatography–APcI+-time-of-flight mass spectrometry. Postharvest Biol. Technol. 2008, 50, 145–152. [Google Scholar] [CrossRef]
- Medlicott, A.; Sigrist, J.; Reynolds, S.; Thompson, A. Effect of ethylene and acetylene on mango fruit ripening. Ann. Appl. Biol. 2008, 111, 439–444. [Google Scholar] [CrossRef]
- Su, L.; Diretto, G.; Purgatto, E.; Danoun, S.; Zouine, M.; Li, Z.; Roustan, J.-P.; Bouzayen, M.; Giuliano, G.; Chervin, C. Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol. 2015, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Gorial, B.Y.; O’Callaghan, J.R. Aerodynamic properties of grain/straw materials. J. Agric. Eng. Res. 1990, 46, 275–290. [Google Scholar] [CrossRef]
- Sunmonu, M.O.; Iyanda, M.O.; Odewole, M.; Ajala, O.; Aduba, J. Determination of physical properties of almond seed related to the design of food processing machines. Niger. J. Pure Appl. Sci. 2016, 29, 2730–2740. [Google Scholar]
- Osadare, T.; Koyenikan, O.; Akinola, F. Physical and mechanical properties of three varieties of mango. Asian Food Sci. J. 2019, 1–8. [Google Scholar] [CrossRef]
- Spreer, W.; Müller, J. Estimating the mass of mango fruit (Mangifera indica, cv. ChokAnan) from its geometric dimensions by optical measurement. Comput. Electron. Agric. 2011, 75, 125–131. [Google Scholar] [CrossRef]
- Navaphattra, N.; Suesut, T. Measuring geometric mean diameter of fruits and vegetables using light sectioning method. Songklanakarin J. Sci. Technol. 2010, 31, 629–633. [Google Scholar]
- Nordey, T.; Mathieu, L.; Saudreau, M.; Joas, J.; Genard, M. Model-assisted analysis of spatial and temporal variations in fruit temperature and transpiration highlighting the role of fruit development. PLoS ONE 2014, 9, e92532. [Google Scholar] [CrossRef][Green Version]
- Athmaselvi, K.; Jenney, P.; Pavithra, C.; Roy, I. Physical and biochemical properties of selected tropical fruits. Int. Agrophys. 2014, 28. [Google Scholar] [CrossRef]
- Nguyen, H.D.H.; Nguyen, H.V.H.; Savage, G.P. Properties of pectin extracted from Vietnamese mango peels. Foods 2019, 8, 629. [Google Scholar] [CrossRef] [PubMed]
- Sista Kameshwar, A.; Qin, W. Structural and functional properties of pectin and lignin–carbohydrate complexes de-esterases: A review. Bioresour. Bioprocess. 2018, 5. [Google Scholar] [CrossRef]
- Voragen, A.; Coenen, G.-J.; Verhoef, R.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Abdualrahm, M. Physico-chemical characteristics of different types of mango (Mangifera indica L.) fruits grown in drafur regions and its use in jam processing. Sci. Int. 2013, 1, 144–147. [Google Scholar] [CrossRef]
- Anila, R.; Radha, T. Physico-chemical analysis of mango varieties under Kerala conditions. J. Trop. Agric. 2006, 41, 20–22. [Google Scholar]
- Rodriguez, C.; Durán Zuazo, V.; Fernández, J.; Tarifa, D. Physico-chemical quality parameters of mango (Mangifera indica L.) fruits grown in a mediterranean subtropical climate (SE Spain). J. Agric. Sci. Technol. 2012, 14, 365–374. [Google Scholar]
- Tesfaye, T. Valorisation of mango fruit by-products: Physicochemical characterisation and future prospect. Chem. Process Eng. Res. 2017, 50, 22–34. [Google Scholar]
- Jiang, Y.; Du, Y.; Zhu, X.; Xiong, H.; Woo, M.W.; Hu, J. Physicochemical and comparative properties of pectins extracted from Akebia trifoliata var. australis peel. Carbohydr. Polym. 2012, 87, 1663–1669. [Google Scholar] [CrossRef]
- Khaskheli, M.I.; Memon, S.Q.; Siyal, A.N.; Khuhawar, M.Y. Use of orange peel waste for arsenic remediation of drinking water. Waste Biomass Valoriz. 2011, 2, 423. [Google Scholar] [CrossRef]
- Posé, S.; Kirby, A.; Mercado, J.; Morris, V.; Quesada, M. Structural characterization of cell wall pectin fractions in ripe strawberry fruits using AFM. Carbohydr. Polym. 2012, 88, 882–890. [Google Scholar] [CrossRef]
- Abid, M.; Cheikhrouhou, S.; Renard, C.; Sylvie, B.; Cuvelier, G.; Attia, H.; Ayadi, M. Characterization of pectins extracted from pomegranate peel and their gelling properties. Food Chem. 2016, 215. [Google Scholar] [CrossRef]
- Černá, M.; Barros, A.S.; Nunes, A.; Rocha, S.l.M.; Delgadillo, I.; Čopı́ková, J.; Coimbra, M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003, 51, 383–389. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef]
- Devi, S.; Nand, K. Microbiological pretreatment of mango peel for biogas production. J. Microb. Biotechnol. 1989, 4, 110–115. [Google Scholar]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Mori, B.; Bellani, L.M. Differential staining for cellulosic and modified plant cell walls. Biotech. Histochem. 1996, 71, 71–72. [Google Scholar] [CrossRef]
- Amnuaysin, N.; Seraypheap, K.; Kidyoo, M. Anatomical changes in peel structure of ‘Hom Thong’ banana during fruit development and ripening. Trop. Nat. Hist. 2012, 12, 127–136. [Google Scholar]
- Harker, F.R.; Redgwell, R.J.; Hallett, I.C.; Murray, S.H.; Carter, G. Texture of fresh fruit. Hortic. Rev. 1997, 20, 121–224. [Google Scholar]
- Ratule, M.; Osman, A.; Saari, N.; Ahmad, H.S. Microstructure of peel cell wall and selected physico-chemical characteristics of ‘Berangan’ banana (Musa cv. Berangan (AAA)) ripened at high temperature. Asia Pac. J. Mol. Biol. Biotechnol. 2007, 15, 8–13. [Google Scholar]
- Brummell, D.; Harpster, M. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–340. [Google Scholar] [CrossRef]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Mercado, J.; Pliego-Alfaro, F.; Quesada, M. Fruit shelf life and potential for its genetic improvement. In Breeding for Fruit Quality; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 81–104. [Google Scholar]
- Lohani, S.; Trivedi, P.K.; Nath, P. Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: Effect of 1-MCP, ABA and IAA. Postharvest Biol. Technol. 2004, 31, 119–126. [Google Scholar] [CrossRef]
- Paniagua, C.; Posé, S.; Morris, V.J.; Kirby, A.R.; Quesada, M.A.; Mercado, J.A. Fruit softening and pectin disassembly: An overview of nanostructural pectin modifications assessed by atomic force microscopy. Ann. Bot. 2014, 114, 1375–1383. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Chun, J.-P.; Huber, D.J. Extensive solubilization and depolymerization of cell wall polysaccharides during avocado (Perseaamericana) ripening involves concerted action of polygalacturonase and pectinmethylesterase. Physiol. Plant. 2000, 108, 345–352. [Google Scholar] [CrossRef]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef]
- Offem, J.O.; Thomas, O.O. Chemical changes in relation to mode and degree of maturation of plantain (Musa paradisiaca) and banana (Musa sapientum) fruits. Food Res. Int. 1993, 26, 187–193. [Google Scholar] [CrossRef]
- John, K.S.; Bhat, S.G.; Prasada Rao, U.J. Biochemical characterization of sap (latex) of a few Indian mango varieties. Phytochemistry 2003, 62, 13–19. [Google Scholar] [CrossRef]
- Tokas, J.; Punia, H.; Baloda, S.; Sheokand, R.N. Mango peel: A potential source of bioactive compounds. Austin Food Sci. 2020, 5, 1–7. [Google Scholar]
- Bello-Pérez, L.A.; Garcia-Suarez, F.; Agama-Acevedo, E. Mango Carbohydrates. Food 2009, 1, 36–40. [Google Scholar]
- Saleem Dar, M.; Oak, P.; Chidley, H.; Deshpande, A.; Giri, A.; Gupta, V. Chapter 19—Nutrient and flavor content of mango (Mangifera indica L.) cultivars: An appurtenance to the list of staple foods. In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 445–467. [Google Scholar]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Pectic polysaccharides of mango (Mangifera indica L): Structural studies. J. Sci. Food Agric. 2004, 84, 1731–1735. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.D.P.; Gutiérrez, L.-F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Melton, L.D.; Brasch, D.J. Cell wall dissolution in ripening kiwifruit (Actinidia deliciosa): Solubilization of the pectic polymers. Plant Physiol. 1992, 98, 71–81. [Google Scholar] [CrossRef]
- Carrington, C.M.S.; Greve, L.C.; Labavitch, J.M. Cell wall metabolism in ripening fruit (vi. effect of the antisense polygalacturonase gene on cell wall changes accompanying ripening in transgenic tomatoes). Plant Physiol. 1993, 103, 429–434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, G.W.; Huber, D.J. Alterations in structural polysaccharides during liquefaction of tomato locule tissue. Plant Physiol. 1996, 111, 447–457. [Google Scholar] [CrossRef]
- Rose, J.K.; Hadfield, K.A.; Labavitch, J.M.; Bennett, A.B. Temporal sequence of cell wall disassembly in rapidly ripening melon fruit. Plant Physiol. 1998, 117, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Muda, P.; Seymour, G.B.; Errington, N.; Tucker, G.A. Compositional changes in cell wall polymers during mango fruit ripening. Carbohydr. Polym. 1995, 26, 255–260. [Google Scholar] [CrossRef]
- Roe, B.; Bruemmer, J.H. Changes in pectic substances and enzymes during ripening and storage of “Keitt” mangos. J. Food Sci. 1981, 46, 186–189. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Romelle, F.D.; Rani, A.; Manohar, R.S. Chemical composition of some selected fruit peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Giraldo, L.M.; Correa, H.M.; Gutiérrez, J.B.; Castano, C.C. Aprovechamiento del residuo agroindustrial del mango común (Mangifera indica L.) en la obtención de azúcares fermentables. Ingeniería Ciencia 2007, 3, 41–62. [Google Scholar]
- Bandyopadhyay, C.; Gholap, A.S. Changes in fatty acids in ripening mango pulp (var Alphonso). J. Agric. Food Chem. 1973, 21, 496–497. [Google Scholar] [CrossRef]
- Khan, D.N.; Ruqia, B.; Hussain, J.; Jamila, D.N.; Rehman, N.; Hussain, S. Nutritional assessment and proximate analysis of selected vegetables from parachinar kurram agency. Am. J. Res. Commun. 2013, 1, 184–198. [Google Scholar]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Kumar, C.S.C.; Mythily, R.; Chandraju, S. Utilization of mango peels (Mangifera indica) for the extraction of sugars. Der Pharma Chem. 2012, 4, 2422–2426. [Google Scholar]
- Taylor, K.A.C.C. A colorimetric method for the quantitation of galacturonic acid. Appl. Biochem. Biotechnol. 1993, 43, 51–54. [Google Scholar] [CrossRef]
- Wathoni, N.; Shan, C.Y.; Shan, W.Y.; Rostinawati, T.; Indradi, R.B.; Pratiwi, R.; Muchtaridi, M. Characterization and antioxidant activity of pectin from Indonesian mangosteen (Garcinia mangostana L.) rind. Heliyon 2019, 5, e02299. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Ali, M.; Akter, M.; Rahman, M.J.; Ahmed, M. Isolation and characterization of pectin extracted from lemon pomace during ripening. J. Food Nutr. Sci. 2014, 2, 30–35. [Google Scholar] [CrossRef]
- Selvaraj, Y.; Kumar, R. Studies on fruit softening enzymes and polyphenol oxidase activity in ripening mango (Mangifera indica L.) fruit. J. Food Sci. Technol. 1989, 26, 218–222. [Google Scholar]
- Shaha, R.K.; Nayagi, Y.; Punichelvana, A.; Afandi, A. Optimized extraction condition and characterization of pectin from kaffir lime (Citrus hystrix). Res. J. Agric. For. Sci. 2013, 1, 1–11. [Google Scholar]
- Israel, K.A.; Baguio, S.F.; Diasanta, M.D.B.; Lizardo, R.C.; Dizon, E.; Mejico, M.I.F. Extraction and characterization of pectin from Saba banana [Musa ‘saba’ (Musa acuminata × Musa balbisiana)] peel wastes: A preliminary study. Int. Food Res. J. 2015, 22, 202–207. [Google Scholar]
- Constenla, D.; Lozano, J. Kinetic model of pectin demethylation. Lat. Am. Appl. Res. 2003, 33, 91–95. [Google Scholar]
- Rouse, A.H.; Atkins, C.D.; Moore, E.L. The occurrence and evaluation of pectin in component parts of valencia oranges during maturation. Proc. Fla. State Hortic. Soc. 2007, 75, 307–311. [Google Scholar]
- Mesbahi, G.; Jamalian, J.; Farahnaky, A. A comparative study on functional properties of beet and citrus pectins in food systems. Food Hydrocoll. 2005, 19, 731–738. [Google Scholar] [CrossRef]
- Tandee, K.; Kittiwachana, S.; Mahatheeranont, S. Antioxidant activities and volatile compounds in longan (Dimocarpus longan Lour.) wine produced by incorporating longan seeds. Food Chem. 2021, 348, 128921. [Google Scholar] [CrossRef] [PubMed]
- Mamiro, P.; Fweja, L.; Chove, B.; Kinabo, J.; George, V.; Mtebe, K. Physical and chemical characteristics of off vine ripened mango (Mangifera indica L.) fruit (Dodo). Afr. J. Biotechnol. 2007, 6. [Google Scholar] [CrossRef]
- Appiah, F.; Patrick, K.; Idun, I. Effect of ripening stage on composition, sensory qualities and acceptability of keitt mango (Mangifera indica L.) chips. Afr. J. Food Agric. Nutr. Dev. 2011, 11, 5096–5109. [Google Scholar] [CrossRef]
- Othman, O.; Mbogo, G. Physico-chemical characteristics of storage-ripened mango (Mangifera indica L.) fruits varieties of Eastern Tanzania. Tanzan. J. Sci. 2009, 35, 57–66. [Google Scholar]
- Tscheuschner, H.-D.N.N. Mohsenin: Physical properties of plant and animal materials: Structure, physical characteristics and mechanical properties. 2. Aufl. 891 Seiten, zahlr. Abb. und Table Gordon and Breach Science Publishers, New York u. a. 1986. Preis: 140—£. Food Nahrung 1987, 31, 702. [Google Scholar] [CrossRef]
- Razavi, S.; Bahram-Parvar, M. Some physical and mechanical properties of kiwifruit. Int. J. Food Eng. 2007, 3, 1–16. [Google Scholar] [CrossRef]
- Tabar, F.J.; Lorestani, A.N.; Gholami, R.; Behzadi, A.; Fereidoni, M. Physical and mechanical properties of Oak (Quercus Persica) fruits. Agric. Eng. Int. CIGR J. 2012, 13, 1–4. [Google Scholar]
- Pandit, S.G.; Vijayanand, P.; Kulkarni, S.G. Pectic principles of mango peel from mango processing waste as influenced by microwave energy. LWT Food Sci. Technol. 2015, 64, 1010–1014. [Google Scholar] [CrossRef]
- Bagherian, H.; Zokaee Ashtiani, F.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Maran, J.P.; Swathi, K.; Jeevitha, P.; Jayalakshmi, J.; Ashvini, G. Microwave-assisted extraction of pectic polysaccharide from waste mango peel. Carbohydr. Polym. 2015, 123, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Rongkaumpan, G.; Amsbury, S.; Andablo-Reyes, E.; Linford, H.; Connell, S.; Knox, J.P.; Sarkar, A.; Benitez-Alfonso, Y.; Orfila, C. Cell wall polymer composition and spatial distribution in ripe banana and mango fruit: Implications for cell adhesion and texture perception. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Kumar, C.S.C.; Mythily, R.; Chandraju, S. A rapid and sensitive extraction of sugars from papaya peels (Carica Papaya). Der Pharma Chem. 2012, 4, 1631–1636. [Google Scholar]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res. Int. 2019, 119, 455–461. [Google Scholar] [CrossRef]
- Pinheiro, E.S.R.; Silva, I.M.D.A.; Gonzaga, L.V.; Amante, E.R.; Teófilo, R.F.; Ferreira, M.M.C.; Amboni, R.D.M.C. Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresour. Technol. 2008, 99, 5561–5566. [Google Scholar] [CrossRef]
- Funsueb, S.; Krongchai, C.; Mahatheeranont, S.; Kittiwachana, S. Prediction of 2-acetyl-1-pyrroline content in grains of Thai Jasmine rice based on planting condition, plant growth and yield component data using chemometrics. Chemom. Intell. Lab. Syst. 2016, 156, 203–210. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



