Biogeographic Overview of Ulmaceae: Diversity, Distribution, Ecological Preferences, and Conservation Status
Abstract
1. Introduction
2. Results
2.1. Taxonomic Division and Species List
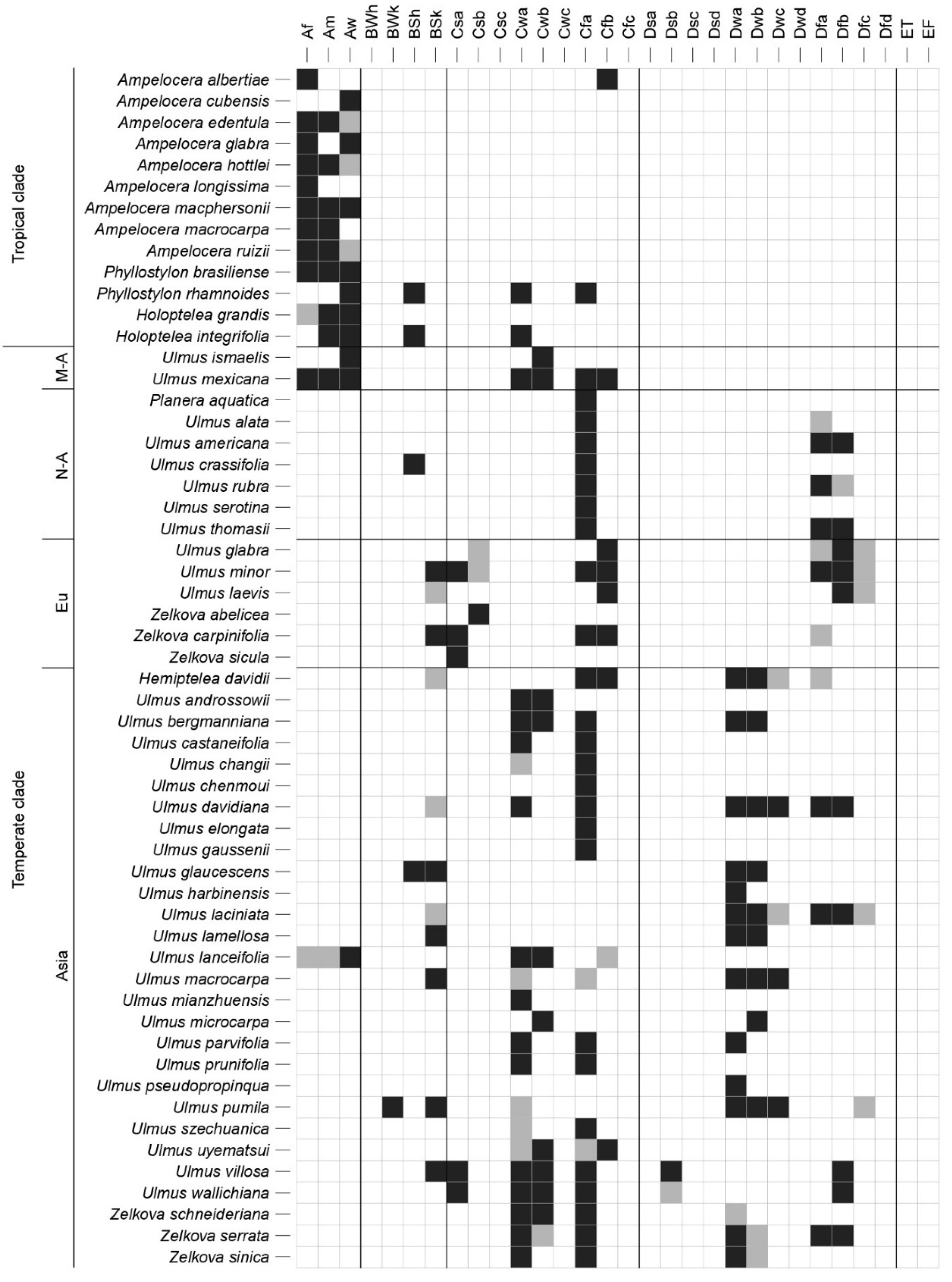
2.2. Species and Genera Distribution
2.3. Species Macroclimatic Niche and Ecological Preferences
2.4. Conservation Status
3. Discussion
3.1. Diversity and Distribution
3.2. Macroclimatic Niche and Ecological Preferences
3.3. Threats and Conservation Status
4. Materials and Methods
4.1. Taxonomic Division and Species List
4.2. Data Collection
4.3. Species Distribution
4.4. Species Macroclimatic Niche
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozlowski, G.; Bétrisey, S.; Song, Y.-G.; Fazan, L.; Garfì, G. The Red List of Zelkova; Natural History Museum Fribourg: Fribourg, Switzerland, 2018; ISBN 2-9701096-2-X. [Google Scholar]
- Simpson, M.G. Plant Systematics, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 0-12-812628-0. [Google Scholar]
- Boratynska, K. Chorology of the family ulmaceae (sensu stricto). Arbor Kornikie 1989, 34, 3–29. [Google Scholar]
- Christenhusz, M.J.; Fay, M.F.; Chase, M.W. Plants of the World: An Illustrated Encyclopedia of Vascular Plants; University of Chicago Press: Chicago, IL, USA, 2017; ISBN 0-226-53670-X. [Google Scholar]
- Fazan, L.; Song, Y.-G.; Kozlowski, G. The woody planet: From past triumph to manmade decline. Plants 2020, 9, 1593. [Google Scholar] [CrossRef]
- Todzia, C.A. A Revision of Ampelocera (Ulmaceae). Ann. Mo. Bot. Gard. 1989, 76, 1087–1102. [Google Scholar] [CrossRef]
- Todzia, C.A. A Reevaluation of the genus Phyllostylon (Ulmaceae). SIDA Contribut. Bot. 1992, 15, 263–270. [Google Scholar]
- Manchester, S.R. Systematics and fossil history of the Ulmaceae. Evolut. Syst. Foss. Hist. Hamamelidae 1989, 2, 221–251. [Google Scholar]
- Todzia, C.A. Ulmaceae. In Flowering Plants·Dicotyledons; Springer: Berlin/Heidelberg, Germany, 1993; pp. 603–611. [Google Scholar]
- Jia, L.-B.; Manchester, S.R.; Su, T.; Xing, Y.-W.; Chen, W.-Y.; Huang, Y.-J.; Zhou, Z.-K. First occurrence of Cedrelospermum (Ulmaceae) in Asia and its biogeographic implications. J. Plant Res. 2015, 128, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.-B.; Su, T.; Huang, Y.-J.; Wu, F.-X.; Deng, T.; Zhou, Z.-K. First fossil record of Cedrelospermum (Ulmaceae) from the Qinghai–Tibetan plateau: Implications for morphological evolution and biogeography. J. Syst. Evol. 2019, 57, 94–104. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Z.; Tang, Z. Atlas of Woody Plants in China: Distribution and Climate; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 1, ISBN 3-642-15017-9. [Google Scholar]
- Wang, Q.; Manchester, S.R.; Li, C.; Geng, B. Fruits and leaves of Ulmus from the paleogene of fushun, Northeastern China. Int. J. Plant Sci. 2010, 171, 221–226. [Google Scholar] [CrossRef]
- De Mirbel, C.F.B. Élements de Physiologie Végétale et de Botanique; Magimel: Paris, France, 1815; Volume 1. [Google Scholar]
- Wiegrefe, S.J.; Sytsma, K.J.; Guries, R.P. The Ulmaceae, one family or two? Evidence from chloroplast DNA restriction site mapping. Plant Syst. Evol. 1998, 210, 249–270. [Google Scholar] [CrossRef]
- Grudzinskaya, I.A. Ulmaceae and reasons for distinguishing celtidoideae as a separate family celtidaceae link. Bot. Zhurn. 1967, 52, 1723–1748. [Google Scholar]
- Yang, M.-Q.; van Velzen, R.; Bakker, F.T.; Sattarian, A.; Li, D.-Z.; Yi, T.-S. Molecular phylogenetics and character evolution of Cannabaceae. Taxon 2013, 62, 473–485. [Google Scholar] [CrossRef]
- Zavada, M.S.; Kim, M. Phylogenetic analysis of Ulmaceae. Plant Syst. Evol. 1996, 200, 13–20. [Google Scholar] [CrossRef]
- Ueda, K.; Kosuge, K.; Tobe, H. A molecular phylogeny of Celtidaceae and Ulmaceae (Urticales) based on RbcL nucleotide sequences. J. Plant Res. 1997, 110, 171–178. [Google Scholar] [CrossRef]
- Zhang, S.; Soltis, D.E.; Yang, Y.; Li, D.; Yi, T. Multi-gene analysis provides a well-supported phylogeny of rosales. Mol. Phylogenet. Evol. 2011, 60, 21–28. [Google Scholar] [CrossRef]
- Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; Stevens, P.F. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- Sytsma, K.J.; Morawetz, J.; Pires, J.C.; Nepokroeff, M.; Conti, E.; Zjhra, M.; Hall, J.C.; Chase, M.W. Urticalean rosids: Circumscription, rosid ancestry, and phylogenetics based on RbcL, TrnL-F, and NdhF sequences. Am. J. Bot. 2002, 89, 1531–1546. [Google Scholar] [CrossRef]
- Neubig, K.; Herrera, F.; Manchester, S.R.; Abbott, J.R. Fossils, biogeography and dates in an expanded phylogeny of Ulmaceae. In Proceedings of the Botany 2012—Annual Meeting of the Botanical Society of America in Columbus, Columbus, OH, USA, 7–11 July 2012. [Google Scholar]
- Zhang, Q.; Deng, M.; Bouchenak-Khelladi, Y.; Zhou, Z.; Hu, G.; Xing, Y. The diversification of the northern temperate woody flora—A case study of the elm family (Ulmaceae) based on phylogenomic and paleobotanical evidence. J. Syst. Evol. 2021. [Google Scholar] [CrossRef]
- Oyama, H.; Fuse, O.; Tomimatsu, H.; Seiwa, K. Variable seed behavior increases recruitment success of a hardwood tree, Zelkova Serrata, in spatially heterogeneous forest environments. For. Ecol. Manag. 2018, 415, 1–9. [Google Scholar] [CrossRef]
- Certini, D.; Fazan, L.; Nakayama, N.; Viola, I.M.; Kozlowski, G. Velocity of the falling dispersal units in Zelkova abelicea: Remarkable evolutionary conservation within the relict tree genus. Am. J. Bot. 2020. [Google Scholar] [CrossRef]
- Leme, F.M.; Staedler, Y.M.; Schönenberger, J.; Teixeira, S.P. Ontogeny and vascularization elucidate the atypical floral structure of Ampelocera Glabra, a tropical species of Ulmaceae. Int. J. Plant Sci. 2018, 179, 461–476. [Google Scholar] [CrossRef]
- Kozlowski, G.; Gratzfeld, J. Zelkova—An Ancient Tree: Global Status and Conservation Action; Natural History Museum Fribourg: Fribourg, Switzerland, 2013. [Google Scholar]
- Mutke, J.; Barthlott, W. Patterns of vascular plant diversity at continental to global scales. Biol. Skr. 2005, 55, 521–531. [Google Scholar]
- Collen, B.; Whitton, F.; Dyer, E.E.; Baillie, J.E.; Cumberlidge, N.; Darwall, W.R.; Pollock, C.; Richman, N.I.; Soulsby, A.-M.; Böhm, M. Global patterns of freshwater species diversity, threat and endemism. Glob. Ecol. Biogeogr. 2014, 23, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Fragnière, Y.; Bétrisey, S.; Cardinaux, L.; Stoffel, M.; Kozlowski, G. Fighting their last stand? A global analysis of the distribution and conservation status of gymnosperms. J. Biogeogr. 2015, 42, 809–820. [Google Scholar] [CrossRef]
- Song, Y.-G.; Fragnière, Y.; Meng, H.-H.; Li, Y.; Bétrisey, S.; Corrales, A.; Manchester, S.R.; Deng, M.; Jasińska, A.K.; Văn Sâm, H. Global biogeographic synthesis and priority conservation regions of the relict tree family Juglandaceae. J. Biogeogr. 2020, 47, 643–657. [Google Scholar] [CrossRef]
- Hassler, M. World plants: Synonymic checklists of the vascular plants of the world (Version Nov 2018). In Species 2000 & ITIS Catalogue of Life, 2020-09-01 Beta; Roskov, Y., Ower, G., Orrell, T., Nicolson, D., Bailly, N., Kirk, P.M., Bourgoin, T., DeWalt, R.E., Decock, W., van Nieukerken, E., et al., Eds.; Naturalis: Leiden, The Netherlands, 2020. [Google Scholar]
- The Plant List A Working List of All Plant Species; Version 1.1. Available online: http://www.theplantlist.org/ (accessed on 15 December 2020).
- Elbert, L.; Little, J. Checklist of United States Trees (Native and Naturalized); Forest Service, US Department of Agriculture: Washington, DC, USA, 1979. [Google Scholar]
- Caudullo, G.; De Rigo, D. Ulmus-elms in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016; pp. 186–188. [Google Scholar]
- Seregin, A.P. Digital herbarium of Moscow State University: The largest Russian biodiversity database. Biol. Bull. 2017, 44, 584–590. [Google Scholar] [CrossRef]
- Dunn, C.P. The Elms: Breeding, Conservation, and Disease Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2000; ISBN 0-7923-7724-9. [Google Scholar]
- Boratyńska, K.; Sękiewicz, M.; Boratyński, A. Morfologia, systematyka i rozmieszczenie geograficzne. In Wiązy (Ulmus); Bugała, W., Boratyński, A., Iszkuło, G., Eds.; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2015; pp. 24–52. [Google Scholar]
- Zhang, Q.; Zhou, Z.; Xing, Y. Phylogeny and biogeographic history of Ulmaceae based on complete chloroplast genome and nuclear sequences. In Proceedings of the 16th National Congress of Botanical Society of China, Kunming, China, 10–13 October 2018. [Google Scholar]
- Kumar, V.; Singh, S.; Bhadouria, R.; Singh, R.; Prakash, O. Phytochemical, analytical and medicinal studies of Holoptelea Integrifolia roxb: Planch—A Review. Curr. Tradit. Med. 2019, 5, 270–277. [Google Scholar] [CrossRef]
- Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [CrossRef]
- Visser, V.; Clayton, W.D.; Simpson, D.A.; Freckleton, R.P.; Osborne, C.P. Mechanisms driving an unusual latitudinal diversity gradient for grasses. Glob. Ecol. Biogeogr. 2014, 23, 61–75. [Google Scholar] [CrossRef]
- Manchester, S.R.; Chen, Z.-D.; Lu, A.-M.; Uemura, K. Eastern Asian endemic seed plant genera and their paleogeographic history throughout the northern hemisphere. J. Syst. Evol. 2009, 47, 1–42. [Google Scholar] [CrossRef]
- Denk, T.; Dillhoff, R.M. Ulmus leaves and fruits from the early-middle Eocene of Northwestern North America: Systematics and implications for character evolution within Ulmaceae. Botany 2005, 83, 1663–1681. [Google Scholar] [CrossRef]
- Magallón-Puebla, S.; Cevallos-Ferriz, S.R. Latest occurrence of the extinct genus Cedrelospermum (Ulmaceae) in North America: Cedrelospermum Manchesteri from Mexico. Rev. Palaeobot. Palynol. 1994, 81, 115–128. [Google Scholar] [CrossRef]
- Garfì, G.; Carimi, F.; Fazan, L.; Gristina, A.S.; Kozlowski, G.; Console Livreri, S.; Motisi, P.; Pasta, S. From glacial refugia to hydrological microrefugia: Factors and processes driving the persistence of the climate relict tree Zelkova sicula. Ecol. Evol. 2021. [Google Scholar] [CrossRef]
- Lykholat, Y.; Khromykh, N.; Didur, O.; Alexeyeva, A.; Lykholat, T.; Davydov, V. Modeling the invasiveness of Ulmus pumila in urban ecosystems in conditions of climate change. Regulat. Mech. Biosyst. 2018, 9, 161–166. [Google Scholar] [CrossRef]
- Sherman-Broyles, S.L.; Barker, W.T.; Schulz, L.M. Ulmaceae. Flora N. Am. 1997, 3, 368–380. [Google Scholar]
- Kozlowski, G.; Frey, D.; Fazan, L.; Egli, B.; Bétrisey, S.; Gratzfeld, J.; Garfì, G.; Pirintsos, S. The tertiary relict tree Zelkova abelicea (Ulmaceae): Distribution, population structure and conservation status on Crete. Oryx 2014, 48, 80–87. [Google Scholar] [CrossRef][Green Version]
- World Conservation Monitoring Centre IUCN Red List of Threatened Species: Ulmus Gaussenii. Available online: https://www.iucnredlist.org/en (accessed on 4 November 2020).
- Garfì, G.; Pasta, S.; Fazan, L.; Kozlowski, G. IUCN Red List of Threatened Species: Zelkova Sicula. Available online: https://www.iucnredlist.org/en (accessed on 6 October 2020).
- Zhang, Q.; Zhang, H.; Li, Q.; Bai, R.; Ning, E.; Cai, X. Characterization of the complete chloroplast genome sequence of an endangered elm species, Ulmus gaussenii (Ulmaceae). Conserv. Genet. Resour. 2019, 11, 71–74. [Google Scholar] [CrossRef]
- Geng, Q.-F.; Yang, J.; He, J.; Wang, D.-B.; Shi, E.; Xu, W.-X.; Jeelani, N.; Wang, Z.-S.; Liu, H. Microsatellite markers for the critically endangered elm species Ulmus gaussenii (Ulmaceae). Genes Genet. Syst. 2016, 91, 11–14. [Google Scholar] [CrossRef][Green Version]
- Garfì, G.; Carimi, F.; Pasta, S.; Rühl, J.; Trigila, S. Additional insights on the ecology of the relic tree Zelkova sicula di Pasquale, garfì et quézel (Ulmaceae) after the finding of a new population. Flora Morphol. Distribut. Funct. Ecol. Plants 2011, 206, 407–417. [Google Scholar] [CrossRef]
- Garfì, G.; Buord, S. Relict species and the challenges for conservation: The emblematic Case of Zelkova sicula di pasquale, garfì et quézel and the efforts to save it from extinction. Biodivers. J. 2012, 3, 281–296. [Google Scholar]
- Christe, C.; Kozlowski, G.; Frey, D.; Bétrisey, S.; Maharramova, E.; Garfì, G.; Pirintsos, S.; Naciri, Y. Footprints of past intensive diversification and structuring in the genus Zelkova (Ulmaceae) in south-western Eurasia. J. Biogeogr. 2014, 41, 1081–1093. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 11 December 2020).
- Fazan, L.; Guillet, S.; Corona, C.; Kozlowski, G.; Stoffel, M. Imprisoned in the Cretan mountains: How relict Zelkova abelicea (Ulmaceae) trees cope with Mediterranean climate. Sci. Total Environ. 2017, 599, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Stritch, L.; Rivers, M.C.; Barstow, M. IUCN Red List of Threatened Species: Ulmus Americana. Available online: https://www.iucnredlist.org/en (accessed on 20 October 2020).
- Gao, J.-G.; Wu, Y.-H.; Xu, G.-D.; Li, W.-Q.; Yao, G.-H.; Ma, J.; Liu, P. Phylogeography of Ulmus elongata based on fourier transform-infrared spectroscopy (FTIR), thermal gravimetric and differential thermal analyses. Biochem. Syst. Ecol. 2012, 40, 184–191. [Google Scholar] [CrossRef]
- Song, J.; Chen, L.; Chen, F.; Ye, J. Edaphic and host plant factors are linked to the composition of arbuscular mycorrhizal fungal communities in the root zone of endangered Ulmus chenmoui Cheng in China. Ecol. Evol. 2019, 9, 8900–8910. [Google Scholar] [CrossRef] [PubMed]
- Mughal, A.H.; Mugloo, J.A. Elm (Ulmus wallichiana): A vulnerable lesser known multipurpose tree species of Kashmir valley. SKUAST J. Res. 2016, 18, 73–79. [Google Scholar]
- Lopez-Gallego, C.; Morales, M.P.A. IUCN Red List of Threatened Species: Ampelocera albertiae. Available online: https://www.iucnredlist.org/en (accessed on 9 February 2021).
- Todzia, C.A.; Panero, J.L. A New species of Ulmus (Ulmaceae) from Southern Mexico and a synopsis of the species in Mexico. Brittonia 1998, 50, 343–347. [Google Scholar] [CrossRef]
- Linares, J.L. Primer registro de Ulmus ismaelis (Ulmaceae) para Centroamérica. Rev. Mexicana Biodivers. 2005, 76, 95–96. [Google Scholar] [CrossRef]
- Hou, H.; Ye, H.; Wang, Z.; Wu, J.; Gao, Y.; Han, W.; Na, D.; Sun, G.; Wang, Y. Demographic history and genetic differentiation of an endemic and endangered Ulmus lamellosa (Ulmus). BMC Plant Biol. 2020, 20, 526. [Google Scholar] [CrossRef]
- Manchester, S.R.; Tiffney, B.H. Integration of paleobotanical and neobotanical data in the assessment of phytogeographic history of holarctic angiosperm clades. Int. J. Plant Sci. 2001, 162, 19–27. [Google Scholar] [CrossRef]
- Wu, Z.; Raven, P.H.; Hong, D. Flora of China—Ulmaceae through Basellaceae; Science Press: Beijing, China, 2003; Volume 5. [Google Scholar]
- Naciri, Y.; Christe, C.; Bétrisey, S.; Song, Y.-G.; Deng, M.; Garfì, G.; Kozlowski, G. Species delimitation in the East Asian species of the relict tree genus Zelkova (Ulmaceae): A complex history of diversification and admixture among species. Mol. Phylogenet. Evol. 2019, 134, 172–185. [Google Scholar] [CrossRef]
- Nee, M. Ulmaceae—Flora Mesoamericana. Available online: http://legacy.tropicos.org/Project/FM (accessed on 11 December 2020).
- Akhter, R. Ulmaecae in Flora of Pakistan. Available online: www.eFloras.org (accessed on 11 December 2020).
- Wiegrefe, S.J.; Sytsma, K.J.; Guries, R.P. Phylogeny of elms (Ulmus, Ulmaceae): Molecular evidence for a sectional classification. Syst. Bot. 1994, 19, 590–612. [Google Scholar] [CrossRef]
- Buchel, A.S. The species of the genus Ulmus. In The Elms, Breeding, Conservation and Disease Management; Kluwer Academic Publishers: Berlin, Germany, 2000; pp. 351–358. [Google Scholar]
- Xiao, Y.; Watson, M. Guidance on conducting—A systematic literature review. J. Plann. Educ. Res. 2019, 39, 93–112. [Google Scholar] [CrossRef]
- Little, J.; Elbert, L. Atlas of United States Trees. In Conifers and Important Hardwoods; USDA Forest Service Miscellaneous Publication: Washington, DC, USA, 1971; Volume 1, p. 1146. [Google Scholar]
- Melville, R.; Heybroek, H.M. The elms of the Himalaya. Kew Bull. 1971, 26, 5–28. [Google Scholar] [CrossRef]
- Fu, L.; Xin, Y. Elms of China. In The Elms; Springer: Boston, MA, USA, 2000; pp. 21–44. [Google Scholar]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Pederneiras, L.C.; Machado, A.F.P.; Pederneiras, L.C.; Machado, A.F.P. Flora do estado do Rio de Janeiro: Ulmaceae. Rodriguésia 2017, 68, 541–543. [Google Scholar] [CrossRef][Green Version]
- GBIF. GBIF: The Global Biodiversity Information Facility. Available online: https://www.gbif.org (accessed on 29 September 2020).
- Gradstein, S.R. Catálogo de Plantas y Líquenes de Colombia; En Bernal, R., Gradstein, S.R., Celis, M., Eds.; Instituto de Ciencias Naturales, Universidad Nacional de Colombia: Bogotá, Colombia, 2015. [Google Scholar]
- Royal Botanic Gardens Kew. Plants of the World Online. Available online: http://www.plantsoftheworldonline.org (accessed on 29 September 2020).
- QGIS. Development Team QGIS Geographic Information System. Available online: http://qgis.org (accessed on 12 November 2020).
- GADM. GADM Database of Global Administrative Areas. Available online: https://gadm.org/ (accessed on 26 February 2021).
- Natural Earth Data. Natural Earth—Free Vector and Raster Map Data at 1:10 m, 1:50 m, and 1:110 m Scales. Available online: https://www.naturalearthdata.com/ (accessed on 26 February 2021).
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 1–12. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Earth Resources Observation and Science (EROS). Center shuttle radar topography mission (SRTM) 1 arc-second global. USGS 2017. [Google Scholar] [CrossRef]
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic predictors for supporting ecological applications in the conterminous United States. US Geol. Surv. Data Ser. 2012, 691, 4–9. [Google Scholar]
- Goedecke, F.; Bergmeier, E. Ecology and potential distribution of the Cretan endemic tree species Zelkova abelicea. J. Mediterr. Ecol. 2018, 16, 15–26. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Kruskal, J.B. Nonmetric multidimensional scaling: A numerical method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The Vegan Package. Commun. Ecol. Package 2007, 10, 719. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2018; ISBN 3-319-71404-X. [Google Scholar]




| Clade | Genus | # Species | General Distribution |
|---|---|---|---|
| Tropical clade | Ampelocera | 9 | South America and Mesoamerica (8), Caribbean (1) |
| Holoptelea | 2 | Africa (1) Asia (1) | |
| Phyllostylon | 2 | South America (1) South America, Mesoamerica and Caribbean (1) | |
| Temperate clade | Hemiptelea | 1 | Eastern Asia |
| Planera | 1 | North America | |
| Ulmus | 35 | North America (6) Mesoamerica (2) Europe and Western Asia (3) Asia (24) | |
| Zelkova | 6 | Mediterranean Europe and Western Asia (3) Eastern Asia (3) | |
| Total | 56 |
| Category | # Species | % |
|---|---|---|
| Species of humid macrohabitats (equatorial and tropical humid rainforests, large amount (>1500 mm) of annual precipitation) | 15 | 26.8 |
| Species of moist microhabitats (alluvial and riparian forests, moist ravines), generally exclusively | 11 | 19.6 |
| Species often occurring in moist microhabitats but also in other habitat types | 13 | 23.2 |
| Species occurring in other habitat types or scarce information available | 17 | 30.4 |
| IUCN Category | # Species | Species |
|---|---|---|
| CR | 2 | Ulmus gaussenii, Zelkova sicula |
| EN | 4 | Ampelocera albertiae, Ulmus americana, U. chenmoui, Zelkova abelicea |
| VU | 5 | Ulmus elongata, U. wallichiana, Zelkova carpinifolia, Z. sinica, Z. schneideriana |
| NT | 2 | Ampelocera longissima, Zelkova serrata |
| LC | 22 | Ampelocera edentula, A. hottlei, A. macphersonii, A. macrocarpa, A. ruizii, Hemiptelea davidii, Holoptelea grandis, Phyllostylon rhamnoides, Planera aquatica, Ulmus alata, U. castaneifolia, U. crassifolia, U. davidiana, U. laciniata, U. rubra, U. macrocarpa, U. parvifolia, U. pumila, U. serotina, U. szechuanica, U. thomasii, U. mexicana |
| DD | 3 | Ulmus glabra, U. laevis, U. minor |
| Not assessed | 18 | Ampelocera cubensis, A. glabra, Holoptelea integrifolia, Phyllostylon brasiliense, Ulmus androssowii, U. bergmanniana, U. changii, U. glaucescens, U. harbinensis, U. ismaelis, U. lamellosa, U. lanceifolia, U. mianzhuensis, U. microcarpa, U. prunifolia, U. pseudopropinqua, U. uyematsui, U. villosa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragnière, Y.; Song, Y.-G.; Fazan, L.; Manchester, S.R.; Garfì, G.; Kozlowski, G. Biogeographic Overview of Ulmaceae: Diversity, Distribution, Ecological Preferences, and Conservation Status. Plants 2021, 10, 1111. https://doi.org/10.3390/plants10061111
Fragnière Y, Song Y-G, Fazan L, Manchester SR, Garfì G, Kozlowski G. Biogeographic Overview of Ulmaceae: Diversity, Distribution, Ecological Preferences, and Conservation Status. Plants. 2021; 10(6):1111. https://doi.org/10.3390/plants10061111
Chicago/Turabian StyleFragnière, Yann, Yi-Gang Song, Laurence Fazan, Steven R. Manchester, Giuseppe Garfì, and Gregor Kozlowski. 2021. "Biogeographic Overview of Ulmaceae: Diversity, Distribution, Ecological Preferences, and Conservation Status" Plants 10, no. 6: 1111. https://doi.org/10.3390/plants10061111
APA StyleFragnière, Y., Song, Y.-G., Fazan, L., Manchester, S. R., Garfì, G., & Kozlowski, G. (2021). Biogeographic Overview of Ulmaceae: Diversity, Distribution, Ecological Preferences, and Conservation Status. Plants, 10(6), 1111. https://doi.org/10.3390/plants10061111









