Functional Traits of Olive Varieties and Their Relationship with the Tolerance Level towards Verticillium Wilt
Abstract
1. Introduction
2. Results
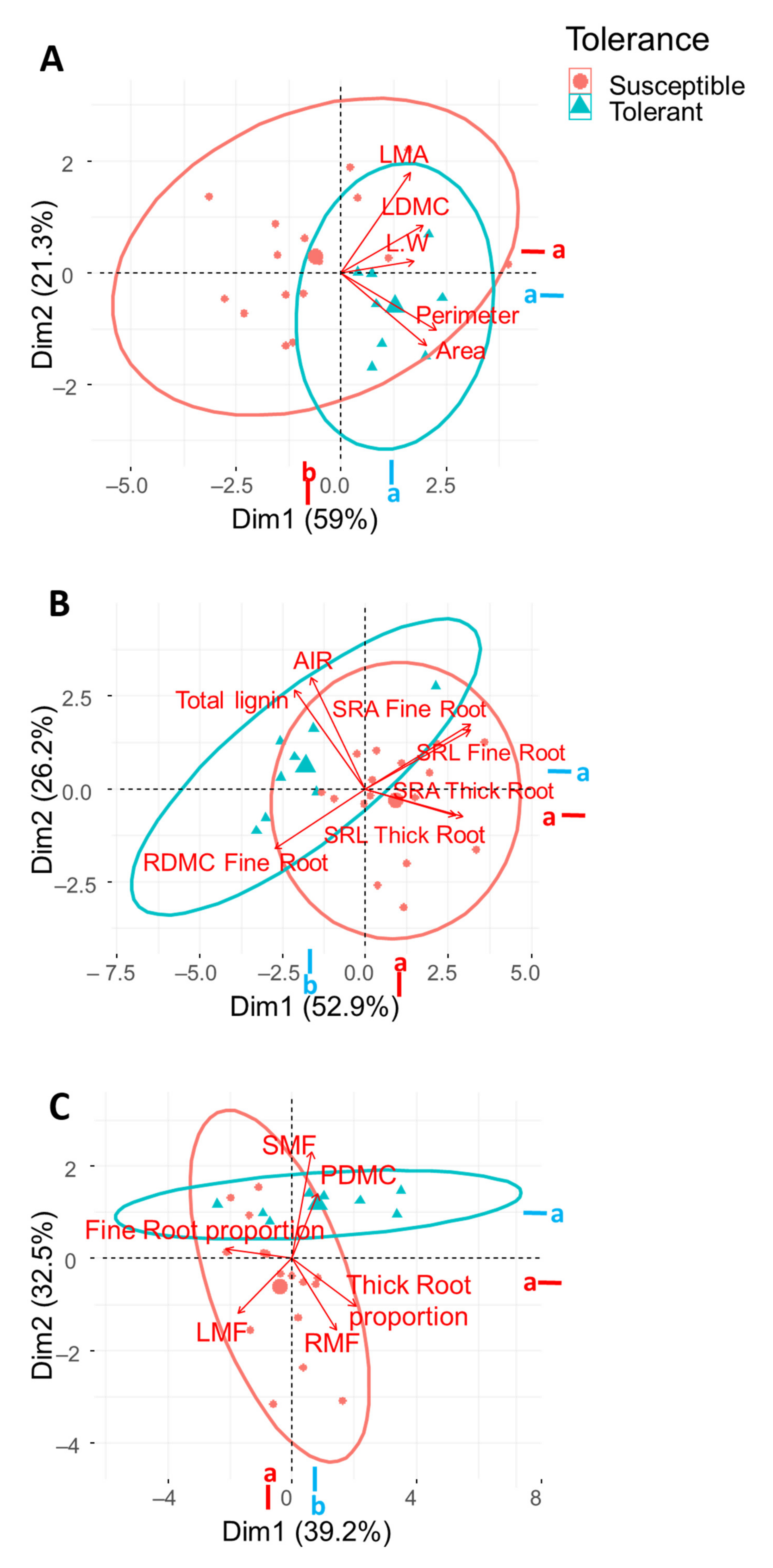
2.1. Variability in Functional Traits among Olive Varieties and between VWO Susceptibility/Tolerance Level
2.2. Differences in Leaf and Stem Functional Traits
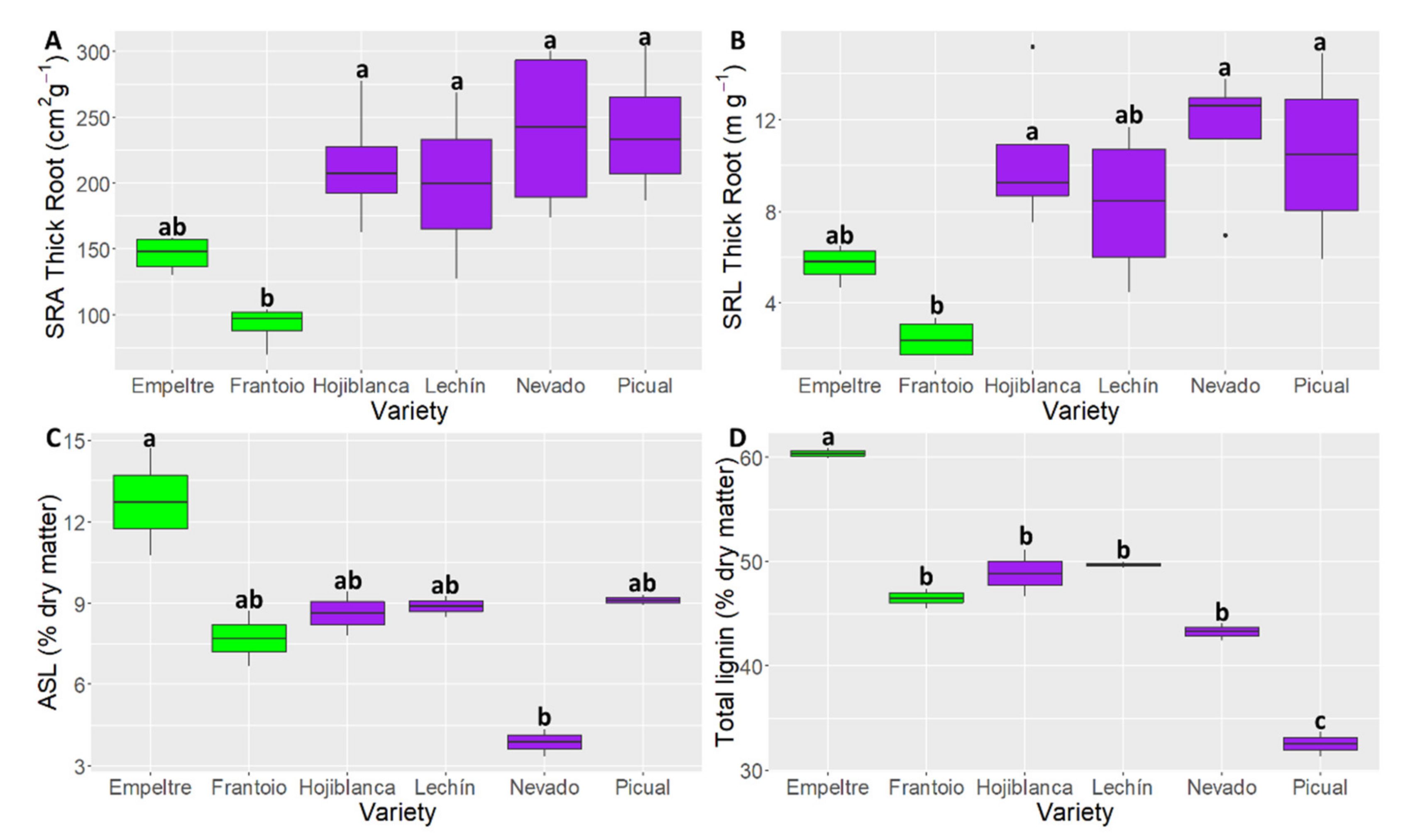
2.3. Differences in Root Functional Traits
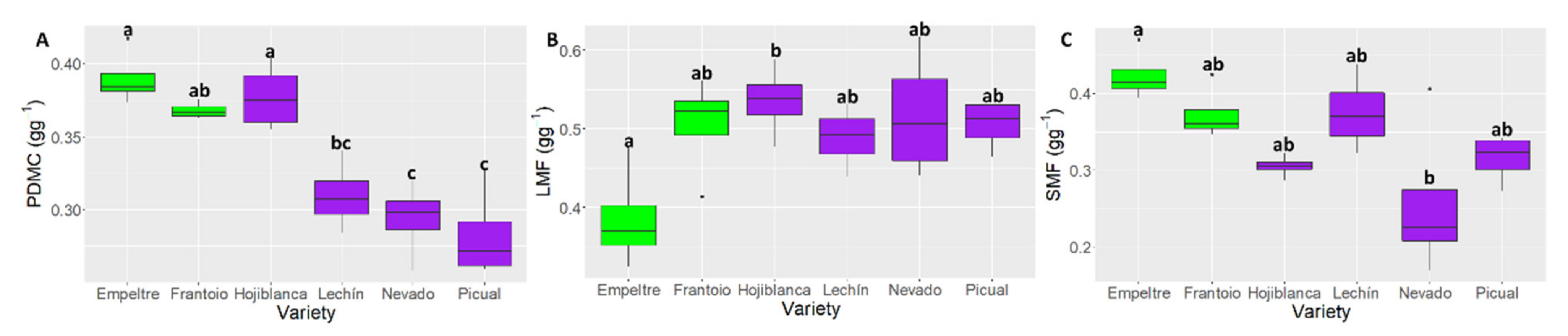
2.4. Differences in Plant Functional Traits
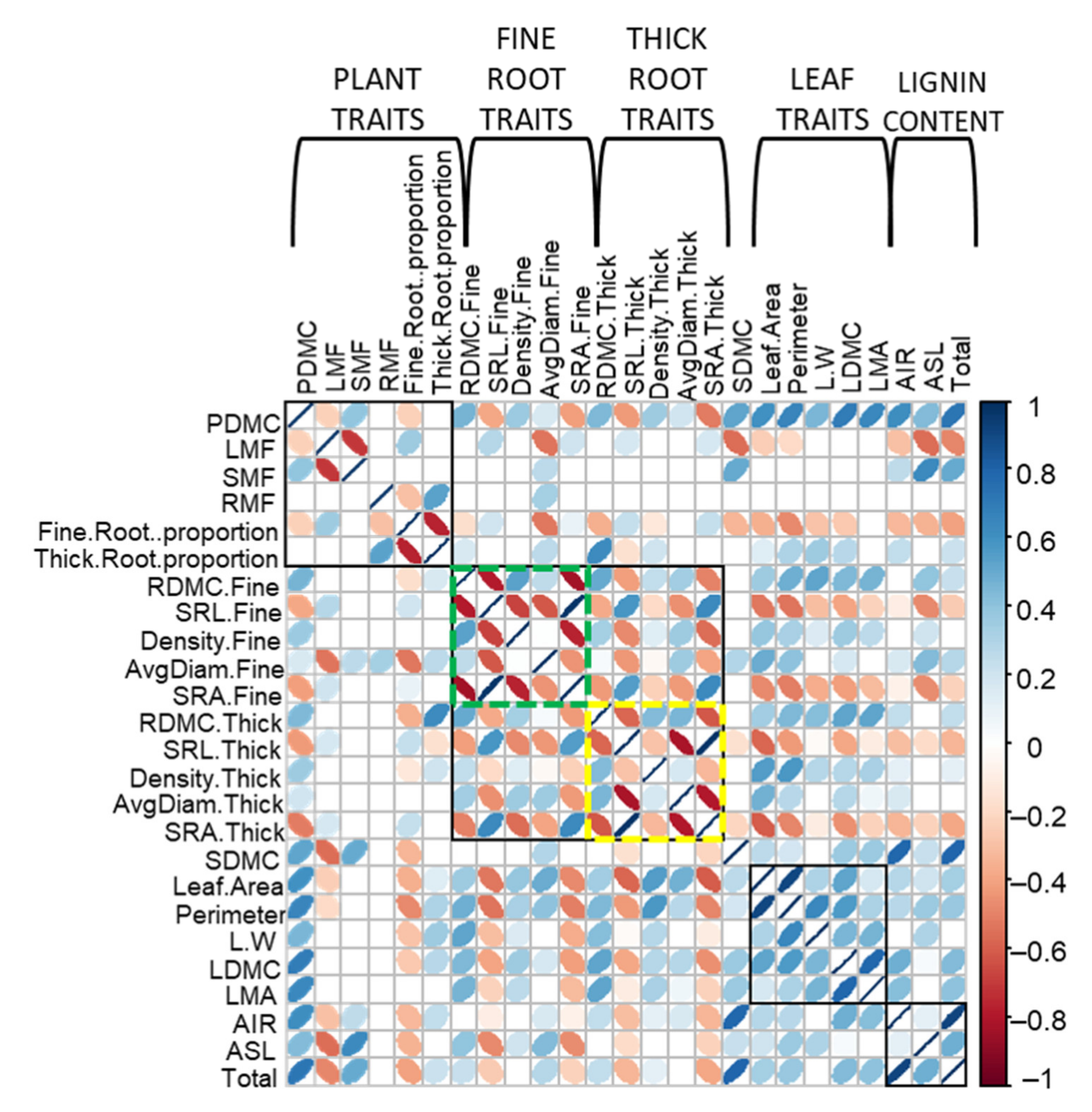
2.5. Correlation among Functional Traits
3. Discussion
4. Materials and Methods
4.1. Olive Varieties and Plant Tissue Manipulation
| Cultivar | Origin | Susceptibility | Reference(s) |
|---|---|---|---|
| Empeltre | Spain | T | [3,70] |
| Frantoio | Italy | T | [13,46,71,72] |
| Hojiblanca | Spain | E | [9,70] |
| Lechín de Sevilla | Spain | S | [3] |
| Nevado fino | Spain | S | [3,73] |
| Picual | Spain | E | [13,46,71,74] |
4.2. Measurements of Functional Traits of Leaves, Stems and Roots
4.3. Determination of Acid-Insoluble Additionally, Acid-Soluble Lignin Content
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nation (FAO). FAOSTAT, Production Statistics. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 18 July 2020).
- Montes-Osuna, N.; Mercado-Blanco, J. Verticillium wilt of olive and its control: What did we learn during the last decade? Plants 2020, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Trapero, C.; Rallo, L.; López-Escudero, F.J.; Barranco, D.; Díez, C.M. Variability and selection of verticillium wilt resistant genotypes in cultivated olive and in the Olea genus. Plant Pathol. 2015, 64, 890–900. [Google Scholar] [CrossRef]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, pathogenicity, and management of Verticillium species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef]
- Fernández-González, A.J.; Cardoni, M.; Gómez-Lama Cabanás, C.; Valverde-Corredor, A.; Villadas, P.J.; Fernández-López, M.; Mercado-Blanco, J. Linking belowground microbial network changes to different tolerance level towards Verticillium wilt of olive. Microbiome 2020, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- López-Escudero, F.J.; Mercado-Blanco, J. Verticillium wilt of olive: A case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil 2011, 344, 1–50. [Google Scholar] [CrossRef]
- Garcia-Ruiz, G.M.; Trapero, C.; Del Rio, C.; Lopez-Escudero, F.J. Evaluation of resistance of Spanish olive cultivars to Verticillium dahliae in inoculations conducted in greenhouse. Phytoparasitica 2014, 42, 205–212. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Orfanoudakis, M.; Alifragis, D.; Therios, I. Colonization of Greek olive cultivars’ root system by arbuscular mycorrhiza fungus: Root morphology, growth, and mineral nutrition of olive plants. Sci. Agric. 2013, 70, 185–194. [Google Scholar] [CrossRef][Green Version]
- Trapero, C.; Serrano, N.; Arquero, O.; Del Río, C.; Trapero, A.; López-Escudero, F.J. Field resistance to Verticillium wilt in selected olive cultivars grown in two naturally infested soils. Plant Dis. 2012, 97, 668–674. [Google Scholar] [CrossRef]
- Prieto, P.; Navarro-Raya, C.; Valverde-Corredor, A.; Amyotte, S.G.; Dobinson, K.F.; Mercado-Blanco, J. Colonization process of olive tissues by Verticillium dahliae and its in planta interaction with the biocontrol root endophyte Pseudomonas fluorescens PICF7. Microb. Biotechnol. 2009, 2, 499–511. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, J.; Ramírez-Tejero, J.A.; Fernández-Pozo, N.; Leyva-Pérez, M.D.L.O.; Yan, H.; de la Rosa, R.; Belaj, A.; Montes, E.; Rodríguez-Ariza, M.O.; Navarro, F.; et al. Transposon activation is a major driver in the genome evolution of cultivated olive trees (Olea europaea L.). Plant Genome 2020, 13, 1–19. [Google Scholar] [CrossRef]
- Gharbi, Y.; Barkallah, M.; Bouazizi, E.; Hibar, K.; Gdoura, R.; Triki, M.A. Lignification, phenols accumulation, induction of PR proteins and antioxidant-related enzymes are key factors in the resistance of Olea europaea to Verticillium wilt of olive. Acta Physiol. Plant. 2017, 39, 1–15. [Google Scholar] [CrossRef]
- Trapero, C.; Alcántara, E.; Jiménez, J.; Amaro-Ventura, M.C.; Romero, J.; Koopmann, B.; Karlovsky, P.; Von Tiedemann, A.; Pérez-Rodríguez, M.; López-Escudero, F.J. Starch hydrolysis and vessel occlusion related to wilt symptoms in olive stems of susceptible cultivars infected by Verticillium dahliae. Front. Plant Sci. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- De la Riva, E.G.; Tosto, A.; Pérez-Ramos, I.M.; Navarro-Fernández, C.M.; Olmo, M.; Anten, N.P.R.; Marañón, T.; Villar, R. A plant economics spectrum in Mediterranean forests along environmental gradients: Is there coordination among leaf, stem and root traits? J. Veg. Sci. 2016, 27, 187–199. [Google Scholar] [CrossRef]
- Booth, R.E.; Grime, J.P. Effects of genetic impoverishment on plant community diversity. J. Ecol. 2003, 91, 721–730. [Google Scholar] [CrossRef]
- Ackerly, D.D.; Cornwell, W.K. A trait-based approach to community assembly: Partitioning of species trait values into within- and among-community components. Ecol. Lett. 2007, 10, 135–145. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Douzet, R.; Aubert, S.; Lavorel, S.; Ando, K.; Grumet, R.; Terpstra, K.; Kelly, J.D. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2010, 24, 1192–1201. [Google Scholar] [CrossRef]
- De Bello, F.; Lavorel, S.; Albert, C.H.; Thuiller, W.; Grigulis, K.; Dolezal, J.; Janeček, Š.; Lepš, J. Quantifying the relevance of intraspecific trait variability for functional diversity. Methods Ecol. Evol. 2011, 2, 163–174. [Google Scholar] [CrossRef]
- Boege, K.; Dirzo, R. Intraspecific variation in growth, defense and herbivory in Dialium guianense (Caesalpiniaceae) mediated by edaphic heterogeneity. Plant Ecol. 2004, 175, 59–69. [Google Scholar] [CrossRef]
- Crutsinger, G.M.; Collins, M.D.; Fordyce, J.A.; Gompert, Z.; Nice, C.C.; Sanders, N.J. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 2006, 647, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Lecerf, A.; Chauvet, E. Intraspecific variability in leaf traits strongly affects alder leaf decomposition in a stream. Basic Appl. Ecol. 2008, 9, 598–605. [Google Scholar] [CrossRef]
- Faucon, M.P.; Houben, D.; Lambers, H. Plant functional traits: Soil and ecosystem services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef]
- Ando, K.; Grumet, R.; Terpstra, K.; Kelly, J.D. Manipulation of plant architecture to enhance crop disease control. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 2, 1–8. [Google Scholar] [CrossRef]
- Alameda, D.; Villar, R. Linking root traits to plant physiology and growth in Fraxinus angustifolia Vahl. seedlings under soil compaction conditions. Environ. Exp. Bot. 2012, 79, 49–57. [Google Scholar] [CrossRef]
- Hummel, I.; Vile, D.; Violle, C.; Devaux, J.; Ricci, B.; Blanchard, A.; Garnier, É.; Roumet, C. Relating root structure and anatomy to whole-plant functioning in 14 herbaceous Mediterranean species. New Phytol. 2007, 173, 313–321. [Google Scholar] [CrossRef]
- Dias, R.D.C.S.; Picó, B.; Espinos, A.; Nuez, F. Resistance to melon vine decline derived from Cucumis melo ssp. agrestis: Genetic analysis of root structure and root response. Plant Breed. 2004, 123, 66–72. [Google Scholar]
- Román-Avilés, B.; Snapp, S.S.; Kelly, J.D. Assessing root traits associated with root rot resistance in common bean. Field Crop. Res. 2004, 86, 147–156. [Google Scholar]
- Emmett, B.; Nelson, E.B.; Kessler, A.; Bauerle, T.L. Fine-root system development and susceptibility to pathogen colonization. Planta 2014, 239, 325–340. [Google Scholar] [CrossRef]
- Valentines, M.C.; Vilaplana, R.; Torres, R.; Usall, J.; Larrigaudière, C. Specific roles of enzymatic browning and lignification in apple disease resistance. Postharvest Biol. Technol. 2005, 36, 227–234. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L.; Tu, L.; Liu, L.; Yuan, D.; Jin, L.; Long, L.; Zhang, X. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. J. Exp. Bot. 2011, 62, 5607–5621. [Google Scholar] [CrossRef] [PubMed]
- Sabella, E.; Luvisi, A.; Aprile, A.; Negro, C.; Vergine, M.; Nicolì, F.; Miceli, A.; De Bellis, L. Xylella fastidiosa induces differential expression of lignification related-genes and lignin accumulation in tolerant olive trees cv. Leccino. J. Plant Physiol. 2018, 220, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Eynck, C.; Koopmann, B.; Karlovsky, P.; Von Tiedemann, A. Internal resistance in winter oilseed rape inhibits systemic spread of the vascular pathogen Verticillium longisporum. Phytopathology 2009, 99, 802–811. [Google Scholar] [CrossRef]
- Zhang, C.B.; Chen, L.H.; Jiang, J. Why fine tree roots are stronger than thicker roots: The role of cellulose and lignin in relation to slope stability. Geomorphology 2014, 206, 196–202. [Google Scholar] [CrossRef]
- Maire, V.; Gross, N.; Hill, D.; Martin, R.; Wirth, C.; Wright, I.J.; Soussana, J.F. Disentangling coordination among functional traits using an individual-centred model: Impact on plant performance at intra- and inter-specific levels. PLoS ONE 2013, 8, e77372. [Google Scholar] [CrossRef]
- Liu, G.; Freschet, G.T.; Pan, X.; Cornelissen, J.H.C.; Li, Y.; Dong, M. Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytol. 2010, 188, 543–553. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Olmo, M.; López-Iglesias, B.; Villar, R. Drought changes the structure and elemental composition of very fine roots in seedlings of ten woody tree species. Implications for a drier climate. Plant Soil 2014, 384, 113–129. [Google Scholar] [CrossRef]
- Lloret, F.; Casanovas, C.; Peñuelas, J. Seedling survival of Mediterranean shrubland species in relation to root: Shoot ratio, seed size and water and nitrogen use. Funct. Ecol. 1999, 13, 210–216. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Oh, S.; Oh, M.M.; Son, J.E. Estimation of individual leaf area, fresh weight, and dry weight of hydroponically grown cucumbers (Cucumis sativus L.) using leaf length, width, and SPAD value. Sci. Hortic. 2007, 111, 330–334. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, B.; Villar, R.; Poorter, L. Functional traits predict drought performance and distribution of mediterranean woody species. Acta Oecologica 2014, 56, 10–18. [Google Scholar] [CrossRef]
- Rodríguez-Jurado, D. Interacciones Huésped Parásito en la Verticilosis del Olivo (Olea europea L.) Inducida por Verticillium dahliae Kleb. Ph.D. Thesis, University of Córdoba, Córdoba, Spain, 1993. [Google Scholar]
- Leyva-Pérez, M.D.L.O.; Jiménez-Ruiz, J.; Gómez-Lama Cabanás, C.; Valverde-Corredor, A.; Barroso, J.B.; Luque, F.; Mercado-Blanco, J. Tolerance of olive (Olea europaea) cv Frantoio to Verticillium dahliae relies on both basal and pathogen-induced differential transcriptomic responses. New Phytol. 2018, 217, 671–686. [Google Scholar] [CrossRef]
- Olmo, M.; Villar, R. Changes in root traits explain the variability of biochar effects on fruit production in eight agronomic species. Org. Agric. 2019, 9, 139–153. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, D. The significance of lateral roots in phosphorus (P) acquisition of water hyacinth (Eichhornia crassipes). Aquat. Bot. 2003, 75, 311–321. [Google Scholar] [CrossRef]
- Lõhmus, K.; Oja, T.; Lasn, R. Specific root area: A soil characteristic. Plant Soil 1989, 119, 245–249. [Google Scholar] [CrossRef]
- Boot, R.G.A.; Mensink, M. Size and morphology of root systems of perennial grasses from contrasting habitats as affected by nitrogen supply. Plant Soil 1990, 129, 291–299. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations in Plants and Soils, 1st ed.; Academic Press: Lewes, DE, USA, 1995; pp. 1–152. [Google Scholar]
- Ryser, P. The mysterious root length. Plant Soil 2006, 286, 1–6. [Google Scholar] [CrossRef]
- Ostonen, I.; Lõhmus, K.; Helmisaari, H.S.; Truu, J.; Meel, S. Fine root morphological adaptations in Scots pine, Norway spruce and silver birch along a latitudinal gradient in boreal forests. Tree Physiol. 2007, 27, 1627–1634. [Google Scholar] [CrossRef]
- Eissenstat, D.M. Costs and benefits of constructing roots of small diameter. J. Plant Nutr. 1992, 15, 763–782. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Caldwell, M.M. Invasive root growth into disturbed soil of two tussock grasses that differ in competitive effectiveness. Funct. Ecol. 1989, 3, 345. [Google Scholar] [CrossRef]
- Mengel, K.; Steffens, D. Potassium uptake of rye-grass (Lolium perenne) and red clover (Trifolium pratense) as related to root parameters. Biol. Fertil. Soils 1985, 1, 53–58. [Google Scholar] [CrossRef]
- Hamblin, A.P.; Tennant, D. Root length density and water uptake in cereals and grain legumes: How well are they correlated. Aust. J. Agric. Res. 1987, 38, 513–527. [Google Scholar] [CrossRef]
- Ramírez-Tejero, J.A.; Jiménez-Ruiz, J.; Serrano, A.; Belaj, A.; León, L.; de la Rosa, R.; Mercado-Blanco, J.; Luque, F. Verticillium wilt resistant and susceptible olive cultivars express a very different basal set of genes in roots. BMC Genom. 2021, 22, 229. [Google Scholar] [CrossRef]
- Markakis, E.A.; Tjamos, S.E.; Antoniou, P.P.; Roussos, P.A.; Paplomatas, E.J.; Tjamos, E.C. Phenolic responses of resistant and susceptible olive cultivars induced by defoliating and nondefoliating Verticillium dahliae pathotypes. Plant Dis. 2010, 94, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O.K. Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. EMBO J. 2019, 38, e101948. [Google Scholar] [CrossRef]
- Lozovaya, V.V.; Lygin, A.V.; Li, S.; Hartman, G.L.; Widholm, J.M. Biochemical response of soybean roots to Fusarium solani f. sp. glycines infection. Crop Sci. 2004, 44, 819–826. [Google Scholar] [CrossRef]
- Ride, J. Role of cell wall alterations in resistance to fungi. Ann. Appl. Biol. 1978, 89, 302–306. [Google Scholar]
- Shi, H.; Liu, Z.; Zhu, L.; Zhang, C.; Chen, Y.; Zhou, Y.; Li, F.; Li, X. Overexpression of cotton (Gossypium hirsutum) dirigent1 gene enhances lignification that blocks the spread of Verticillium dahliae. Acta Biochim. Biophys. Sin. 2012, 44, 555–564. [Google Scholar] [CrossRef]
- Vile, D.; Garnier, É.; Shipley, B.; Laurent, G.; Navas, M.L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef]
- Westoby, M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 1998, 199, 213–227. [Google Scholar] [CrossRef]
- Elger, A.; Willby, N.J. Leaf dry matter content as an integrative expression of plant palatability: The case of freshwater macrophytes. Funct. Ecol. 2003, 17, 58–65. [Google Scholar] [CrossRef]
- Saura-Mas, S.; Lloret, F. Leaf and shoot water content and leaf dry matter content of Mediterranean woody species with different post-fire regenerative strategies. Ann. Bot. 2007, 99, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Cianciaruso, M.V.; Batalha, M.A.; Gaston, K.J.; Petchey, O.L. Including intraspecific variability in functional diversity. Ecology 2009, 90, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Cortés, F.; Parfitt, E.E.; Romero, C.; Struss, D.; Llácer, G.; Badenes, M.L. Intraspecific olive diversity assessed with AFLP. Plant Breed. 2003, 122, 173–177. [Google Scholar] [CrossRef]
- López-Escudero, F.J.; Del Río, C.; Caballero, J.M.; Blanco-López, M.A. Evaluation of olive cultivars for resistance to Verticillium dahliae. Eur. J. Plant Pathol. 2004, 110, 79–85. [Google Scholar] [CrossRef]
- Gómez-Lama Cabanás, C.; Schilirò, E.; Valverde-Corredor, A.; Mercado-Blanco, J. Systemic responses in a tolerant olive (Olea europaea L.) cultivar upon root colonization by the vascular pathogen Verticillium dahliae. Front. Microbiol. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Bubici, G.; Cirulli, M. Control of Verticillium wilt of olive by resistant rootstocks. Plant Soil 2012, 352, 363–376. [Google Scholar] [CrossRef]
- Trapero, C.; Serrano, N.; Arquero, O.; Del Rio, C.; Trapero, A.; López-Escudero, F.J. Resistencia de variedades de olivo en un campo infestado por Verticillium dahliae. Vida Rural 2011, 334, 34–39. [Google Scholar]
- Jiménez-Ruiz, J.; Leyva-Pérez, M.D.L.O.; Schilirò, E.; Barroso, J.B.; Bombarely, A.; Mueller, L.; Mercado-Blanco, J.; Luque, F. Transcriptomic analysis of Olea europaea L. roots during the Verticillium dahliae early infection process. Plant Genome 2017, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- TAPPI. Acid-insoluble Lignin in Wood and Pulp, Technical Association of the Pulp and Paper Industry, TAPPI Standard Methods T222 om-02; TAPPI: Atlanta, GA, USA, 2011. [Google Scholar]
- TAPPI. Solvent Extractives of Wood and Pulp, Technical Association of the Pulp and Paper Industry, TAPPI Standard Methods T204 cm-07; TAPPI: Atlanta, GA, USA, 2007. [Google Scholar]
- Cara, C.; Ruiz, E.; Oliva, J.M.; Sáez, F.; Castro, E. Conversion of olive tree biomass into fermentable sugars by dilute acid pretreatment and enzymatic saccharification. Bioresour. Technol. 2008, 99, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Kaar, W.E.; Brink, D.L. Simplified analysis of acid soluble lignin. J. Wood Chem. Technol. 1991, 11, 465–477. [Google Scholar] [CrossRef]
- De Mendiburu, F.; Simon, R. Agricolae—Ten years of an open source statistical tool for experiments in breeding, agriculture and biology. PeerJ 2015, 3, 1–17. [Google Scholar]
- Kassambara, A. Practical Guide to Principal Component Methods in R: PCA, M (CA), FAMD, MFA, HCPC, Factoextra, 1st ed. Statistical Tools for High-throughput Data Analysis (STHDA). 2016, pp. 1–154. Available online: http://www.sthda.com/english/ (accessed on 18 January 2021).
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. R package “corrplot”: Visualization of a correlation matrix. Statistician 2017, 56, 316–324. [Google Scholar]
- R. Team, RStudio: Integrated Development Environment for R. 2015. Available online: https://www.rstudio.com (accessed on 18 January 2021).

| Plant Part | Trait | Abbreviation | Units | Explained Variance (‘variety’) (%) | Explained Variance (‘tolerance’) (%) |
|---|---|---|---|---|---|
| Leaf | Area | Area | cm2 | 62.97 *** 1 | 97.11 ***(+) 1 |
| Perimeter | Perimeter | cm | 50.43 ** | 92.14 **(+) | |
| Green density | Green density | - | 72.40 ** | 2.83 | |
| Length/width ratio | L/W | - | 73.05 ** | 8.12 | |
| Leaf dry matter content | LDMC | g∙g−1 | 69.34 ** | 87.78 *(+) | |
| Leaf mass per area | LMA | g∙m−2 | 74.05 *** | 9.38 | |
| Stem | Stem dry matter content | SDMC | g∙g−1 | 68.13 *** | 85.18 *(+) |
| Plant | Plant dry matter content | PDMC | g∙g−1 | 89.43 *** | 92.65 **(+) |
| Leaf mass fraction | LMF | g∙g−1 | 62.80 * | 57.84 *(-) | |
| Stem mass fraction | SMF | g∙g−1 | 72.27 * | 87.63 **(+) | |
| Root mass fraction | RMF | g∙g−1 | 49.52 | 11.07 | |
| Fine root proportion | Fine root prop. | g∙g−1 | 58.73 | 24.31 | |
| Thick root proportion | Thick root prop. | g∙g−1 | 24.78 | 0.07 | |
| Fine root | Fine root specific length | SRL Fine Root | m∙g−1 | 47.56 | 67.55 *(-) |
| Fine root tissue density | Density Fine Root | g∙cm−3 | 44.46 | 62.85 | |
| Fine root average diameter | AvgDiam Fine Root | cm | 67.60 *** | 78.91 **(+) | |
| Fine root specific area | SRA Fine Root | cm2∙g−1 | 60.32 | 12.97 | |
| Fine root dry matter content | RDMC Fine Root | g∙g−1 | 62.28 | 43.51 | |
| Thick root | Thick root specific length | SRL Thick Root | m∙g−1 | 45.16 ** | 85.58 ***(-) |
| Thick root tissue density | Density Thick Root | g∙cm−3 | 17.91 | 19.37 | |
| Thick root average diameter | AvgDiam Thick Root | cm | 42.45 | 51.94 | |
| Thick root specific area | SRA Thick Root | cm2∙g−1 | 51.19 ** | 90.56 ***(-) | |
| Thick root dry matter content | RDMC Thick Root | g∙g−1 | 1.42 | 28.63 | |
| Root lignin content | Acid insoluble residue | AIR | % dry matter | 97.04 *** | 68.76 |
| Acid soluble lignin | ASL | % dry matter | 86.17 * | 85.72 * (+) | |
| Total lignin | Total lignin | % dry matter | 97.19 *** | 84.6 * (+) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoni, M.; Mercado-Blanco, J.; Villar, R. Functional Traits of Olive Varieties and Their Relationship with the Tolerance Level towards Verticillium Wilt. Plants 2021, 10, 1079. https://doi.org/10.3390/plants10061079
Cardoni M, Mercado-Blanco J, Villar R. Functional Traits of Olive Varieties and Their Relationship with the Tolerance Level towards Verticillium Wilt. Plants. 2021; 10(6):1079. https://doi.org/10.3390/plants10061079
Chicago/Turabian StyleCardoni, Martina, Jesús Mercado-Blanco, and Rafael Villar. 2021. "Functional Traits of Olive Varieties and Their Relationship with the Tolerance Level towards Verticillium Wilt" Plants 10, no. 6: 1079. https://doi.org/10.3390/plants10061079
APA StyleCardoni, M., Mercado-Blanco, J., & Villar, R. (2021). Functional Traits of Olive Varieties and Their Relationship with the Tolerance Level towards Verticillium Wilt. Plants, 10(6), 1079. https://doi.org/10.3390/plants10061079








