Sulfur Deprivation Modulates Salicylic Acid Responses via Nonexpressor of Pathogenesis-Related Gene 1 in Arabidopsis thaliana
Abstract
1. Introduction
2. Results
2.1. The Absence of Sulfur Induces SA-mediated PR1 Expression
2.2. Excess of Sulfur Inhibits SA-mediated PR1 Expression
2.3. Transient Expression of PR1 under Sulfur Deficiency
2.4. A Total Absence of Sulfur Induces PR1 Expression
2.5. SA Accumulation and Functional NPR1 Is Required for PR1 Expression Induced by Sulfur Deprivation
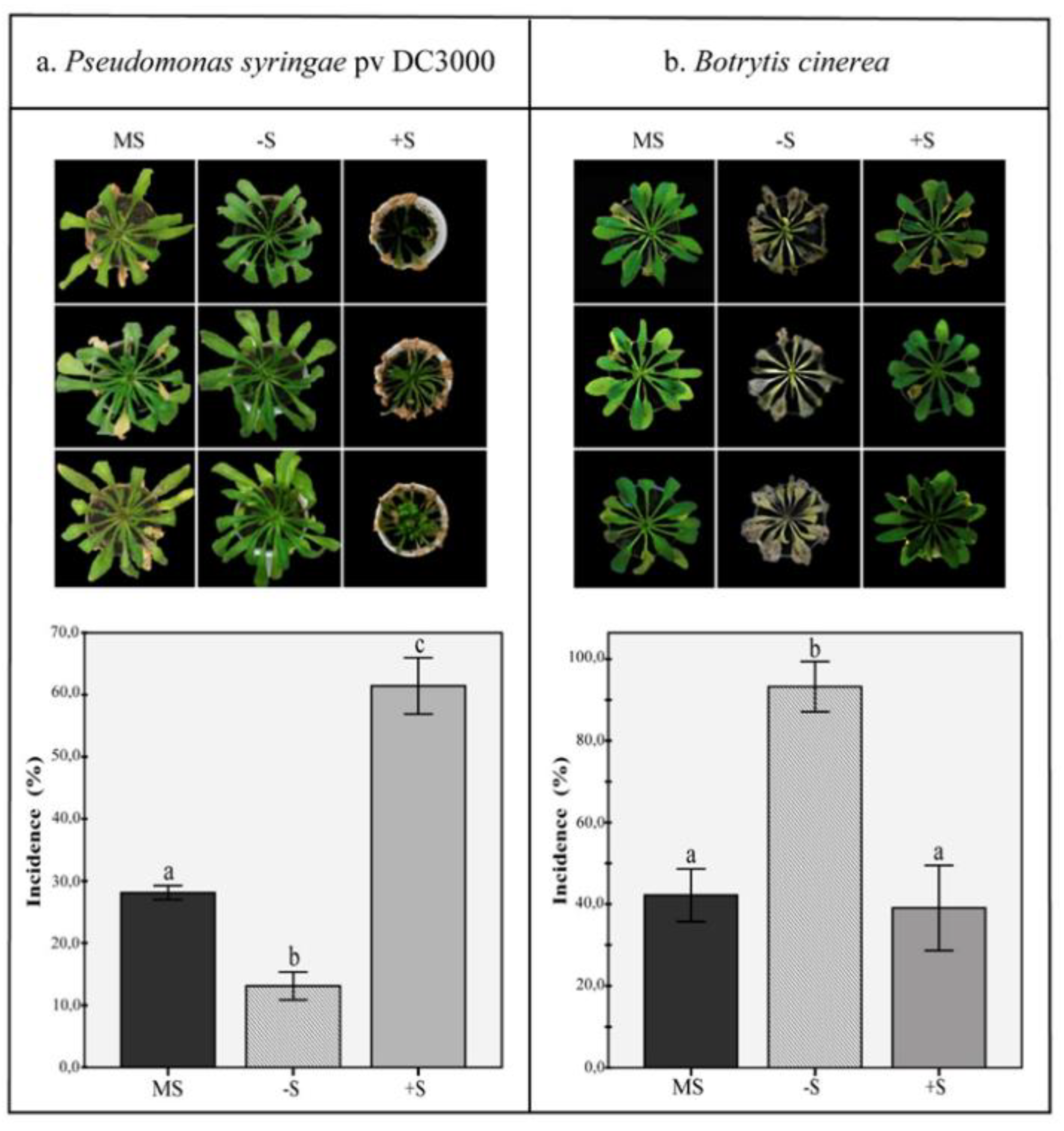
2.6. Disease Resistance under Deficiency and Excess of Sulfur
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Plant Material
5.1.1. Growth Conditions and Nutrient Treatment for A. thaliana In Vitro Assays
5.1.2. A. thaliana In Vitro Assays: Sulfur Stress and SA-Mediated Pathways Evaluation
5.2. A. thaliana In Vivo Assays: Greenhouse Conditions and Nutrient Treatments
5.3. Histochemical GUS Assays
5.4. RNA Extraction and RT-PCR
5.5. Evaluation of Pseudomonas syringae and Botrytis cinerea Disease Resistance on Sulfur-Manipulated Diets
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fixen, P.E.; Brentrup, F.; Bruulsema, T.; Garcia, F.; Norton, R.; Zingore, S. Nutrient/fertilizer use efficiency: Measurement, current situation and trends. In Managing Water and Fertilizer for Sustainable Agricultural Intensification; International Fertilizer Industry Association (IFA); International Water Management Institute (IWMI); International Plant Nutrition Institute (IPNI); International Potash Institute (IPI): Paris, France, 2015; pp. 1–30. [Google Scholar]
- Adams, F. Nutritional imbalances and constraints to plant growth on acid soils. J. Plant Nutr. 1981, 4, 81–87. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3544. [Google Scholar] [CrossRef]
- Bhattacharyya-Pakrasi, M. A. Pareek, S. Sopory, H.J. Bohnert; Govindjee (Eds) Abiotic stress adaptation in plants: Physiological, molecular and genomic foundation. Photosynth. Res. 2011, 108, 247–248. [Google Scholar] [CrossRef]
- A Grusak, M. Plant Macro- and Micronutrient Minerals. eLS 2001. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nat. Cell Biol. 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, L.; Birch, P.R.J. The zigzag model of plant–microbe interactions: Is it time to move on? Mol. Plant Pathol. 2014, 15, 865–870. [Google Scholar] [CrossRef]
- Lukasik, E.; Takken, F.L. STANDing strong, resistance proteins instigators of plant defense. Curr. Opin. Plant Biol. 2009, 12, 427–436. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van Der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Catinot, J.; Buchala, A.; Mansour, E.A.; Métraux, J.-P. Salicylic acid production in response to biotic and abiotic stress depends on isochorismate inNicotiana benthamiana. FEBS Lett. 2008, 582, 473–478. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Park, S.-W.; Kaimoyo, E.; Kumar, D.; Mosher, S.; Klessig, D.F. Methyl Salicylate Is a Critical Mobile Signal for Plant Systemic Acquired Resistance. Science 2007, 318, 113–116. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of Inducible Defense-related Proteins in Infected Plants. Annu. Rev. Phytopathol. 2006, 44, 135–161. [Google Scholar] [CrossRef]
- Gamir, J.; Darwiche, R.; Hof, P.V.; Choudhary, V.; Stumpe, M.; Schneiter, R.; Mauch, F. The sterol-binding activity of PATHOGENESIS-RELATED PROTEIN 1 reveals the mode of action of an antimicrobial protein. Plant J. 2017, 89, 502–509. [Google Scholar] [CrossRef]
- Métraux, J.-P.; Nawrath, C.; Genoud, T. Systemic acquired resistance. Euphytica 2002, 124, 237–243. [Google Scholar] [CrossRef]
- Withers, J.; Dong, X. Posttranslational modifications of NPR1: A single protein playing multiple roles in plant immunity and physiology. PLoS Pathog. 2016, 12, e1005707. [Google Scholar] [CrossRef]
- Pokotylo, I.; Kravets, V.; Ruelland, E. Salicylic acid binding proteins (SABPs): The hidden forefront of salicylic acid signalling. Int. J. Mol. Sci. 2019, 20, 4377. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- McConn, M.; Creelman, R.A.; Bell, E.; Mullet, J.E.; Browse, J. Jasmonate is essential for insect defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 1997, 94, 5473–5477. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Melotto, M.; Yao, J.; He, S.Y. Jasmonate signaling and manipulation by pathogens and insects. J. Exp. Bot. 2017, 68, 1371–1385. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D. Hormone Crosstalk in Plant Disease and Defense: More Than Just JASMONATE-SALICYLATE Antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Naseem, M.; Dandekar, T. The Role of Auxin-Cytokinin Antagonism in Plant-Pathogen Interactions. PLOS Pathog. 2012, 8, e1003026. [Google Scholar] [CrossRef]
- Broekgaarden, C.; Caarls, L.; Vos, I.A.; Pieterse, C.M.; Van Wees, S. Ethylene: Traffic controller on hormonal crossroads to defense. Plant Physiol. 2015, 169, 2371–2379. [Google Scholar] [CrossRef]
- Walters, D.; Bingham, I. Influence of nutrition on disease development caused by fungal pathogens: Implications for plant disease control. Ann. Appl. Biol. 2007, 151, 307–324. [Google Scholar] [CrossRef]
- Garcia-Mina, J.M. Plant Nutrition and Defense Mechanism: Frontier Knowledge. In Advances in Citrus Nutrition; Springer: Dodrecht, The Netherlands, 2012; pp. 1–12. ISBN 9789400741713. [Google Scholar]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C. A Role for Zinc in Plant Defense Against Patho-gens and Herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Mur, L.A.J.; Shen, Q.; Guo, S. Unravelling the roles of nitrogen nutrition in plant disease defenses. Int. J. Mol. Sci. 2020, 21, 572. [Google Scholar] [CrossRef]
- Reuveni, R.; Reuveni, M.; Agapov, V. Induction of Growth Increase and Systemic Resistance to Exserohilum turcicum in Maize by Foliar Spray of Phosphates. J. Phytopathol. 1994, 141, 337–346. [Google Scholar] [CrossRef]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 2008, 133, 682–691. [Google Scholar] [CrossRef]
- Greenshields, D.L.; Liu, G.; Wei, Y. Roles of iron in plant defense and fungal virulence (Plant Signaling and Behavior). Plant Signal. Behav. 2007, 2, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Ebloem, E.; Ehaneklaus, S.; Eschnug, E. Milestones in plant sulfur research on sulfur-induced-resistance (SIR) in Europe. Front. Plant Sci. 2015, 5, 779. [Google Scholar] [CrossRef]
- Trapet, P.; Avoscan, L.; Klinguer, A.; Pateyron, S.; Citerne, S.; Chervin, C.; Mazurier, S.; Lemanceau, P.; Wendehenne, D.; Besson-Bard, A. The Pseudomonas fluorescens Siderophore Pyoverdine Weakens Arabidopsis thaliana Defense in Favor of Growth in Iron-Deficient Conditions. Plant Physiol. 2016, 171, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Aznar, A.; Chen, N.W.; Thomine, S.; Dellagi, A. Immunity to plant pathogens and iron homeostasis. Plant Sci. 2015, 240, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.J.; Simpson, C.; Kumari, A.; Gupta, A.K.; Gupta, K.J. Moving nitrogen to the centre of plant defence against patho-gens. Ann. Bot. 2017, 119, 703–709. [Google Scholar]
- Anjum, N.A.; Gill, R.; Kaushik, M.; Hasanuzzaman, M.; Pereira, E.; Ahmad, I.; Tuteja, N.; Gill, S.S. ATP-sulfurylase, sulfur-compounds, and plant stress tolerance. Front. Plant Sci. 2015, 6, 210. [Google Scholar] [CrossRef]
- Capaldi, F.R.; Gratão, P.L.; Reis, A.R.; Lima, L.W.; Azevedo, R.A. Sulfur Metabolism and Stress Defense Responses in Plants. Trop. Plant Biol. 2015, 8, 60–73. [Google Scholar] [CrossRef]
- Rausch, T.; Wachter, A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef]
- Ryan, C.A. Protease Inhibitors in Plants: Genes for Improving Defenses Against Insects and Pathogens. Annu. Rev. Phytopathol. 1990, 28, 425–449. [Google Scholar] [CrossRef]
- McCauley, A.; Jones, C.; Jacobsen, J. Plant nutrient functions and deficiency and toxicity symptons. Nutr. Manag. 2009, 9, 1–16. [Google Scholar]
- Mohd, M.; Khan, A.; Rewiev, M.; Mazid, M.; Zeba, K.H.; Quddusi, S.; Ahmed Khan, T.; Mohammad, F. Significance of Sul-phur nutrition against metal induced oxidative stress in plants. J. Stress Physiol. 2011, 7, 165–184. [Google Scholar]
- Ausma, T.; De Kok, L.J. Atmospheric H2S: Impact on plant functioning. Front. Plant Sci. 2019, 10, 743. [Google Scholar] [CrossRef]
- Henríquez-Valencia, C.; Arenas-M, A.; Medina, J.; Canales, J. Integrative Transcriptomic Analysis Uncovers Novel Gene Modules That Underlie the Sulfate Response in Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 470. [Google Scholar] [CrossRef]
- Kopriva, S.; Calderwood, A.; Weckopp, S.C.; Koprivova, A. Plant sulfur and Big Data. Plant Sci. 2015, 241, 1–10. [Google Scholar] [CrossRef]
- Abrol, Y.P.; Ahmad, A. Sulphur in Plants; Springer: Dodrecht, The Netherlands, 2003; ISBN 9789048162765. [Google Scholar]
- Ernst, W.H.O.; Krauss, G.; Verkleij, J.A.C.; Wesenberg, D. Interaction of heavy metals with the sulphur metabolism in angiosperms from an ecological point of view. Plant Cell Environ. 2007, 31, 123–143. [Google Scholar] [CrossRef]
- Bashir, H.; Ibrahim, M.M.; Bagheri, R.; Ahmad, J.; Arif, I.; Baig, A.; Qureshi, M.I. Influence of sulfur and cadmium on antioxidants, phytochelatins and growth in Indian mustard. AoB PLANTS 2015, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Lara, L.O.; Medrano-Macías, J.; Pérez-Labrada, F.; Rivas-Martínez, E.N.; García-Enciso, E.L.; González-Morales, S.; Juárez-Maldonado, A.; Rincón-Sánchez, F.; Benavides-Mendoza, A. From Elemental Sulfur to Hydrogen Sulfide in Agricultural Soils and Plants. Molecules 2019, 24, 2282. [Google Scholar] [CrossRef] [PubMed]
- Keinath, A.; Loria, R. Management of common scab of potato with plant nutrients. Manag. Dis. Macro Microelements 1989, 1989, 152–166. [Google Scholar]
- Huber, D. Sulphur and Plant Disease; Washington State University: Washington, DC, USA, 2007. [Google Scholar]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Bollig, K.; Specht, A.; Myint, S.S.; Zahn, M.; Horst, W.J. Sulphur supply impairs spread of Verticillium dahliae in tomato. Eur. J. Plant Pathol. 2012, 135, 81–96. [Google Scholar] [CrossRef]
- Van Wees, S.C.M.; Glazebrook, J. Loss of non-host resistance of Arabidopsis NahG to Pseudomonas syringae pv. phaseolicola is due to degradation products of salicylic acid. Plant J. 2003, 33, 733–742. [Google Scholar] [CrossRef]
- Uquillas, C.; Letelier, I.; Blanco, F.; Jordana, X.; Holuigue, L. NPR1-Independent Activation of Immediate Early Salicylic Acid-Responsive Genes in Arabidopsis. Mol. Plant Microbe Interact. 2004, 17, 34–42. [Google Scholar] [CrossRef]
- Schuhegger, R.; Ihring, A.; Gantner, S.; Bahnweg, G.; Knappe, C.; Vogg, G.; Hutzler, P.; Schmid, M.; Van Breusegem, F.; Eberl, L.; et al. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006, 29, 909–918. [Google Scholar] [CrossRef]
- Ferrari, S.; Galletti, R.; Denoux, C.; De Lorenzo, G.; Ausubel, F.M.; Dewdney, J. Resistance to Botrytis cinerea Induced in Arabidopsis by Elicitors Is Independent of Salicylic Acid, Ethylene, or Jasmonate Signaling But Requires PHYTOALEXIN DEFICIENT. Plant Physiol. 2007, 144, 367–379. [Google Scholar] [CrossRef]
- Zhou, N.; Tootle, T.L.; Glazebrook, J. Arabidopsis PAD3, a Gene Required for Camalexin Biosynthesis, Encodes a Putative Cytochrome P450 Monooxygenase. Plant Cell 1999, 11, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- E Clark, S.; Williams, R.W.; Meyerowitz, E.M. The CLAVATA1Gene Encodes a Putative Receptor Kinase That Controls Shoot and Floral Meristem Size in Arabidopsis. Cell 1997, 89, 575–585. [Google Scholar] [CrossRef]
- von Liebig, J. Organic Chemistry in Its Applications to Agriculture and Physiology, 1st ed.; Taylor and Walton: London, UK, 1840; p. 387. [Google Scholar]
- Havlin, J.; Beaton, J.; Tisdale, S.; Nelson, W. Soil Fertility and Fertilizers: An Introduction to Nutrient Management; Pearson Education India: Indianapolis, IN, USA, 2005. [Google Scholar]
- Parikh, S.J.; James, B.R. Soil: The Foundation of Agriculture. Nat. Educ. Knowl. 2012, 3, 2. [Google Scholar]
- Martos, S.; Gallego, B.; Cabot, C.; Llugany, M.; Barceló, J.; Poschenrieder, C. Zinc triggers signaling mechanisms and defense responses promoting resistance to Alternaria brassicicola in Arabidopsis thaliana. Plant Sci. 2016, 249, 13–24. [Google Scholar] [CrossRef]
- Gupta, K.J.; Brotman, Y.; Segu, S.; Zeier, T.; Zeier, J.; Persijn, S.; Cristescu, S.M.; Harren, F.J.M.; Bauwe, H.; Fernie, A.R.; et al. The form of nitrogen nutrition affects resistance against Pseudomonas syringae pv. phaseolicola in tobacco. J. Exp. Bot. 2012, 64, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Troufflard, S.; Mullen, W.; Larson, T.R.; A Graham, I.; Crozier, A.; Amtmann, A.; Armengaud, P. Potassium deficiency induces the biosynthesis of oxylipins and glucosinolates in Arabidopsis thaliana. BMC Plant Biol. 2010, 10, 172. [Google Scholar] [CrossRef]
- Kruse, C.; Haas, F.H.; Jost, R.; Reiser, B.; Reichelt, M.; Wirtz, M.; Gershenzon, J.; Schnug, E.; Hell, R. Improved sulfur nutrition provides the basis for enhanced production of sulfur-containing defense compounds in Arabidopsis thaliana upon inoculation with Alternaria brassicicola. J. Plant Physiol. 2012, 169, 740–743. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Ma, Y.; Wang, L.; Yang, L.; Shi, S. Crop resistance to diseases as influenced by sulphur application rates. In Proceedings of the 12th World Fertilizer Congress, Beijing, China, 3–9 August 2001; pp. 1285–1296. [Google Scholar]
- Klikocka, H.; Haneklaus, S.; Bloem, E.; Schnug, E. Influence of Sulfur Fertilization on Infection of Potato Tubers withRhizoctonia solaniandStreptomyces scabies. J. Plant Nutr. 2005, 28, 819–833. [Google Scholar] [CrossRef]
- Salac, I.; Haneklaus, S.H.; Bloem, E.; Booth, E.J.; Sutherland, K.G.; Walker, K.C.; Schnug, E. Sulfur nutrition and its signifi-cance for crop resistance—a case study from Scotland. Landbauforsch. Völkenrode 2005, 283, 111–119. [Google Scholar]
- Bouranis, D.L.; Venieraki, A.; Chorianopoulou, S.N.; Katinakis, P. Impact of Elemental Sulfur on the Rhizospheric Bacteria of Durum Wheat Crop Cultivated on a Calcareous Soil. Plants 2019, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Bouranis, D.L.; Malagoli, M.; Avice, J.-C.; Bloem, E. Advances in Plant Sulfur Research. Plants 2020, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.; Accotto, G.P.; Bechtold, U.; Creissen, G.; Funck, D.; Jimenez, A.; Kular, B.; Leyland, N.; Mejia-Carranza, J.; Reynolds, H.; et al. Evidence for a Direct Link between Glutathione Biosynthesis and Stress Defense Gene Expression in Arabidopsis. Plant Cell 2004, 16, 2448–2462. [Google Scholar] [CrossRef]
- Bai, Y.; Sunarti, S.; Kissoudis, C.; Visser, R.G.F.; Van Der Linden, C.G. The Role of Tomato WRKY Genes in Plant Responses to Combined Abiotic and Biotic Stresses. Front. Plant Sci. 2018, 9, 801. [Google Scholar] [CrossRef]
- Mou, Z.; Fan, W.; Dong, X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef]
- Le Henanff, G.; Heitz, T.; Mestre, P.; Mutterer, J.; Walter, B.; Chong, J. Characterization of Vitis vinifera NPR1 homologs involved in the regulation of Pathogenesis-Related gene expression. BMC Plant Biol. 2009, 9, 54. [Google Scholar] [CrossRef]
- Ohshima, M.; Itoh, H.; Matsuoka, M.; Murakami, T.; Ohashi, Y. Analysis of stress-induced or salicylic acid-induced expression of the pathogenesis-related 1a protein gene in transgenic tobacco. Plant Cell 1990, 2, 95–106. [Google Scholar] [CrossRef]
- Somssich, I.E.; Bollmann, J.; Hahlbrock, K.; Kombrink, E.; Schulz, W. Differential early activation of defense-related genes in elicitor-treated parsley cells. Plant Mol. Biol. 1989, 12, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Dröge-Laser, W.; A Dixon, R.; Lamb, C. Transcriptional activation of plant defense genes. Curr. Opin. Genet. Dev. 1996, 6, 624–630. [Google Scholar] [CrossRef]
- Herrera-Vã¡squez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171. [Google Scholar] [CrossRef]
- Leon-Reyes, A.; Spoel, S.H.; De Lange, E.S.; Abe, H.; Kobayashi, M.; Tsuda, S.; Millenaar, F.F.; Welschen, R.A.; Ritsema, T.; Pieterse, C.M. Ethylene Modulates the Role of NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 in Cross Talk between Salicylate and Jasmonate Signaling. Plant Physiol. 2009, 149, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Van der Does, D.; Leon-Reyes, A.; Koornneef, A.; Van Verk, M.C.; Rodenburg, N.; Pauwels, L.; Goossens, A.; Körbes, A.P.; Memelink, J.; Ritsema, T.; et al. Salicylic Acid Suppresses Jasmonic Acid Signaling Downstream of SCFCOI1-JAZ by Targeting GCC Promoter Motifs via Transcription Factor ORA. Plant Cell 2013, 25, 744–761. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, A.; Leon-Reyes, A.; Ritsema, T.; Verhage, A.; Otter, F.C.D.; Van Loon, L.; Pieterse, C.M. Kinetics of Salicylate-Mediated Suppression of Jasmonate Signaling Reveal a Role for Redox Modulation. Plant Physiol. 2008, 147, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Natl. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef]
- Nikiforova, V.; Freitag, J.; Kempa, S.; Bielecka, M.; Hesse, H.; Hoefgen, R. Transcriptome analysis of sulfur depletion inArabidopsis thaliana: Interlacing of biosynthetic pathways provides response specificity. Plant J. 2003, 33, 633–650. [Google Scholar] [CrossRef]
- Dubuis, P.-H.; Marazzi, C.; Städler, E.; Mauch, F. Sulphur Deficiency Causes a Reduction in Antimicrobial Potential and Leads to Increased Disease Susceptibility of Oilseed Rape. J. Phytopathol. 2005, 153, 27–36. [Google Scholar] [CrossRef]
- Koornneef, A.; Verhage, A.; Leon-Reyes, A.; Snetselaar, R.; Van Loon, L.; Pieterse, C.M. Towards a reporter system to identify regulators of cross-talk between salicylate and jasmonate signaling pathways in Arabidopsis. Plant Signal. Behav. 2008, 3, 543–546. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method forAgrobacterium-mediated transformation ofArabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1938, 347, 32. [Google Scholar]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef]
- Proietti, S.; Caarls, L.; Coolen, S.; Van Pelt, J.A.; Van Wees, S.; Pieterse, C.M. Genome-wide association study reveals novel players in defense hormone crosstalk in Arabidopsis. Plant Cell Environ. 2018, 41, 2342–2356. [Google Scholar] [CrossRef]
- Herrera-Romero, I.; Ruales, C.; Caviedes, M.; Leon-Reyes, A. Postharvest evaluation of natural coatings and antifungal agents to control Botrytis cinerea in Rosa sp. Phytoparasitica 2017, 45, 9–20. [Google Scholar] [CrossRef]
- Van Wees, S.; Van Pelt, J.A.; Bakker, P.A.H.M.; Pieterse, C.M.J. Bioassays for Assessing Jasmonate-Dependent Defenses Triggered by Pathogens, Herbivorous Insects, or Beneficial Rhizobacteria. Methods Mol. Biol. 2013, 1011, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Denby, K.J.; Kumar, P.; Kliebenstein, D.J. Identification ofBotrytis cinereasusceptibility loci inArabidopsis thaliana. Plant J. 2004, 38, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Corwin, J.A.; Subedy, A.; Eshbaugh, R.; Kliebenstein, D.J. Expansive Phenotypic Landscape of Botrytis cinerea Shows Differential Contribution of Genetic Diversity and Plasticity. Mol. Plant Microbe Interact. 2016, 29, 287–298. [Google Scholar] [CrossRef] [PubMed]






| Deficiency (mM) | Standard (mM) | Excess (mM) | ||||
|---|---|---|---|---|---|---|
| Nitrogen | -N | 0 | MS | 60 | +N | 120 |
| Potassium | -K | 0 | 20 | +K | 80 | |
| Sulfur | -S | 0 | 1.6 | +S | 6.4 | |
| Magnesium | -Mg | 0 | 1.5 | +Mg | 6 | |
| Phosphorous | -P | 0 | 1.25 | +P | 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Criollo-Arteaga, S.; Moya-Jimenez, S.; Jimenez-Meza, M.; Gonzalez-Vera, V.; Gordon-Nunez, J.; Llerena-Llerena, S.; Ramirez-Villacis, D.X.; van ‘t Hof, P.; Leon-Reyes, A. Sulfur Deprivation Modulates Salicylic Acid Responses via Nonexpressor of Pathogenesis-Related Gene 1 in Arabidopsis thaliana. Plants 2021, 10, 1065. https://doi.org/10.3390/plants10061065
Criollo-Arteaga S, Moya-Jimenez S, Jimenez-Meza M, Gonzalez-Vera V, Gordon-Nunez J, Llerena-Llerena S, Ramirez-Villacis DX, van ‘t Hof P, Leon-Reyes A. Sulfur Deprivation Modulates Salicylic Acid Responses via Nonexpressor of Pathogenesis-Related Gene 1 in Arabidopsis thaliana. Plants. 2021; 10(6):1065. https://doi.org/10.3390/plants10061065
Chicago/Turabian StyleCriollo-Arteaga, Steven, Sofia Moya-Jimenez, Martin Jimenez-Meza, Victor Gonzalez-Vera, Jessica Gordon-Nunez, Sol Llerena-Llerena, Dario X. Ramirez-Villacis, Pieter van ‘t Hof, and Antonio Leon-Reyes. 2021. "Sulfur Deprivation Modulates Salicylic Acid Responses via Nonexpressor of Pathogenesis-Related Gene 1 in Arabidopsis thaliana" Plants 10, no. 6: 1065. https://doi.org/10.3390/plants10061065
APA StyleCriollo-Arteaga, S., Moya-Jimenez, S., Jimenez-Meza, M., Gonzalez-Vera, V., Gordon-Nunez, J., Llerena-Llerena, S., Ramirez-Villacis, D. X., van ‘t Hof, P., & Leon-Reyes, A. (2021). Sulfur Deprivation Modulates Salicylic Acid Responses via Nonexpressor of Pathogenesis-Related Gene 1 in Arabidopsis thaliana. Plants, 10(6), 1065. https://doi.org/10.3390/plants10061065







