The Impact of Heat Stress on Morpho-Physiological Response and Expression of Specific Genes in the Heat Stress-Responsive Transcriptional Regulatory Network in Brassica oleracea
Abstract
1. Introduction
2. Results: Comparison of Different Brassica oleracea Cultivars on Heat Stress Tolerance Using Morphological and Physiological Measurements
2.1. The Morphological Differences between Heat-Sensitive Cabbage and Heat-Tolerant Cabbage Cultivars at High Temperature
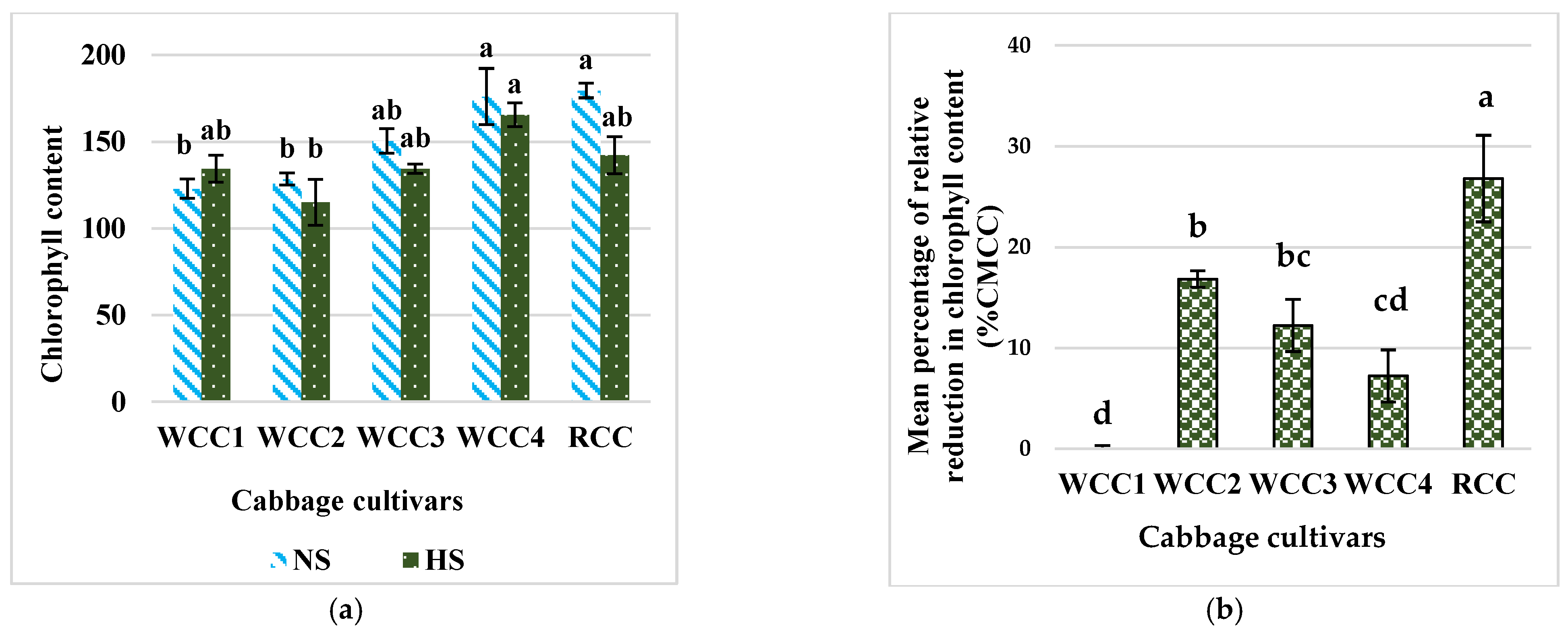
2.2. Effect of Heat Stress on Chlorophyll Content in Brassica oleracea Cultivars
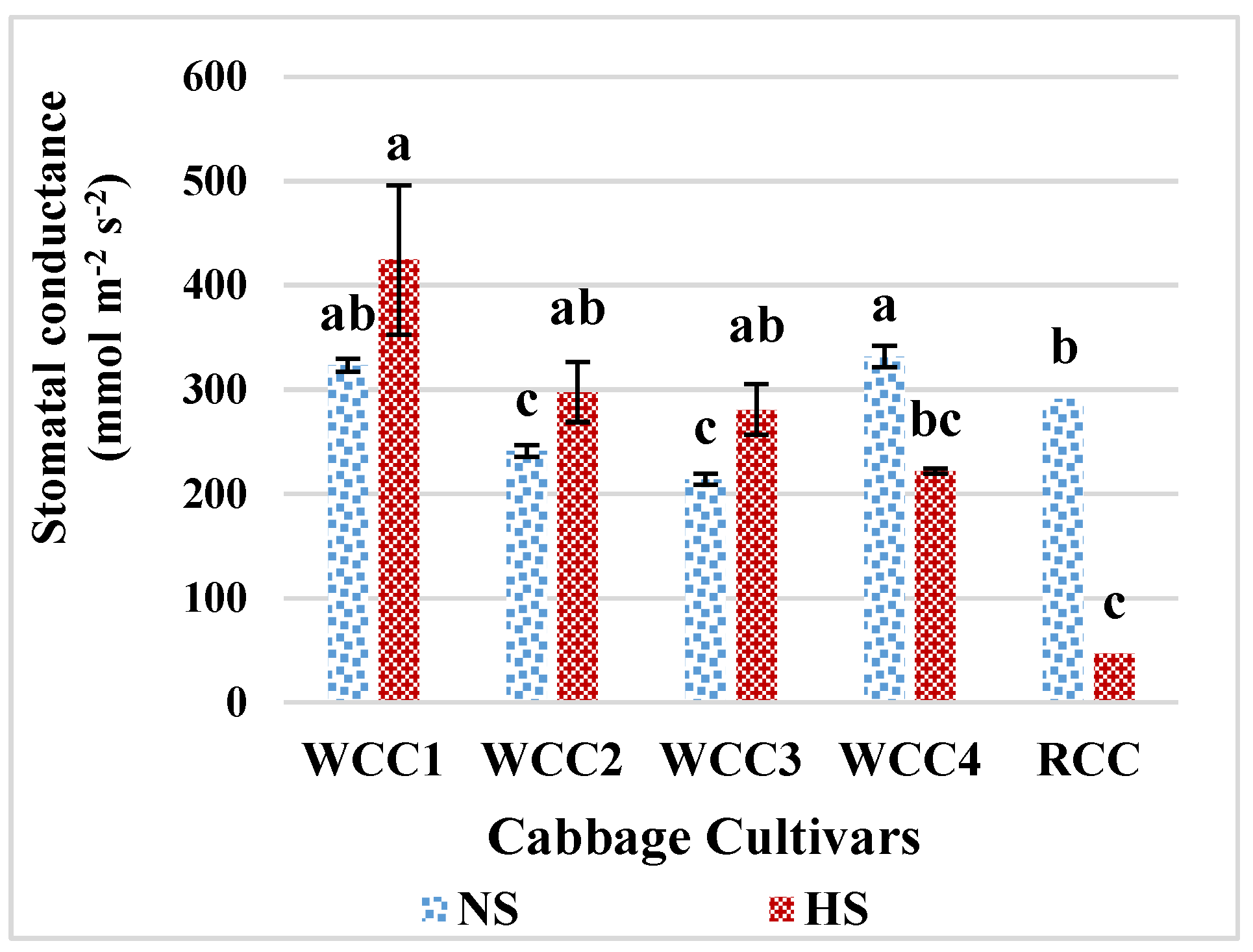
2.3. Effect of Heat Stress on Stomatal Conductance in Brassica oleracea Cultivars
2.4. Effect of Heat Stress on Chlorophyll fluorescence in Brassica oleracea Cultivars
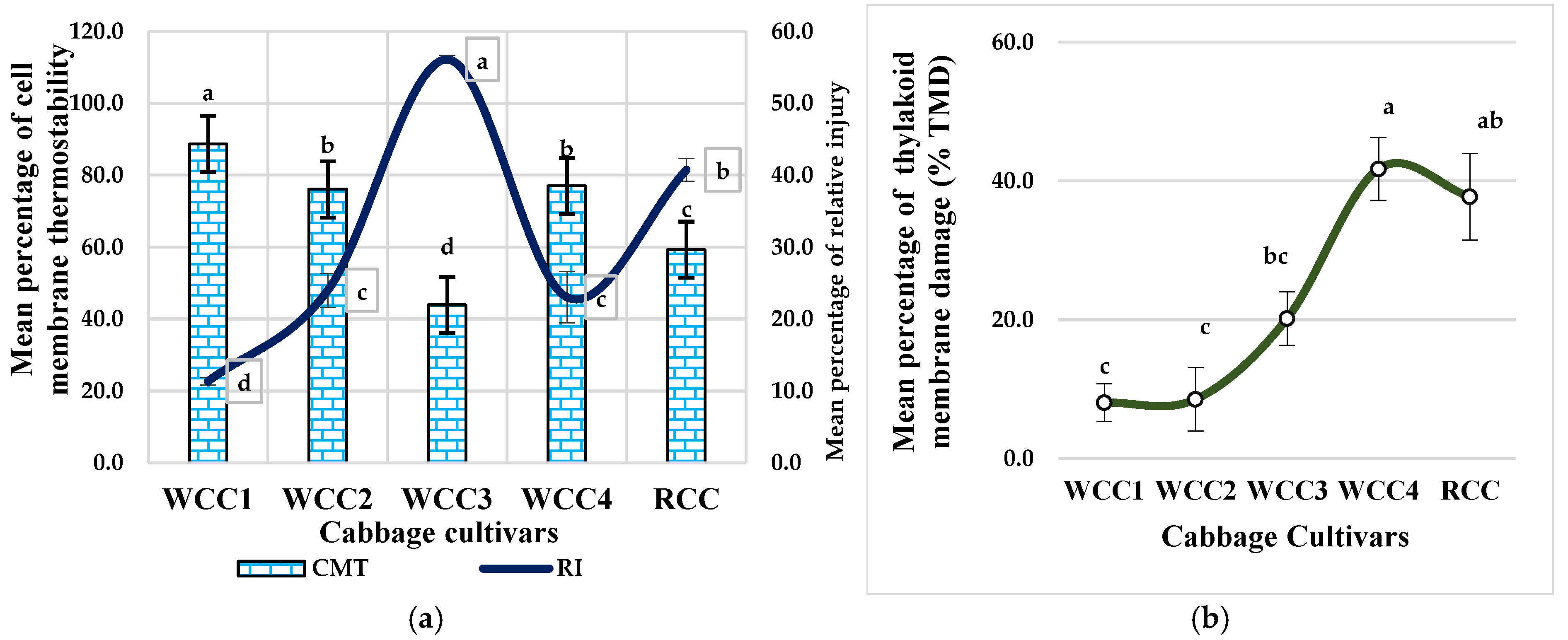
2.5. Effect of Heat Stress on Cell Membrane Thermostability (CMT) and Relative Thylakoid Damage in Brassica oleracea Cultivars
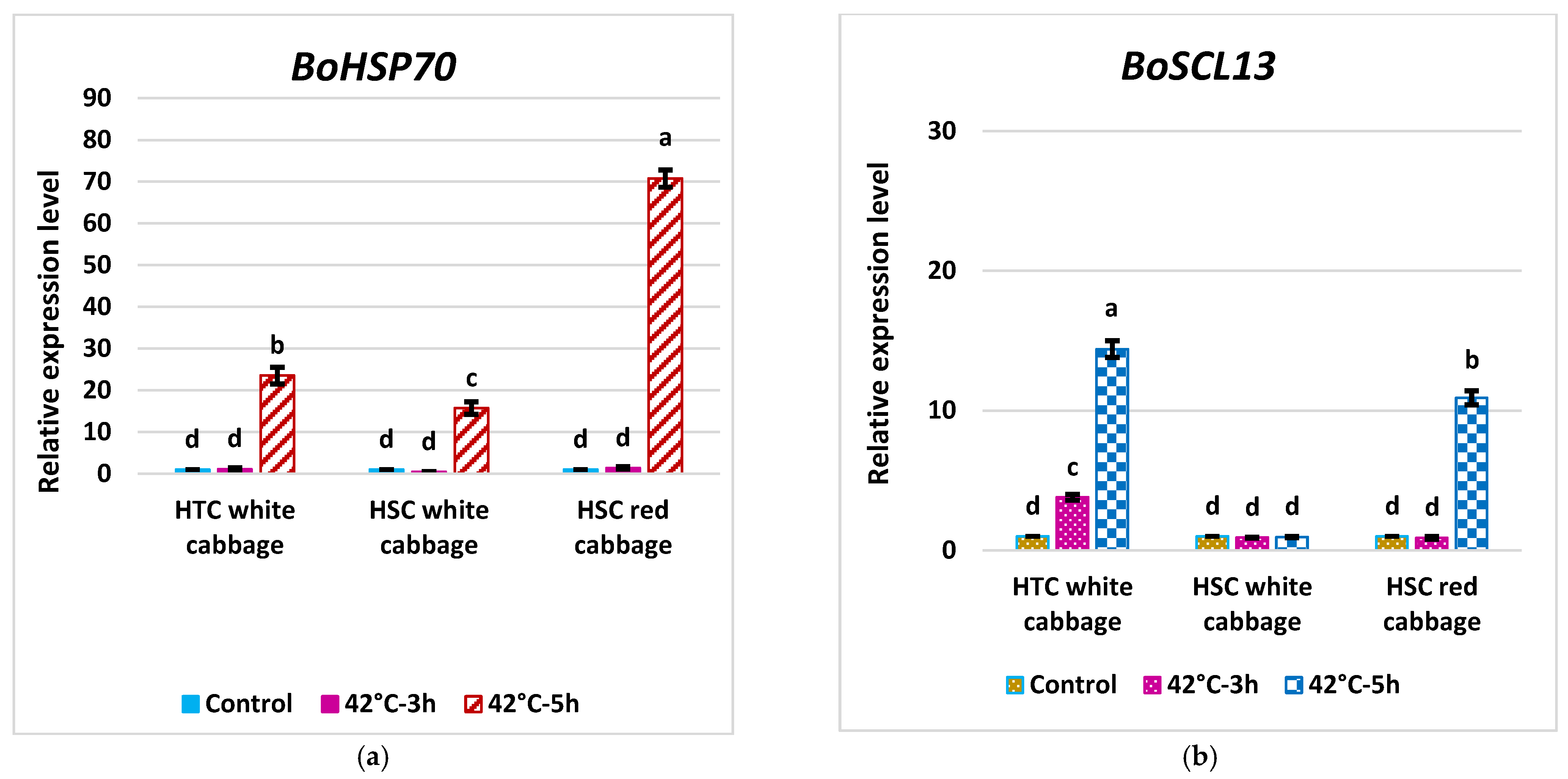
2.6. Expression Pattern of BoHSP70 and BoSCL13 as Specific Marker Genes for the Heat Tolerance Trait in Cabbage
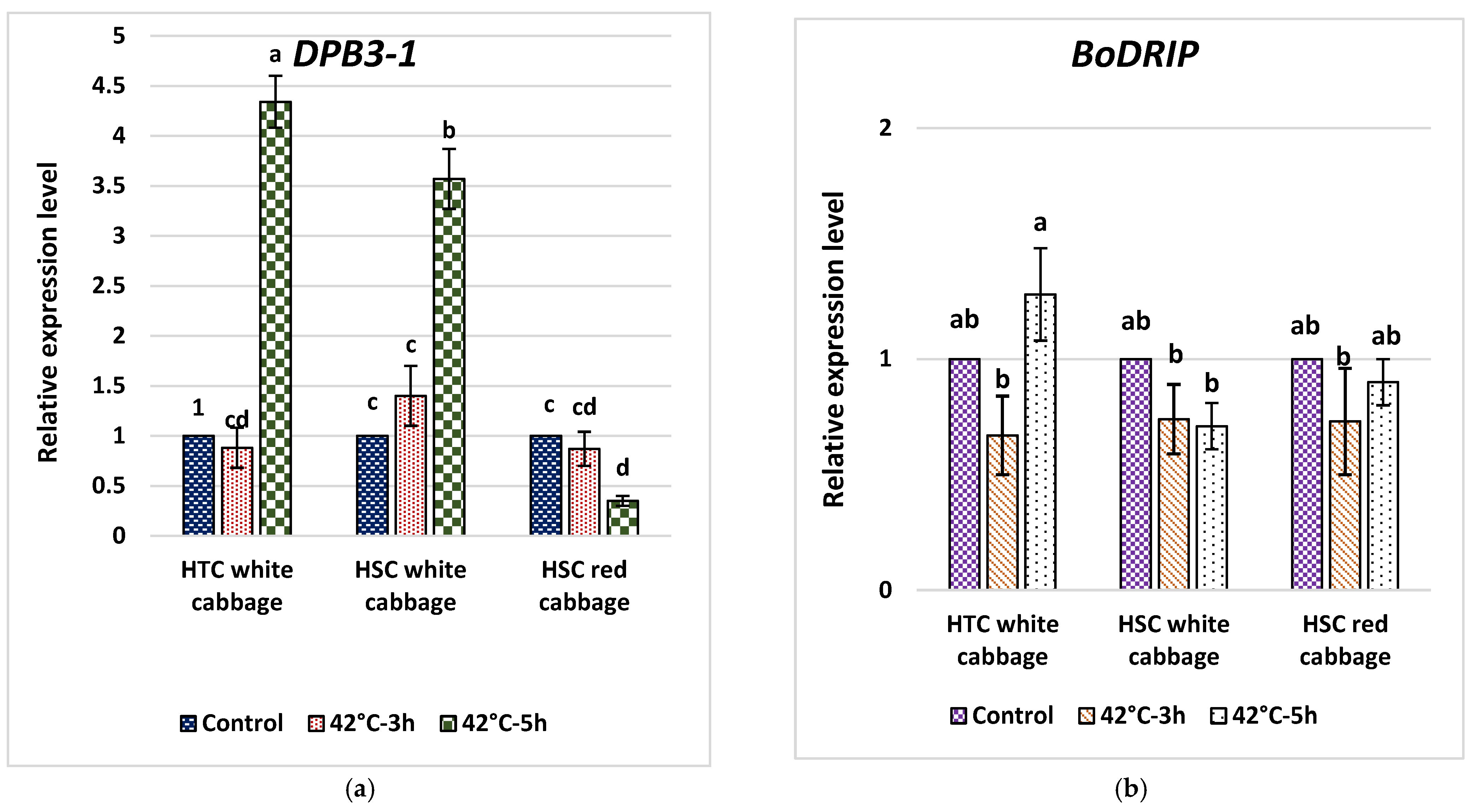
2.7. Analysis of Fold Change in Expression of DPB3-1 and DRIP1 in Heat-Tolerant and Heat-Sensitive Cabbage Cultivars
3. Discussion
3.1. High Temperatures Decrease the Content of Chlorophyll in Brassica oleracea Cultivars
3.2. High Temperatures Damage the Thylakoid Membrane of the Ultrastructure of Chloroplasts in Brassica oleracea Cultivars
3.3. High Temperatures Damage the PSII and REDUCE Fv/Fm in Brassica oleracea Cultivars
3.4. High Temperature and Its Impact on Stomatal Conductance, Photorespiration and Photosynthesis in Brassica oleracea Cultivars
3.5. GeneMANIA Helps to Predict the Function of Genes and Gene Sets
3.6. High Temperature and the Phenotypic Differences between HTCs and HSCs
3.7. Analysis of Fold Change in the Expression of Key Genes in the HS Response Network and Evaluating Potential Molecular Indicators to Differentiate HTC from HSC in Brassica oleracea
3.8. The Expression of BoDRIP as a Negative Regulator Domain of DREB2A Contrary to BoDPB3-1 as a Positive Interactor of DREB2A
3.9. Opportunities for Crop Improvement for Heat Stress Tolerance
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Heat Treatments
4.3. Chlorophyll Measurements
4.4. Stomatal Conductance Measurements
4.5. Chlorophyll Fluorescence (CF) Test
4.6. Cell Membrane Thermostability (CMT) Test
4.7. Determination of the Morphological Characteristics of Heat-Stressed Brassica oleracea
4.8. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.9. Statistical Analysis
4.10. Drawing Co-Expressed Gene Networks for Query Genes in This Study through GeneMANIA Tools
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Name | Primer 5’--3’ | Reference |
|---|---|---|
| UBCF | TTATGAAGGCGGAGTGTTT | [54] |
| UBCR | TGAACCCTCTCGCATCTC | |
| UNK1F | TTACACACCACAAAGAGAGT | [54] |
| UNK1R | TACAACACCTGAAACCCATG | |
| ACT F | GAATCCACGAGACAACATAT | [54] |
| ACT R | AGGGAAGCAAGAATGGAAC | |
| Hsp70 F | GGGAAGGCTGTCCAAGGAAGAG | [2] |
| Hsp70 R | CCATGCCACCAGCTCCACCCATATC | |
| SCL13 F | CCGAAACTCGTGACGCTAGTGGAGC | [2] |
| SCL13 R | GCGGAACATGTAGCCATGGGTCTCC | |
| DPB3 F | CGACGAGAGATACGAGTTCC | Designing specific primers |
| DPB3 R | GCTTCTTTCCCATTCCTCCA | |
| DRIP F | TGAAACACGAGAGGAGAAAGG | Designing specific primers |
| DRIP R | TGGAATCTGAGGCAAGGATG |
| Classification | Source of Seeds | Characteristics |
|---|---|---|
| White Cabbage | KAGAYAKI | Mikado Kyowa seed Round shaped head with bright green color. Extra early maturity of 43–48 days, 1.0–1.3 kg in weight. Compact plant with semi-open, compact outer leaves suitable for density planting. Tolerant to Cabbage Yellows (B type) and Black Rot. |
| P S Hybrid Cabbage Petoseed | Early maturity tropical type with excellent yield, uniformity, disease resistance, shippability. Resistance: yellows, Xanthomonas campestris. | |
| Green World Cabbage | High weather conditions. One round shape and average weight of 1.5 kg | |
| Hybrid Cabbage giant Takii & co., LTD Kyoto, Japan | Hybrid, midseason, Bravo class, denser heads, bright green. Resistance: yellows; high tolerance to black rot, tolerance to thrips, tipburn, heat, cold. | |
| Red Cabbage | Red Globe Mikado Kyowa Seed | Hybrid, round shaped head with deep purplish red color. Medium maturity of 75 days, 1.5–2.0 kg in weight. Uniform, compact, short cored head with thick and tender leaves. Good quality and taste. Slow bursting. |
References
- Cardi, T.; D’Agostino, N.; Tripodi, P. Genetic transformation and genomic resources for next-generation precise genome engineering in vegetable crops. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Park, H.J.; Jung, W.Y.; Lee, S.S.; Song, J.H.; Kwon, S.-Y.; Kim, H.; Kim, C.; Ahn, J.C.; Cho, H.S. Use of Heat Stress Responsive Gene Expression Levels for Early Selection of Heat Tolerant Cabbage (Brassica oleracea L.). Int. J. Mol. Sci. 2013, 14, 11871–11894. [Google Scholar] [CrossRef]
- Ravanfar, S.A.; Orbovic, V.; Moradpour, M.; Abdul Aziz, M.; Karan, R.; Wallace, S.; Parajuli, S. Improvement of tissue culture, genetic transformation, and applications of biotechnology to Brassica. Biotechnol. Genet. Eng. Rev. 2017, 33, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. Genetic Engineering for Modern Agriculture: Challenges and Perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Ruelland, E.; Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Nurniwalis, A.W.; Suhaimi, N.; Siti Nor Akmar, A.; Aminah, S.; Mohamad Arif, M.A. Gene discovery via expressed sequence tags from the oil palm (elaeis guineensis Jacq.) mesocarp. J. Oil Palm Res. 2008, 87–96. [Google Scholar]
- Lin, K.H.; Huang, H.C.; Lin, C.Y. Cloning, expression and physiological analysis of broccoli catalase gene and Chinese cabbage ascorbate peroxidase gene under heat stress. Plant Cell Rep. 2010, 29, 575–593. [Google Scholar] [CrossRef]
- Hirsch, S.; Oldroyd, G.E.D. GRAS-domain transcription factors that regulate plant development. Plant Signal. Behav. 2009, 4, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Bahari, M.N.A.; Sakeh, N.M.; Abdullah, S.N.A.; Ramli, R.R.; Kadkhodaei, S. Transciptome profiling at early infection of Elaeis guineensis by Ganoderma boninense provides novel insights on fungal transition from biotrophic to necrotrophic phase. BMC Plant Biol. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Tanaka, H.; Maruyama, K.; Qin, F.; Osakabe, Y.; Morimoto, K.; Ohori, T.; Kusakabe, K.; Nagata, M.; et al. Arabidopsis DPB3-1, A DREB2A Interactor, Specifically Enhances Heat Stress-Induced Gene Expression by Forming a Heat Stress-Specific Transcriptional Complex with NF-Y Subunitsc w. Plant Cell 2014, 26, 4954–4973. [Google Scholar] [CrossRef]
- Sato, H.; Suzuki, T.; Takahashi, F.; Shinozaki, K.; Yamaguchi-Shinozaki, K. NF-YB2 and NF-YB3 have functionally diverged and differentially induce drought and heat stress-specific genes. Plant Physiol. 2019, 180, 1677–1690. [Google Scholar] [CrossRef]
- Sato, H.; Todaka, D.; Kudo, M.; Mizoi, J.; Kidokoro, S.; Zhao, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. The Arabidopsis transcriptional regulator DPB3-1 enhances heat stress tolerance without growth retardation in rice. Plant Biotechnol. J. 2016, 14, 1756–1767. [Google Scholar] [CrossRef]
- Qin, F.; Sakuma, Y.; Tran, L.S.P.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Fujita, M.; Umezawa, T.; Sawano, Y.; Miyazono, K.I.; et al. Arabidopsis DREB2A-interacting proteins function as Ring E3 ligases and negatively regulate plant drought stress-responsive gene expression. Plant Cell 2008, 20, 1693–1707. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Abdullah, S.N.A.; Abdul Aziz, M.; Namasivayam, P. Oil palm EgCBF3 conferred stress tolerance in transgenic tomato plants through modulation of the ethylene signaling pathway. J. Plant Physiol. 2016, 202, 107–120. [Google Scholar] [CrossRef]
- Yuan, Y.; Fang, L.; Karungo, S.K.; Zhang, L.; Gao, Y.; Li, S.; Xin, H. Overexpression of VaPAT1, a GRAS transcription factor from Vitis amurensis, confers abiotic stress tolerance in Arabidopsis. Plant Cell Rep. 2016, 35, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Torres-Galea, P.; Huang, L.F.; Chua, N.H.; Bolle, C. The GRAS protein SCL13 is a positive regulator of phytochrome-dependent red light signaling, but can also modulate phytochrome A responses. Mol. Genet. Genom. 2006, 276, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Ingwers, M.W.; McGuire, M.A.; Teskey, R.O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J. Exp. Bot. 2017, 68, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Nyarko, G.; Alderson, P.G.; Craigon, J.; Murchie, E.; Sparkes, D.L. Comparison of cell membrane thermostability and chlorophyll fluorescence parameters for the determination of heat tolerance in ten cabbage lines. J. Horticult. Sci. Biotechnol. 2008, 83, 678–682. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J.; Salvucci, M.E. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 2002, 129, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Haworth, M.; Marino, G.; Brunetti, C.; Killi, D.; De Carlo, A.; Centritto, M. The impact of heat stress and water deficit on the photosynthetic and stomatal physiology of olive (Olea europaea L.)—A case study of the 2017 heat wave. Plants 2018, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Azzeme, A.M.; Abdullah, S.N.A.; Aziz, M.A.; Wahab, P.E.M. Oil palm leaves and roots differ in physiological response, antioxidant enzyme activities and expression of stress-responsive genes upon exposure to drought stress. Acta Physiol. Plant. 2016, 38, 1–12. [Google Scholar] [CrossRef]
- Kuo, C.G.; Shen, B.J.; Chen, H.M.; Chen, H.C.; Opeña, R.T. Associations between heat tolerance, water consumption, and morphological characters in Chinese cabbage. Euphytica 1988, 39, 65–73. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and Responses of Chloroplasts to Heat Stress in Plants. Front. Plant Sci. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Marchand, F.L.; Mertens, S.; Kockelbergh, F.; Beyens, L.; Nijs, I. Performance of High Arctic tundra plants improved during but deteriorated after exposure to a simulated extreme temperature event. Glob. Chang. Biol. 2005, 11, 2078–2089. [Google Scholar] [CrossRef]
- Wang, J.Z.; Cui, L.J.; Wang, Y.; Li, J.L. Growth, lipid peroxidation and photosynthesis in two tall fescue cultivars differing in heat tolerance. Biol. Plant. 2009, 53, 237–242. [Google Scholar] [CrossRef]
- Ashraf, M.; Hafeez, M. Thermotolerance of pearl millet and maize at early growth stages: Growth and nutrient relations. Biol. Plant. 2004, 48, 81–86. [Google Scholar] [CrossRef]
- Rodríguez, M.; Canales, E.; Borrás-Hidalgo, O. Molecular aspects of abiotic stress in plants. Biotecnol. Aplic. 2005, 22, 1–10. [Google Scholar]
- Morales, D.; Rodríguez, P.; Dell’Amico, J.; Nicolás, E.; Torrecillas, A.; Sánchez-Blanco, M.J. High-temperature preconditioning and thermal shock imposition affects water relations, gas exchange and root hydraulic conductivity in tomato. Biol. Plant. 2003, 47, 203–208. [Google Scholar] [CrossRef]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [PubMed]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; Van Der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef]
- Tan, W.; Meng, Q.W.; Brestic, M.; Olsovska, K.; Yang, X. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol. 2011, 168, 2063–2071. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Craufurd, P.Q.; Prasad, P.V.V.; Kakani, V.G.; Wheeler, T.R.; Nigam, S.N. Heat tolerance in groundnut. Field Crop. Res. 2003, 80, 63–77. [Google Scholar] [CrossRef]
- Agarie, S.; Hanaoka, N.; Kubota, F.; Agata, W.; Kaufman, P. Measurement of Cell Membrane Stability Evaluated by Electrolyte Leakage as a Drought and Heat Tolerance Test in Rice (Oryza sativa L.). J. Fac. Agric. Kyushu Univ. 1995, 40, 233–240. [Google Scholar] [CrossRef]
- Usman, M.G.; Rafii, M.Y.; Ismail, M.R.; Malek, M.A.; Latif, M.A. Expression of Target Gene HSP70 and Membrane Stability Determine Heat Tolerance in chili pepper. J. Am. Soc. Horticult. Sci. 2015, 140, 144–150. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucl. Acids Res. 2010, 38, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Sabaghian, E.; Drebert, Z.; Inze, D.; Saeys, Y. An integrated network of Arabidopsis growth regulators and its use for gene prioritization. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef][Green Version]
- Yuan, Y.; Chiu, L.W.; Li, L. Transcriptional regulation of anthocyanin biosynthesis in red cabbage. Planta 2009, 230, 1141–1153. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, Y.; Vainstein, A.; Chen, S.; Ma, H. Regulation of fig (Ficus carica L.) fruit color: Metabolomic and transcriptomic analyses of the flavonoid biosynthetic pathway. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Mizoi, J.; Yoshida, T.; Fujita, Y.; Nakajima, J.; Ohori, T.; Todaka, D.; Nakashima, K.; Hirayama, T.; Shinozaki, K.; et al. An ABRE promoter sequence is involved in osmotic stress-responsive expression of the DREB2A gene, which encodes a transcription factor regulating drought-inducible genes in Arabidopsis. Plant Cell Physiol. 2011, 52, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Maruyama, K.; Qin, F.; Osakabe, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18822–18827. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.A.; Jin, S.B.; Byoung, C.K.; Hyo, G.P. Production of somatic hybrids between satsuma mandarin (Citrus unshiu) and navel orange (Citrus sinensis) by protoplast fusion. J. Plant Biol. 2008, 51, 186–191. [Google Scholar] [CrossRef]
- Moradpour, M.; Abdulah, S.N.A. CRISPR/dCas9 platforms in plants: Strategies and applications beyond genome editing. Plant Biotechnol. J. 2020, 18, 32–44. [Google Scholar] [CrossRef]
- Arsenault, E.A.; Yoneda, Y.; Fleming, G.R.; Niyogi, K.K. The role of mixed vibronic Qy-Qx states in green light absorption of light-harvesting complex II. Nat. Commun. 2020, 1–9. [Google Scholar] [CrossRef]
- Molina-Bravo, R.; Arellano, C.; Sosinski, B.R.; Fernandez, G.E. A protocol to assess heat tolerance in a segregating population of raspberry using chlorophyll fluorescence. Sci. Horticult. 2011, 130, 524–530. [Google Scholar] [CrossRef]
- Kadir, S.; Von Weihe, M.; Al-Khatib, K. Photochemical efficiency and recovery of photosystem II in grapes after exposure to sudden and gradual heat stress. J. Am. Soc. Horticult. Sci. 2007, 132, 764–769. [Google Scholar] [CrossRef]
- Thomason, K.; Babar, M.A.; Erickson, J.E.; Mulvaney, M.; Beecher, C.; MacDonald, G. Comparative physiological and metabolomics analysis of wheat (Triticum aestivum L.) following post-anthesis heat stress. PLoS ONE 2018, 13, e0197919. [Google Scholar] [CrossRef] [PubMed]
- MacRae, E. Extraction of plant RNA. Methods Mol. Biol. 2007, 353, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.G.; Zhao, Z.Q.; Yu, H.F.; Wang, J.S.; Zheng, C.F.; Gu, H.H. In-depth analysis of internal control genes for quantitative real-time PCR in Brassica oleracea var. botrytis. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]



| Paramaters | Heat Tolerance Indicators | ||
|---|---|---|---|
| Heat Tolerance | Moderate Tolerance | Sensitive | |
| Reduction of chlorophyll content | X < 10 | X = 10.0–20.0 | X > 20.0 |
| Stomatal conductance | X > 300 | X = 200–300 | X < 300 |
| Fv/Fm | X > 0.75 | X = 0.6–0.75 | X < 0.6 |
| Cell membrane thermostability | X > 60% | X = 30–60% | X < 30% |
| Head formation | Yes | Yes | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradpour, M.; Abdullah, S.N.A.; Namasivayam, P. The Impact of Heat Stress on Morpho-Physiological Response and Expression of Specific Genes in the Heat Stress-Responsive Transcriptional Regulatory Network in Brassica oleracea. Plants 2021, 10, 1064. https://doi.org/10.3390/plants10061064
Moradpour M, Abdullah SNA, Namasivayam P. The Impact of Heat Stress on Morpho-Physiological Response and Expression of Specific Genes in the Heat Stress-Responsive Transcriptional Regulatory Network in Brassica oleracea. Plants. 2021; 10(6):1064. https://doi.org/10.3390/plants10061064
Chicago/Turabian StyleMoradpour, Mahdi, Siti Nor Akmar Abdullah, and Parameswari Namasivayam. 2021. "The Impact of Heat Stress on Morpho-Physiological Response and Expression of Specific Genes in the Heat Stress-Responsive Transcriptional Regulatory Network in Brassica oleracea" Plants 10, no. 6: 1064. https://doi.org/10.3390/plants10061064
APA StyleMoradpour, M., Abdullah, S. N. A., & Namasivayam, P. (2021). The Impact of Heat Stress on Morpho-Physiological Response and Expression of Specific Genes in the Heat Stress-Responsive Transcriptional Regulatory Network in Brassica oleracea. Plants, 10(6), 1064. https://doi.org/10.3390/plants10061064






