In Vitro Root Induction from Argan (Argania spinosa (L.) Skeels) Adventitious Shoots: Influence of Ammonium Nitrate, Auxins, Silver Nitrate and Putrescine, and Evaluation of Plantlet Acclimatization
Abstract
1. Introduction
2. Results
2.1. Effects of Putrescine, Silver Nitrate (AgNO3), Ammonium Nitrate (NH4NO3), Indole-3-Butyric Acid (IBA) and 1-Naphthalene Acetic Acid (NAA) on In Vitro Rooting of Regenerated Shoots
2.1.1. Effects of NH4NO3 on In Vitro Rooting
2.1.2. Effects of IBA, AgNO3 and Putrescine on In Vitro Rooting
2.1.3. Evaluation of the Combined Effects of Putrescine, AgNO3, IBA and NAA on In Vitro Rooting
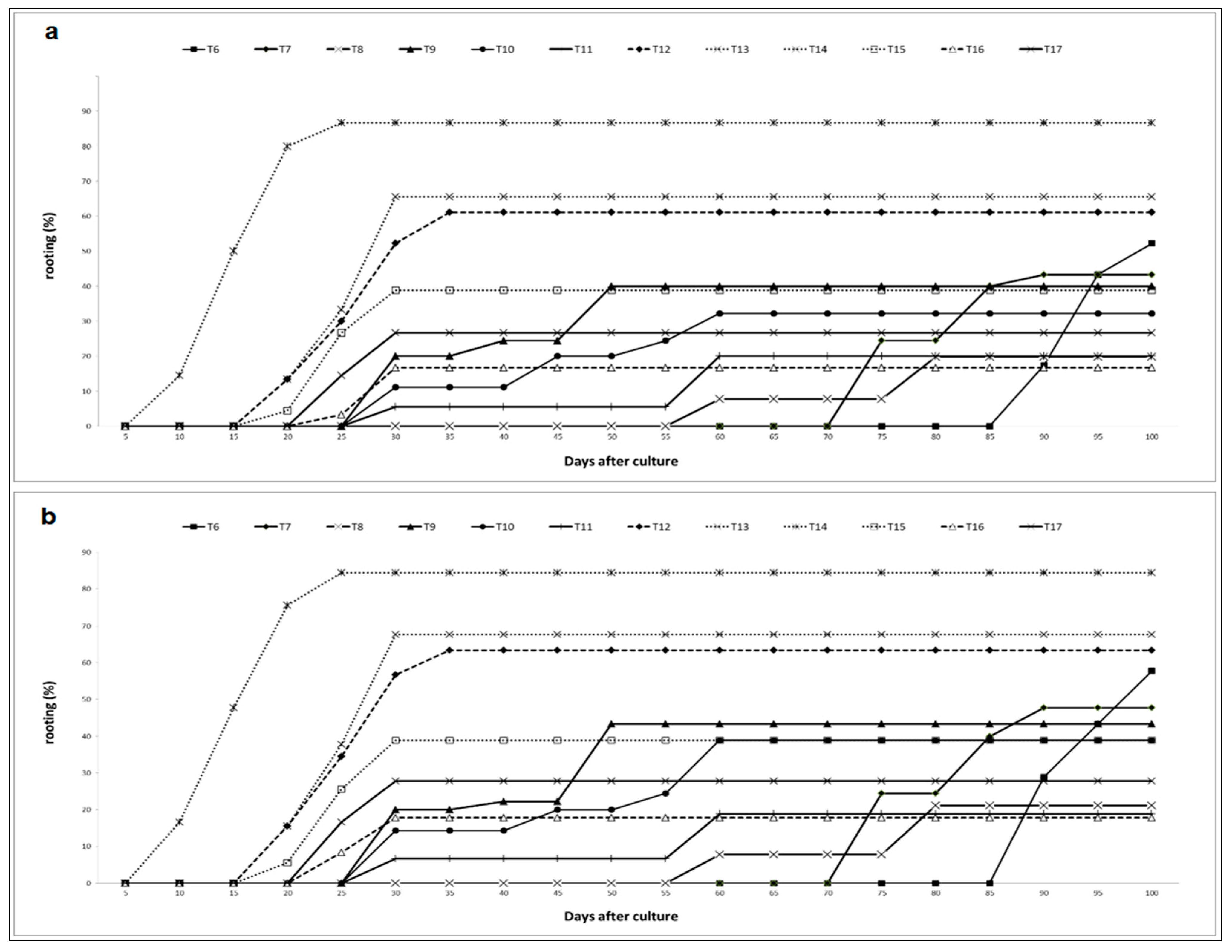
2.1.4. Effects of Putrescine, AgNO3, IBA and NAA on Rooting Kinetics
2.2. Effect of Different Substrate Mixtures on Ex Vitro Acclimatization
3. Discussion
4. Materials and Methods
4.1. Culture Conditions
4.2. Plant Material and Experiments
4.2.1. Origin of Plant Material
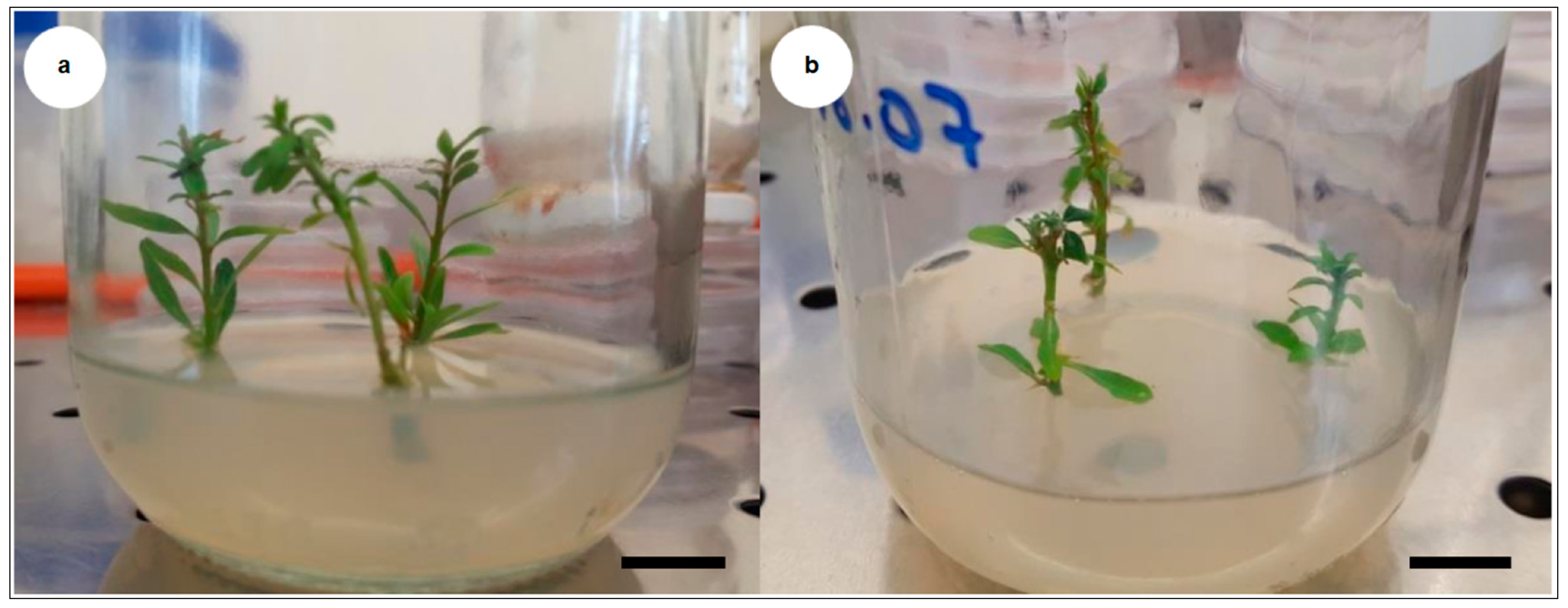
4.2.2. In Vitro Rhizogenesis
4.2.3. Ex Vitro Acclimatization
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zahidi, A.; Bani-Aameur, F.; El Mousadik, A. Morphological variability of the fruiting branches in Argania spinosa: Effects of seasonal variations, locality and genotype. J. Hortic. Forest 2013, 5, 168–182. [Google Scholar] [CrossRef]
- Koufan, M.; Belkoura, I.; Mazri, M.A.; Amarraque, A.; Essatte, A.; Elhorri, H.; Zaddoug, F.; Alaoui, T. Determination of antioxidant activity, total phenolics and fatty acids in essential oils and other extracts from callus culture, seeds and leaves of Argania spinosa (L.) Skeels. Plant Cell Tissue Organ Cult. 2020, 141, 217–227. [Google Scholar] [CrossRef]
- Charrouf, Z.; Harhar, H.; Gharby, S.; Guillaume, D. Enhancing the value of argan oil is the best mean to sustain the argan grove economy and biodiversity. Oleag. Corps Gras Lipides 2008, 15, 269–271. [Google Scholar] [CrossRef]
- El Kharrassi, Y.; Maata, N.; Mazri, M.A.; El Kamouni, S.; Talbi, M.; El Kebbaj, R.; Moustaid, K.; Essamadi, A.K.; Andreoletti, P.; El Mzouri, E.H.; et al. Chemical and phytochemical characterizations of argan oil (Argania spinosa L. skeels), olive oil (Olea europaea L. cv. Moroccan picholine), cactus pear (Opuntia megacantha salm-dyck) seed oil and cactus cladode essential oil. J. Food Meas. Charact. 2018, 12, 747–754. [Google Scholar] [CrossRef]
- De Waroux, Y.L.P.; Lambin, E.F. Niche commodities and rural poverty alleviation: Contextualizing the contribution of argan oil to rural livelihoods in Morocco. Ann. Assoc. Am. Geogr. 2013, 103, 589–607. [Google Scholar] [CrossRef]
- Lybbert, T.J.; Aboudrare, A.; Chaloud, D.; Magnan, N.; Nash, M. Booming markets for Moroccan argan oil appear to benefit some rural households while threatening the endemic argan forest. Proc. Natl. Acad. Sci. USA 2011, 108, 13963–13968. [Google Scholar] [CrossRef] [PubMed]
- Moukrim, S.; Lahssini, S.; Rhazi, M.; Alaoui, H.M.; Benabou, A.; Wahby, I.; El Madihi, M.; Arahou, M.; Rhazi, L. Climate change impacts on potential distribution of multipurpose agro-forestry species: Argania spinosa (L.) Skeels as case study. Agrofor. Syst. 2019, 93, 1209–1219. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Argan oil, the 35-years-of-research product. Eur. J. Lipid Sci. Technol. 2014, 116, 1316–1321. [Google Scholar] [CrossRef]
- Ait Aabd, N.; Bouharroud, R.; Tahiri, A.; Wifaya, A.; Mimouni, A.; El Mousadik, A. Genetic Diversity and Breeding of Argan Tree (Argania spinosa L. Skeels). In Advances in Plant Breeding Strategies: Nut and Beverage Crops; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Cham, Switzerland, 2019; pp. 31–56. [Google Scholar] [CrossRef]
- El Bahloul, Y.; Dauchot, N.; Machtoun, I.; Gaboun, F.; Van Cutsem, P. Development and characterization of microsatellite loci for the Moroccan endemic endangered species Argania spinosa (Sapotaceae). Appl. Plant Sci. 2014, 2, 1300071. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Sustainable development in Northern Africa: The argan forest case. Sustainability 2009, 1, 1012–1022. [Google Scholar] [CrossRef]
- Nouaim, R.; Mangin, G.; Breuil, M.C.; Chaussod, R. The argan tree (Argania spinosa) in Morocco propagation by seeds, cuttings and in-vitro techniques. Agrofor. Syst. 2002, 54, 71–81. [Google Scholar] [CrossRef]
- Koufan, M.; Mazri, M.A.; Essatte, A.; Moussafir, S.; Belkoura, I.; El Rhaffari, L.; Toufik, I. A novel regeneration system through micrografting for Argania spinosa (L.) Skeels, and confirmation of successful rootstock-scion union by histological analysis. Plant Cell Tissue Organ Cult. 2020, 142, 369–378. [Google Scholar] [CrossRef]
- Bellefontaine, R.; Ferradous, A.; Alifriqui, M.; Monteuuis, O. Vegetative propagation of argan tree, Argania spinosa in Morocco: The John Goelet project. Bois. Des. Trop. 2010, 304, 47–59. [Google Scholar] [CrossRef]
- Mazri, M.A.; Meziani, R.; El Fadile, J.; Ezzinbi, A. Optimization of medium composition for in vitro shoot proliferation and growth of date palm cv. Mejhoul. 3 Biotech 2016, 6, 111. [Google Scholar] [CrossRef]
- Mazri, M.A.; Meziani, R.; Belkoura, I.; Mokhless, B.; Nour, S. A combined pathway of organogenesis and somatic embryogenesis for an efficient large-scale propagation in date palm (Phoenix dactylifera L.) cv. Mejhoul. 3 Biotech 2018, 8, 215. [Google Scholar] [CrossRef]
- Koufan, M.; Belkoura, I.; Alaoui, T. The multiplication of the argane tree by microcutting (Argania spinosa L. Skeels). Eur. J. Biotechnol. Biosci. 2018, 6, 47–52. [Google Scholar]
- Justamante, M.S.; Ibáñez, S.; Villanova, J.; Pérez-Pérez, J.M. Vegetative propagation of argan tree (Argania spinosa (L.) Skeels) using in vitro germinated seeds and stem cuttings. Sci. Hortic. 2017, 225, 81–87. [Google Scholar] [CrossRef]
- Lamaoui, M.; Chakhchar, A.; El Kharrassi, Y.; Wahbi, S.; Ferradous, A.; El Mousadik, A.; Ibnsouda-Koraichi, S.; Filali-Maltouf, A.; El Modafar, C. Selection and multiplication of argan (Argania spinosa L.) superior clones for conservation purposes. Acta Sci. Agric. 2019, 3, 116–123. [Google Scholar]
- Bradaï, F.; Almagro-Bastante, J.; Sánchez-Romero, C. Cryopreservation of olive somatic embryos using the droplet-vitrifcation method: The importance of explant culture conditions. Sci. Hortic. 2017, 218, 14–22. [Google Scholar] [CrossRef]
- Mazri, M.A.; Elbakkali, A.; Belkoura, M.; Belkoura, I. Embryogenic competence of calli and embryos regeneration from various explants of Dahbia cv, a Moroccan olive tree (Olea europaea L.). Afr. J. Biotechnol. 2011, 10, 19089–19095. [Google Scholar]
- Mazri, M.A.; Belkoura, I.; Pliego-Alfaro, F.; Belkoura, M. Embryogenic capacity of embryo-derived explants from different olive cultivars. Acta Hortic. 2012, 929, 397–403. [Google Scholar] [CrossRef]
- Pérez-Barranco, G.; Torreblanca, R.; Padilla, I.M.G.; Sánchez-Romero, C.; Pliego-Alfaro, F.; Mercado, J.A. Studies on genetic transformation of olive (Olea europaea L.) somatic embryos: I. Evaluation of different aminoglycoside antibiotics for nptII selection. II. Transient transformation via particle bombardment. Plant Cell Tissue Organ Cult. 2009, 97, 243–251. [Google Scholar] [CrossRef]
- Amghar, I.; Diria, G.; Boumlik, I.; Gaboun, F.; Iraqi, D.; Labhilili, M.; Mentag, R.; Meziani, R.; Mazri, M.A.; Ibriz, M.; et al. An efficient regeneration pathway through adventitious organogenesis for the endangered Argania spinosa (L.) Skeels. Vegetos 2021. [Google Scholar] [CrossRef]
- González, A.; Casares, A.; Sánchez, T.R.; Rodríguez, R. Adventitious root induction in Corylus avellana L. cotyledon. In Vitro Cell. Dev. Biol. Plant 1991, 27, 125–131. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Wang, Z.; Zhang, Y.; Sun, H.; Song, S.; Bai, Z.; Lu, Z.; Li, C. Nitrogen fertilization increases root growth and coordinates the root-shoot relationship in cotton. Front. Plant Sci. 2020, 11, 880. [Google Scholar] [CrossRef]
- Kolbert, Z. Implication of nitric oxide (NO) in excess element-induced morphogenic responses of the root system. Plant Physiol. Biochem. 2016, 101, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Mazri, M.A.; Meziani, R.; Elmaataoui, S.; Alfeddy, M.N.; Jait, F. Assessment of genetic fidelity, biochemical and physiological characteristics of in vitro grown date palm cv. Al-Fayda. Vegetos 2019, 32, 333–344. [Google Scholar] [CrossRef]
- Mazri, M.A.; Naciri, R.; Belkoura, I. Maturation and conversion of somatic embryos derived from seeds of olive (Olea europaea L.) cv. Dahbia: Occurrence of secondary embryogenesis and adventitious bud formation. Plants 2020, 9, 1489. [Google Scholar] [CrossRef]
- Rugini, E.; Luppino, M.; de Agazio, M. Endogenous polyamine and root morphogenesis variations under different treatments in cuttings and in in vitro explants of olive. Acta Hortic. 1992, 300, 225–232. [Google Scholar] [CrossRef]
- Lone, A.B.; Barbosa, C.M.; Takahashi, L.S.A.; Faria, R.T. Acclimatization of the Cattleya (Orchidaceae) in alternative substrates to tree fern fiber and sphagno. Acta Sci. Agron. 2008, 30, 465–469. [Google Scholar] [CrossRef][Green Version]
- Stefanello, S.; Silveira, E.V.; Oliveira, L.K.; Besson, J.C.F.; Dutra, G.M.N. Efficiency of substrates on acclimatization of in vitro propagated Miltonia flavescens Lindl. Rev. Em Agronegócios E Meio Ambiente 2009, 2, 467–476. [Google Scholar]
- Rugini, E.; Fedeli, E. Olive (Olea europaea L.) as an Oilseed Crop. In Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin, Germany, 1990; Volume 10, pp. 593–641. [Google Scholar] [CrossRef]
- Rey, M.; Diaz-Sala, C.; Rodriguez, R. Effect of repeated severe pruning on endogenous polyamine content in hazelnut trees. Physiol. Plant. 1994, 92, 487–492. [Google Scholar] [CrossRef]
- Karimi, S.; Yadollahi, A. Using putrescine to increase rooting ability of hardwood cuttings of the peach×almond hybrid GF677. J. Agrobiol. 2012, 29, 63–69. [Google Scholar] [CrossRef]
- Mendoza de Gyves, E.; Royani, J.I.; Rugini, E. Efficient method of micropropagation and in vitro rooting of teak (Tectona grandis L.) focusing on large-scale industrial plantations. Ann. For. Sci. 2007, 64, 73–78. [Google Scholar] [CrossRef]
- Cristofori, V.; Rouphael, Y.; Rugini, E. Collection time, cutting age, IBA and putrescine effects on root formation in Corylus avellana L. cuttings. Sci. Hortic. 2010, 124, 189–194. [Google Scholar] [CrossRef]
- Chilley, P.M.; Casson, S.A.; Tarkowski, P.; Hawkins, N.; Wang, K.L.; Hussey, P.J.; Beale, M.; Ecker, J.R.; Sandberg, G.K.; Lindsey, K. The POLARIS peptide of Arabidopsis regulates auxin transport and root growth via effects on ethylene signaling. Plant Cell 2006, 18, 3058–3072. [Google Scholar] [CrossRef]
- Weiss, D.; Ori, N. Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol. 2007, 144, 1240–1246. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P.K. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef]
- Galston, A.W.; Kaur-Sawhney, R.; Altabella, T.; Tiburcio, A.F. Plant polyamines in reproductive activity and response to abiotic stress. Bot. Acta 1997, 110, 197–207. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M.I. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J. Food Compos. Anal. 2006, 19, 544–551. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E. Plant Propagation Principles and Practices, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1975. [Google Scholar]
- Hartmann, H.T.; Kester, D.E.; Davies, F.; Geneve, R. Plant Propagation Principles and Practices, 6th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Benbya, A.; Mdarhri Alaoui, M.; Gaboun, F.; Delporte, F.; Chlyah, S.; Cherkaoui, S. Vegetative propagation of Argania spinosa (L.) Skeels cuttings: Effects of auxins and genotype. Adv. Hortic. Sci. 2019, 33, 519–527. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Jinu, U.; Gomathi, M.; Mahendran, D.; Ahmad, N.; Geeth, N.; Sahi, S.V. Role of silver nitrate in plant regeneration from cotyledonary nodal segment explants of Prosopis cineraria (L.) Druce: A recalcitrant medicinal leguminous tree. Biocatal. Agric. Biotechnol. 2017, 12, 286–291. [Google Scholar] [CrossRef]
- Giridhar, P.; Reddy, O.B.; Ravishankar, G.A. Silver nitrate influences in vitro shoot multiplication and root formation in Vanilla planifolia Andr. Curr. Sci. 2001, 81, 1166–1170. [Google Scholar]
- Giridhar, P.; Indu, E.P.; Ramu, D.; Ravishankar, G.A. Effect of silver nitrate on in vitro shoot growth of coffee. Trop. Sci. 2003, 43, 144–146. [Google Scholar] [CrossRef]
- Chithra, M.; Sunandakumari, C.; Martin, K.P.; Madhusoodanan, P.V. Silver nitrate induced rooting and flowering in vitro on rare rhoeophytic woody medicinal plant, Rotula aquatica Lour. Indian J. Biotechnol. 2004, 3, 418–421. [Google Scholar]
- Klíma, P.; Laňková, M.; Vandenbussche, F.; Van Der Straeten, D.; Petrášek, J. Silver ions increase plasma membrane permeability through modulation of intracellular calcium levels in tobacco BY-2 cells. Plant Cell Rep. 2018, 37, 809–818. [Google Scholar] [CrossRef]
- Kumar, V.; Parvatam, G.; Ravishankar, G.A. AgNO3-A potential regulator of ethylene activity and plant growth modulator. Electron. J. Biotechnol. 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Ruzicka, K.; Ljung, K.; Vanneste, S.; Podhorska, R.; Beeckman, T.; Friml, J.; Benkováet, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef]
- Bais, H.P. Studies on Growth of Hairy Root Cultures of Cichorium intybus L. and Production of Phytochemicals. Ph.D. Thesis, University of Mysore, Mysore, India, 2000. [Google Scholar]
- Biddington, N.L. The influence of ethylene in plant tissue culture. Plant Growth Regul. 1992, 11, 173–187. [Google Scholar] [CrossRef]
- Bais, H.; Sudha, G.; Ravishankar, G. Influence of putrescine, silver nitrate and polyamine inhibitors on the morphogenetic response in untransformed and transformed tissues of Cichorium intybus and their regenerants. Plant Cell Rep. 2001, 20, 547–555. [Google Scholar] [CrossRef]
- Corrêa, L.R.; Paim, D.C.; Schwambach, J.; Fett-Neto, A.G. Carbohydrates as regulatory factors on the rooting of Eucalyptus saligna Smith and Eucalyptus globulus Labill. Plant Growth Regul. 2005, 45, 63–73. [Google Scholar] [CrossRef]
- Sriskandarajah, S.; Skirvin, R.M.; Abu-Qaoud, H. The effect of some macronutrients on adventitious root development of scion apple cultivars in vitro. Plant Cell Tissue Organ Cult. 1990, 21, 185–189. [Google Scholar] [CrossRef]
- Vahdati, K.; Leslie, C.; Zamani, Z.; McGranahan, G. Rooting and acclimatization of in vitro grown shoots from mature trees of three Persian walnut cultivars. Hortic. Sci. 2004, 39, 324–327. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. In vitro culture of lavenders (Lavandula spp.) and the production of secondary metabolites. Biotechnol. Adv. 2013, 31, 166–174. [Google Scholar] [CrossRef]
- Pati, P.K.; Rath, S.P.; Sharma, M.; Sood, A.; Ahuja, P.S. In vitro propagation of rose—A review. Biotechnol. Adv. 2006, 24, 94–114. [Google Scholar] [CrossRef]
- Oliveira, Y.; Anselmini, J.I.; Cuquel, F.L.; Pinto, F.; Quoirin, M. In vitro pre-acclimatization of ornamental pineapple. Ciência E Agrotecnologia 2010, 32, 1647–1653. [Google Scholar] [CrossRef]
- Da Silva, K.B.; Reinigern, L.R.S.; Stefanel, C.M.; Rabaiolli, S.M.S. Sucrose and substrates on the acclimatization of micropropagated Luehea divaricata plants. Floresta E Ambiente 2020, 27, e20171170. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agri. Exp. Stn. Circ. 1938, 347, 39. [Google Scholar]


| Treatment | Basal Medium | AgNO3 (mg L−1) | Putrescine (mg L−1) | PGRs (mg L−1) | “Mejji” | “R’zwa” | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IBA | NAA | Rooting Frequency (%) | Average No. of Roots/Shoot | Average Root Length (cm) | Callus Formation | Time for Rooting (Days) | Rooting Frequency (%) | Average No. of Roots/Shoot | Average Root Length (cm) | Callus Formation | Time for Rooting (Days) | ||||
| T0 | MS | - | - | - | - | 0 | 0 | 0 | 0C | - | 0 | 0 | 0 | 0C | - |
| T1 | MS | - | - | 1.5 | - | 0 | 0 | 0 | 0C | - | 0 | 0 | 0 | 0C | - |
| T2 | MS | - | 160 | - | - | 0 | 0 | 0 | 0C | - | 0 | 0 | 0 | 0C | - |
| T3-T4-T5 | MS | 2002/4/6 | - | - | - | 0 | 0 | 0 | 0C | - | 0 | 0 | 0 | 0C | - |
| T6 | MS | - | 160 | 1.5 | - | 52.2 ± 1.1 c | 3.1 ± 0.2 d,e | 2.8 ± 0.2 a | C+++ | 90 | 57.7 ± 2.9 c | 2.8 ± 0.2 a | 3.4 ± 0.2 a,b,c | C+++ | 90 |
| T7 | RM | - | 160 | 1.5 | - | 43.3 ± 3.8 d | 4.6 ± 0.2 c | 3.1 ± 0.2 a | C++ | 75 | 47.7 ± 2.9 d | 4.4 ± 0.2 c | 2.8 ± 0.2 a,b,c | C+++ | 75 |
| T8 | M | - | 160 | 1.5 | - | 19.9 ± 1.9 f,g | 2.5 ± 0.3 e,f,g | 2.0 ± 0.1 b,c | C++ | 60 | 21.1 ± 2.2 f,g | 2.8 ± 0.3 e,f | 3.0 ± 0.1 a,b,c | C+++ | 60 |
| T9 | MS | 2 | - | 1.5 | - | 39.9 ± 1.9 d | 3.3 ± 0.2 d,e | 3.3 ± 0.3 a | 0C | 30 | 43.3 ± 1.9 de | 3.1 ± 0.2 d,e,f | 2.7 ± 0.2 a,b,c | 0C | 30 |
| T10 | MS | 4 | - | 1.5 | - | 32.2 ± 1.1 d,e | 1.9 ± 0.1 f,g | 1.6 ± 0.1 c | 0C | 30 | 38.8 ± 1.1 e | 2.2 ± 0.1 f,g | 2.3 ± 0.1 c | 0C | 30 |
| T11 | MS | 6 | - | 1.5 | - | 19.9 ± 1.9 f,g | 1.9 ± 0.2 f,g | 2.8 ± 0.4 a,b | 0C | 30 | 18.8 ± 2.9 g | 1.5 ± 0.1 g | 2.4 ± 0.3 b,c | 0C | 30 |
| T12 | MS | 2 | 160 | 1.5 | - | 61.1 ± 2.9 b | 3.2 ± 0.1 d,e | 3.3 ± 0.2 a | 0C | 20 | 63.3 ± 3.3 b,c | 3.3 ± 0.1 c,d | 3.2 ± 0.1 a,b | 0C | 20 |
| T13 | RM | 2 | 160 | 1.5 | - | 65.5 ± 4.8 b | 3.7 ± 0.2 c,d | 3.2 ± 0.2 a | 0C | 20 | 67.7 ± 4.8 c | 3.8 ± 0.2 c,d | 3.3 ± 0.2 a | 0C | 20 |
| T14 | RM | 2 | 160 | 1.5 | 0.5 | 86.6 ± 3.8 a | 6.3 ± 0.3 b | 3.5 ± 0.1 a | 0C | 10 | 84.4 ± 2.9 a | 6.7 ± 0.3 b | 3.4 ± 0.1 a | C+ | 10 |
| T15 | RM | 2 | 160 | 1.5 | 1 | 38.8 ± 1.1 c,d | 2.9 ± 0.1 d,e,f | 3.2 ± 0.2 a | C+ | 20 | 38.8 ± 1.1 e | 3.3 ± 0.2 d,e | 2.9 ± 0.2 a,b,c | C++ | 20 |
| T16 | RM | 2 | 160 | 1.5 | 1.5 | 16.6 ± 1.9 g | 1.6 ± 0.1 g | 1.4 ± 0.2 c | C+++ | 25–30 | 17.7 ± 2.9 g | 2.6 ± 0.3 s,f | 1.4 ± 0.1 d | C+++ | 25–30 |
| T17 | RM | 2 | 160 | - | 1.5 | 26.6 ± 1.9 e,f | 10.1 ± 0.6 a | 1.6 ± 0.1 c | C+++ | 25 | 27.7 ± 1.1 f | 10.5 ± 0.4 a | 1.5 ± 0.1 d | C+++ | 25 |
| Substrate Mixture Peat:Sand:Forest Soil | Survival Rate (%) | Average Number of Neoformed Shoots | Stem Growth (cm/Plant) | Stem Growth (cm/Week) | ||||
|---|---|---|---|---|---|---|---|---|
| “Mejji” | “R’zwa” | “Mejji” | “R’zwa” | “Mejji” | “R’zwa” | “Mejji” | “R’zwa” | |
| S1 = 1:1:1 | 73.3 b ± 6.6 | 83.3 b ± 3.3 | 0.2 a,b ± 0.1 | 0.2 b ± 0.1 | 1.9 a,b ± 0.2 | 1.3 a,b ± 0.2 | 0.4 a,b ± 0.0 | 0.3 a ± 0.0 |
| S2 = 1:1:0 | 100.0 a ± 0.0 | 100.0 a ± 0.0 | 0.4 a ± 0.1 | 0.0 a ± 0.0 ǂ | 0.9 b ± 0.2 | 0.9 a ± 0.2 | 0.2 b ± 0.0 | 0.2 a ± 0.0 |
| S3 = 1:0:0 | 100.0 a ± 0.0 | 100.0 a ± 0.0 | 0.0 b ± 0.0 | 0.0 a ± 0.0 | 1.3 b ± 0.2 | 2.0 b ± 0.3 | 0.3 b ± 0.0 | 0.5 a ± 0.0 |
| S4 = 0:1:0 | 100.0 a ± 0.0 | 86.6 b ± 3.3 ǂ | 0.4 a ± 0.1 | 0.0 a ± 0.0 ǂ | 2.7 a ± 0.6 | 0.8 a ± 0.1 ǂ | 0.7 a ± 0.1 | 0.2 a ± 0.0 ǂ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amghar, I.; Ibriz, M.; Ibrahimi, M.; Boudra, A.; Gaboun, F.; Meziani, R.; Iraqi, D.; Mazri, M.A.; Diria, G.; Abdelwahd, R. In Vitro Root Induction from Argan (Argania spinosa (L.) Skeels) Adventitious Shoots: Influence of Ammonium Nitrate, Auxins, Silver Nitrate and Putrescine, and Evaluation of Plantlet Acclimatization. Plants 2021, 10, 1062. https://doi.org/10.3390/plants10061062
Amghar I, Ibriz M, Ibrahimi M, Boudra A, Gaboun F, Meziani R, Iraqi D, Mazri MA, Diria G, Abdelwahd R. In Vitro Root Induction from Argan (Argania spinosa (L.) Skeels) Adventitious Shoots: Influence of Ammonium Nitrate, Auxins, Silver Nitrate and Putrescine, and Evaluation of Plantlet Acclimatization. Plants. 2021; 10(6):1062. https://doi.org/10.3390/plants10061062
Chicago/Turabian StyleAmghar, Ilham, Mohammed Ibriz, Maha Ibrahimi, Abdelaali Boudra, Fatima Gaboun, Reda Meziani, Driss Iraqi, Mouaad Amine Mazri, Ghizlane Diria, and Rabha Abdelwahd. 2021. "In Vitro Root Induction from Argan (Argania spinosa (L.) Skeels) Adventitious Shoots: Influence of Ammonium Nitrate, Auxins, Silver Nitrate and Putrescine, and Evaluation of Plantlet Acclimatization" Plants 10, no. 6: 1062. https://doi.org/10.3390/plants10061062
APA StyleAmghar, I., Ibriz, M., Ibrahimi, M., Boudra, A., Gaboun, F., Meziani, R., Iraqi, D., Mazri, M. A., Diria, G., & Abdelwahd, R. (2021). In Vitro Root Induction from Argan (Argania spinosa (L.) Skeels) Adventitious Shoots: Influence of Ammonium Nitrate, Auxins, Silver Nitrate and Putrescine, and Evaluation of Plantlet Acclimatization. Plants, 10(6), 1062. https://doi.org/10.3390/plants10061062






