Genome-Wide Identification and Expression Analysis of the NRAMP Family Genes in Tea Plant (Camellia sinensis)
Abstract
1. Introduction
2. Results
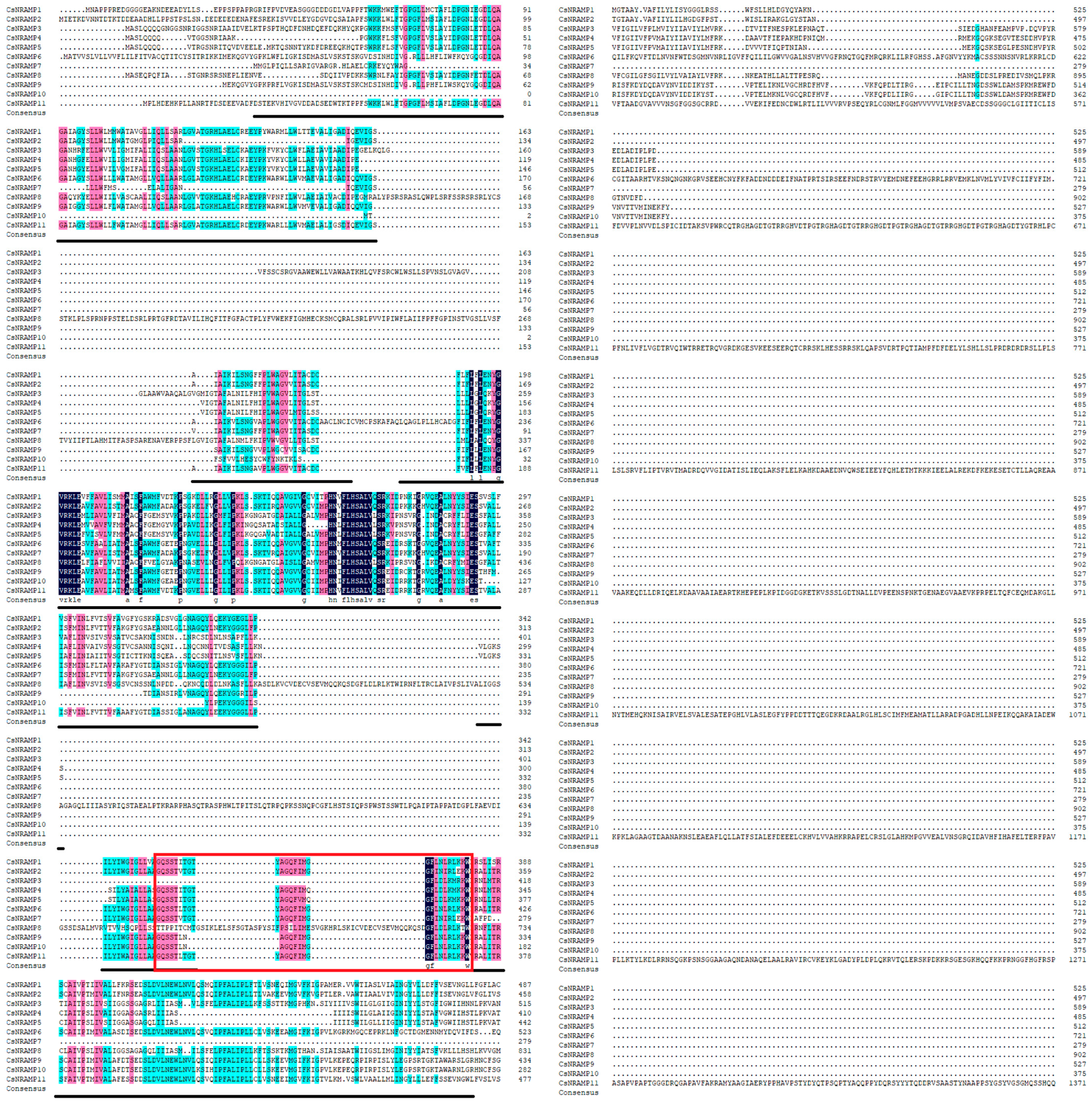
2.1. Identification and Characteristics of CsNRAMPs
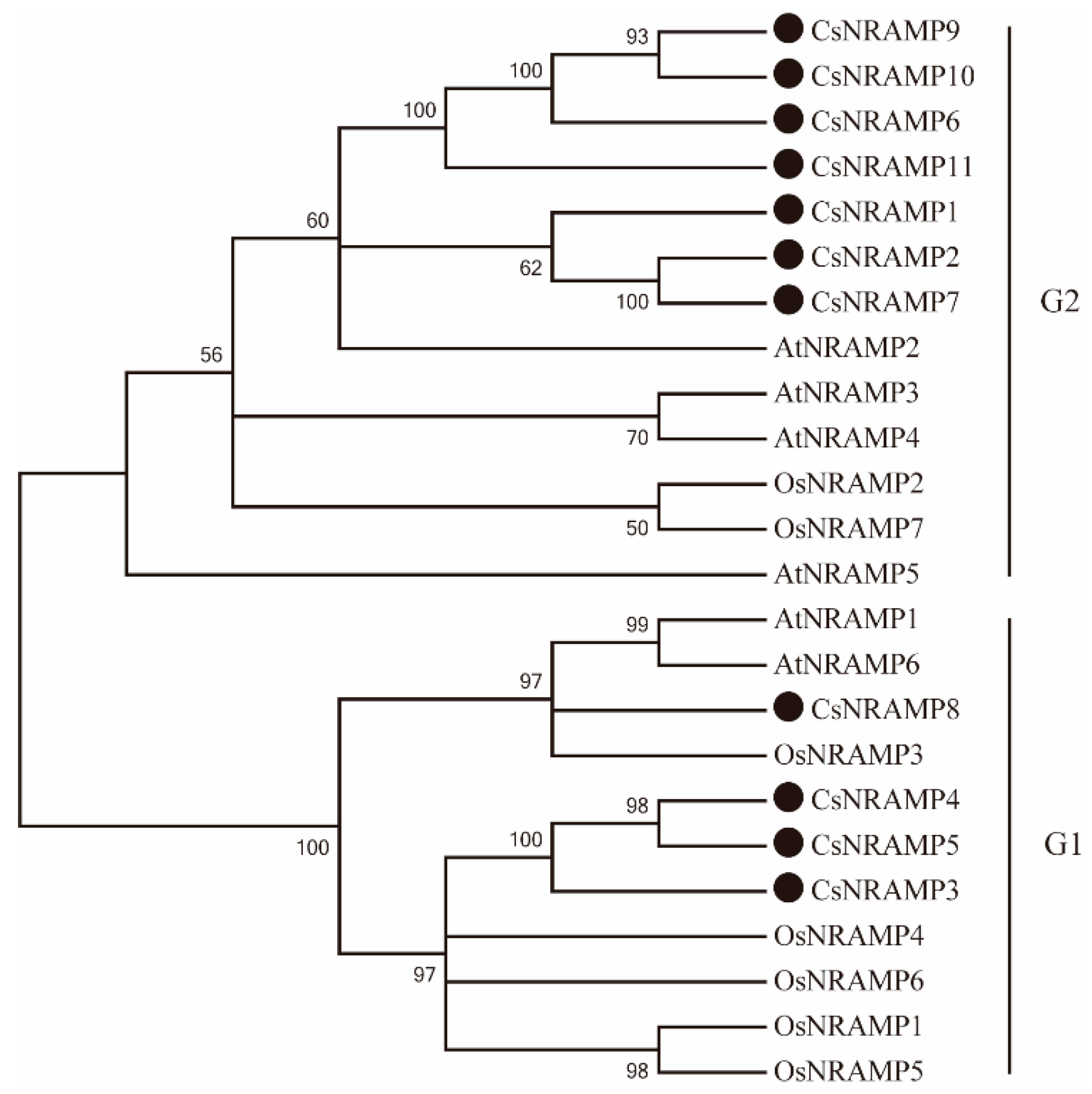
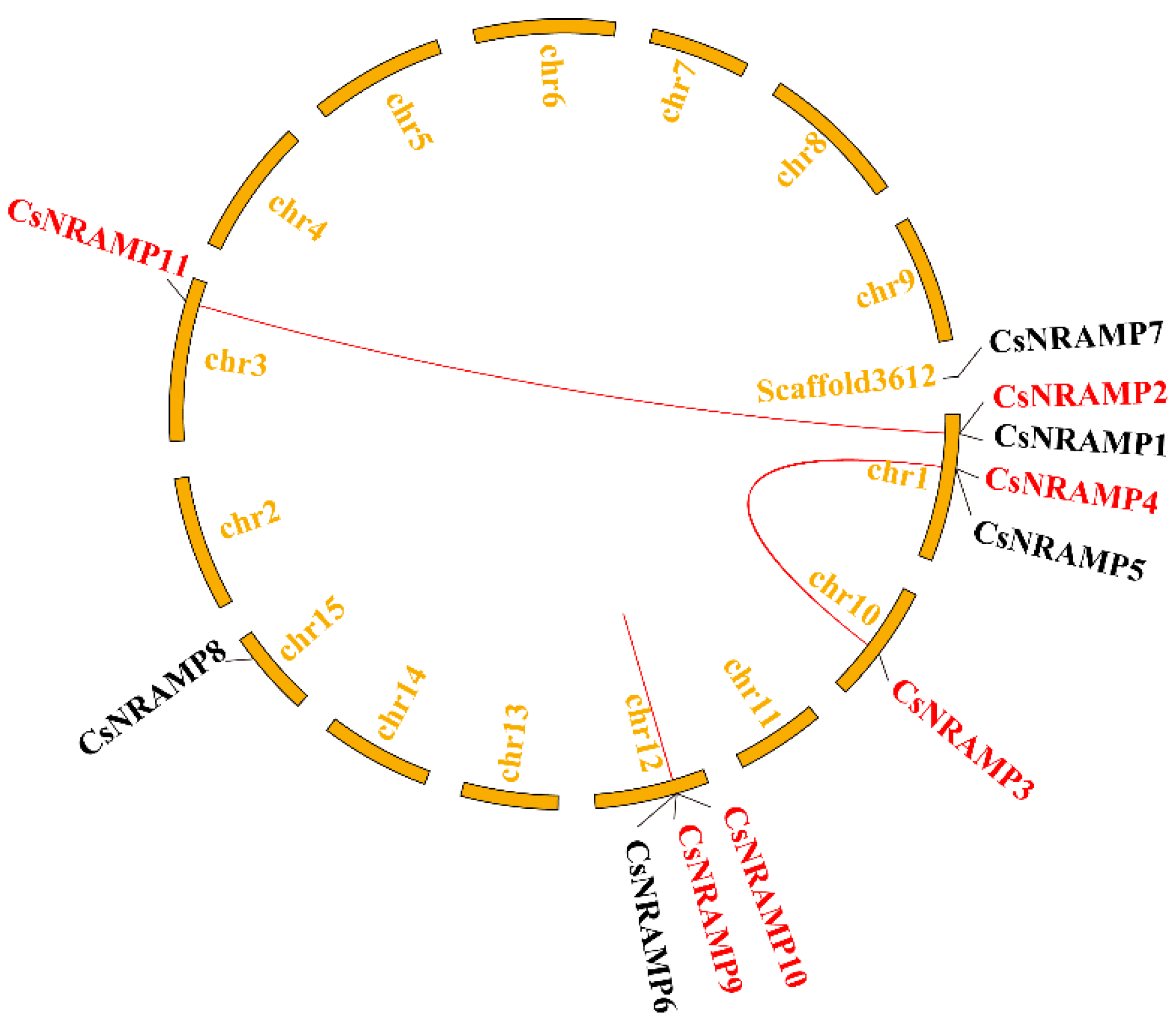
2.2. Phylogenetic Analysis and Duplication Analysis of CsNRAMPs
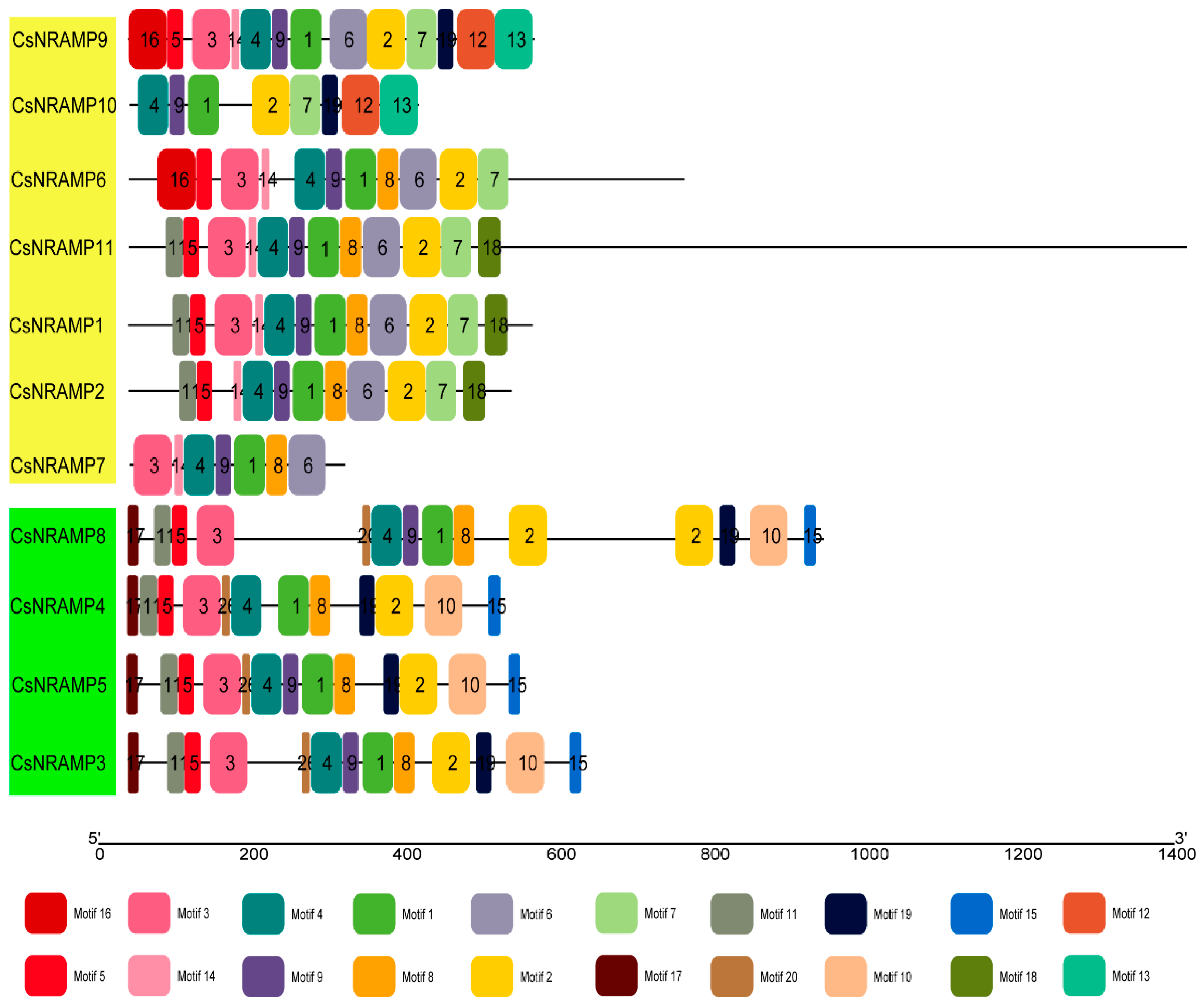
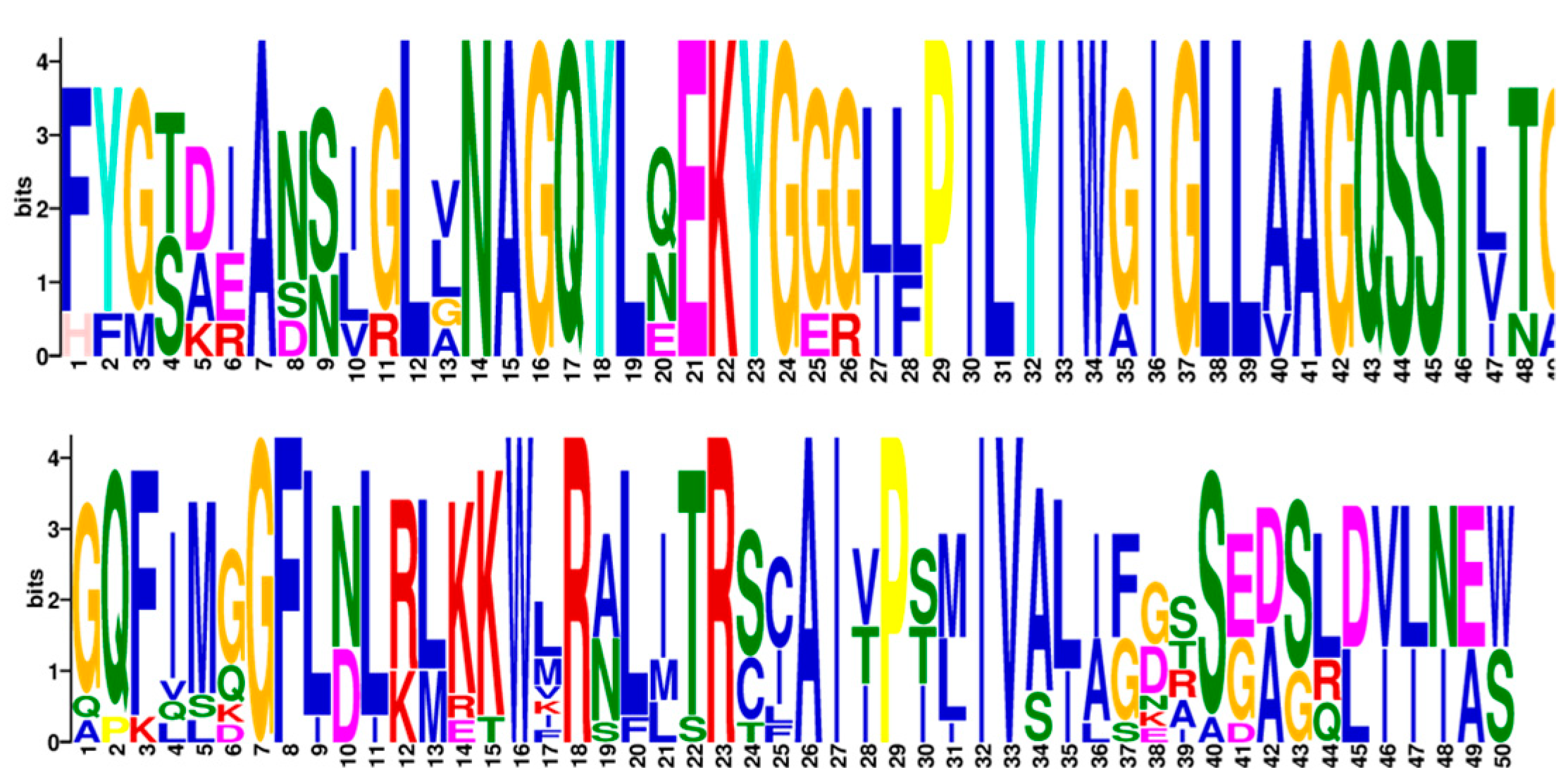
2.3. Gene Structure and Conserved Motifs of CsNRAMP Proteins
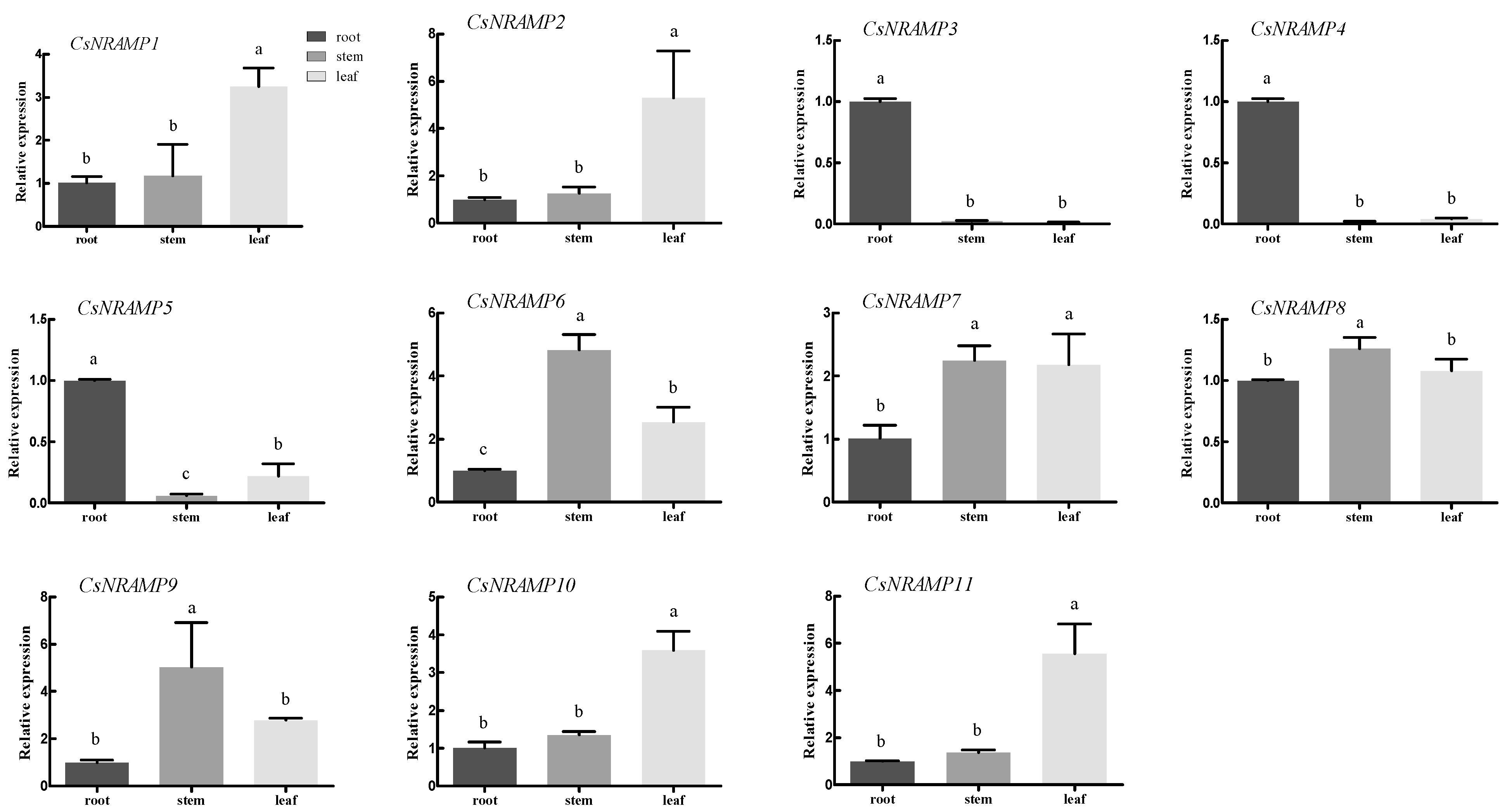
2.4. Expression Analysis of CsNRAMP Genes in Different Tissues
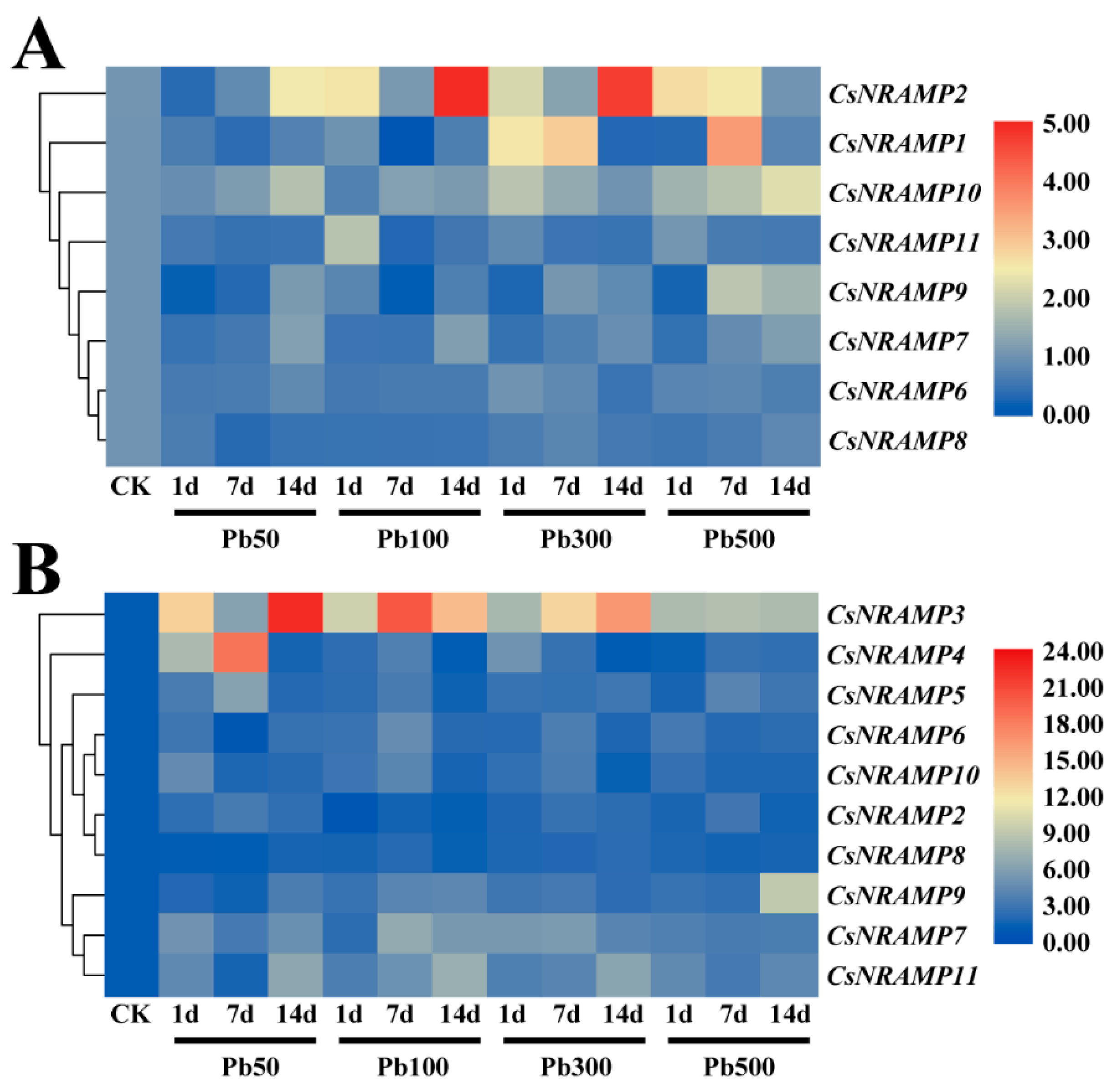
2.5. Expression Analysis of CsNRAMP Genes under Lead Stress
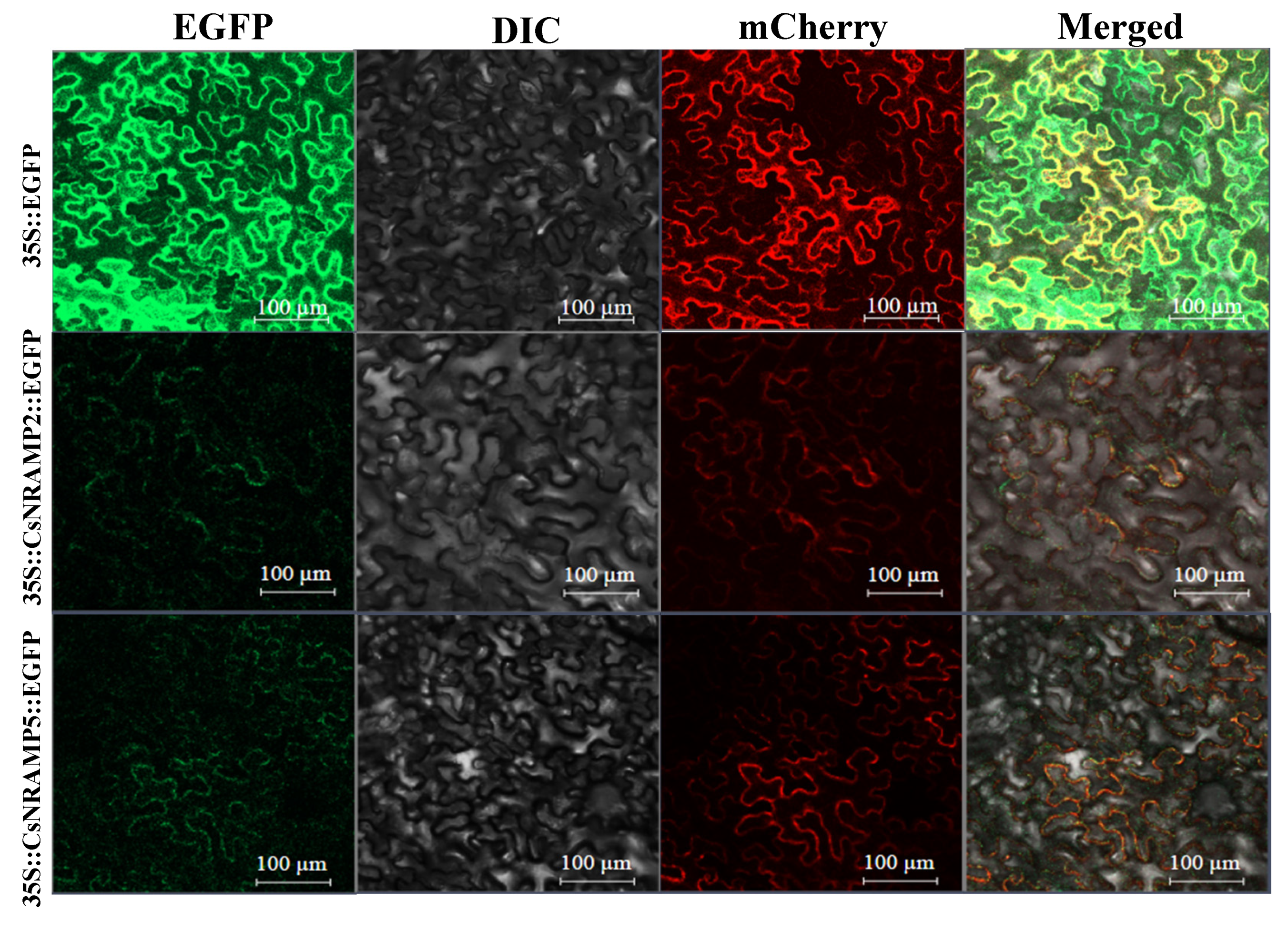
2.6. Cloning of CsNRAMP2 and CsNRAMP5 and Subcellular Localization
3. Discussion
4. Materials and Methods
4.1. Identification of CsNRAMP Family Genes in the Tea Plant
4.2. Characterization of CsNRAMP Proteins
4.3. Plant Materials and Treatments
4.4. Expression Profile Analyses
4.5. Subcellular Location Confirmation of CsNRAMP2 and CsNRAMP5
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, W.-Y.; Shi, Y.-Z.; Ma, L.-F.; Ruan, J.-Y.; Zhao, F.-J. Effect of liming and seasonal variation on lead concentration of tea plant (Camellia sinensis (L.) O. Kuntze). Chemosphere 2007, 66, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.V. Soil–plant nutrient balance of tea crops in the northern mountainous region, Vietnam. Agric. Ecosyst. Environ. 2005, 105, 413–418. [Google Scholar] [CrossRef]
- Alkhatib, R.; Mheidat, M.; Abdo, N.; Tadros, M.; Al-Eitan, L.; Al-Hadid, K. Effect of lead on the physiological, biochemical and ultrastructural properties ofLeucaena leucocephala. Plant Biol. 2019, 21, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.F. EFFECTS OF HEAVY METAL PB AND CD STRESS ON PHYSIOLOGICAL CHARACTERISTICS OF JAPANESE HONEYSUCKLE. Appl. Ecol. Environ. Res. 2019, 17, 6415–6427. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the Links between Heavy Metal Stress and Plant Signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Lal, N. Molecular Mechanisms and Genetic Basis of Heavy Metal Toxicity and Tolerance in Plants. In Plant Adaptation and Phytoremediation; Springer: Berlin/Heidelberg, Germany, 2010; pp. 35–58. [Google Scholar] [CrossRef]
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant. 2016, 38, 257. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Bandpei, A.M.; Maleki, A.; Saberian, M.; Izanloo, H. Determination of Cadmium and Lead Contents in Black Tea and Tea Liquor from Iran. Asian J. Chem. 2010, 22, 1387–1393. [Google Scholar]
- Wen, B.; Li, L.; Duan, Y.; Zhang, Y.; Shen, J.; Xia, M.; Wang, Y.; Fang, W.; Zhu, X. Zn, Ni, Mn, Cr, Pb and Cu in soil-tea ecosystem: The concentrations, spatial relationship and potential control. Chemosphere 2018, 204, 92–100. [Google Scholar] [CrossRef]
- Schwalfenberg, G.; Genuis, S.J.; Rodushkin, I. The Benefits and Risks of Consuming Brewed Tea: Beware of Toxic Element Contamination. J. Toxicol. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Jin, C.W.; He, Y.F.; Zhang, K.; Di Zhou, G.; Shi, J.L.; Zheng, S.J. Lead contamination in tea leaves and non-edaphic factors affecting it. Chemosphere 2005, 61, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.M.; Yang, J.P.; He, J.; Zhao, X.; Ye, X.Y. Effects of Phosphate Fertilizers on Bioavailable Lead in Tea Garden Soil and Lead Absorption and Accumulation by Tea Plants. Appl. Mech. Mater. 2014, 651-653, 231–235. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Yu, M.; Chen, X.; Shi, J. Lead contamination in different varieties of tea plant (Camellia sinensis L.) and factors affecting lead bioavailability. J. Sci. Food Agric. 2010, 90, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy Metal Pollutions: State of the Art and Innovation in Phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef]
- Mani, A.; Sankaranarayanan, K. In Silico Analysis of Natural Resistance-Associated Macrophage Protein (NRAMP) Family of Transporters in Rice. Protein J. 2018, 37, 237–247. [Google Scholar] [CrossRef]
- Vidal, S.M.; Malo, D.; Vogan, K.; Skamene, E.; Gros, P. Natural resistance to infection with intracellular parasites: Isolation of a candidate for Bcg. Cell 1993, 73, 469–485. [Google Scholar] [CrossRef]
- Pinner, E.; Gruenheid, S.; Raymond, M.; Gros, P. Functional Complementation of the Yeast Divalent Cation Transporter Family SMF by NRAMP2, a Member of the Mammalian Natural Resistance-associated Macrophage Protein Family. J. Biol. Chem. 1997, 272, 28933–28938. [Google Scholar] [CrossRef]
- Gunshin, H.; MacKenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nat. Cell Biol. 1997, 388, 482–488. [Google Scholar] [CrossRef]
- Mäser, P.; Thomine, S.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.; Sanders, D.; et al. Phylogenetic Relationships within Cation Transporter Families of Arabidopsis. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef]
- Vatansever, R.; Filiz, E.; Ozyigit, I.I. In silico analysis of Mn transporters (NRAMP1) in various plant species. Mol. Biol. Rep. 2016, 43, 151–163. [Google Scholar] [CrossRef]
- Bressler, J.P.; Olivi, L.; Cheong, J.H.; Kim, Y.; Bannona, D. Divalent Metal Transporter 1 in Lead and Cadmium Transport. Ann. N. Y. Acad. Sci. 2004, 1012, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Huang, S.; Yamaji, N.; Zhang, W.; Ma, J.F.; Zhao, F. OsNRAMP1 transporter contributes to cadmium and manganese uptake in rice. Plant, Cell Environ. 2020, 43, 2476–2491. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Wang, Y.-M.; Yang, H.-L.; Zeng, Q.-Y.; Liu, Y.-J. NRAMP1 promotes iron uptake at the late stage of iron deficiency in poplars. Tree Physiol. 2019, 39, 1235–1250. [Google Scholar] [CrossRef]
- Ishida, J.K.; Caldas, D.G.; Oliveira, L.R.; Frederici, G.C.; Leite, L.M.P.; Mui, T.S. Genome-wide characterization of the NRAMP gene family in Phaseolus vulgaris provides insights into functional implications during common bean development. Genet. Mol. Biol. 2018, 41, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Wang, Y.; Eide, D.J.; Dunwell, J.M. Evolution, and functional analysis of Natural Resistance-Associated Macrophage Proteins (NRAMPs) from Theobroma cacao and their role in cadmium accumulation. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Zhang, X.D.; Meng, J.G.; Zhao, K.X.; Chen, X.; Yang, Z.M. Annotation and characterization of Cd-responsive metal transporter genes in rapeseed (Brassica napus). BioMetals 2017, 31, 107–121. [Google Scholar] [CrossRef]
- Cailliatte, R.; Lapeyre, B.; Briat, J.; Mari, S.; Curie, C. The NRAMP6 metal transporter contributes to cadmium toxicity. Biochem. J. 2009, 422, 217–228. [Google Scholar] [CrossRef]
- Meng, J.G.; Zhang, X.D.; Tan, S.K.; Zhao, K.X.; Yang, Z.M. Genome-wide identification of Cd-responsive NRAMP transporter genes and analyzing expression of NRAMP 1 mediated by miR167 in Brassica napus. BioMetals 2017, 30, 917–931. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Yao, Q.; Long, D.; Fan, X.; Kang, H.; Zeng, J.; Sha, L.; Zhang, H.; Zhou, Y.; et al. Overexpression of TtNRAMP6 enhances the accumulation of Cd in Arabidopsis. Gene 2019, 696, 225–232. [Google Scholar] [CrossRef]
- Williams, L.E.; Pittman, J.K.; Hall, J. Emerging mechanisms for heavy metal transport in plants. Biochim. Biophys. Acta Biomembr. 2000, 1465, 104–126. [Google Scholar] [CrossRef]
- Tiwari, M.; Sharma, D.; Dwivedi, S.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ. 2014, 37, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Zheng, L.; Li, Y.; Zhou, X.; Li, J.; Gu, D.; Xu, E.; Lu, Y.; Chen, X.; et al. The Intracellular Transporter AtNRAMP6 Is Involved in Fe Homeostasis in Arabidopsis. Front. Plant Sci. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Castaings, L.; Caquot, A.; Loubet, S.; Curie, C. The high-affinity metal Transporters NRAMP1 and IRT1 Team up to Take up Iron under Sufficient Metal Provision. Sci. Rep. 2016, 6, 37222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Song, H.; Zhao, J.; Shabala, S.; Tian, S.; Yang, X. A novel plasma membrane-based NRAMP transporter contributes to Cd and Zn hyperaccumulation in Sedum alfredii Hance. Environ. Exp. Bot. 2020, 176, 104121. [Google Scholar] [CrossRef]
- Lanquar, V.; Lelièvre, F.; Bolte, S.; Hamès, C.; Alcon, C.; Neumann, D.; Vansuyt, G.; Curie, C.; Schröder, A.; Krämer, U.; et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005, 24, 4041–4051. [Google Scholar] [CrossRef]
- Mary, V.; Ramos, M.S.; Gillet, C.; Socha, A.L.; Giraudat, J.; Agorio, A.; Merlot, S.; Clairet, C.; Kim, S.A.; Punshon, T.; et al. Bypassing Iron Storage in Endodermal Vacuoles Rescues the Iron Mobilization Defect in the natural resistance associated-macrophage protein3natural resistance associated-macrophage protein4 Double Mutant. Plant Physiol. 2015, 169, 748–759. [Google Scholar] [CrossRef]
- Wang, S.; Sun, J.; Li, S.; Lu, K.; Meng, H.; Xiao, Z.; Zhang, Z.; Li, J.; Luo, F.; Li, N. Physiological, genomic and transcriptomic comparison of two Brassica napus cultivars with contrasting cadmium tolerance. Plant Soil 2019, 441, 71–87. [Google Scholar] [CrossRef]
- Xia, J.; Yamaji, N.; Kasai, T.; Ma, J.F. Plasma membrane-localized transporter for aluminum in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 18381–18385. [Google Scholar] [CrossRef]
- Bereczky, Z.; Wang, H.-Y.; Schubert, V.; Ganal, M.; Bauer, P. Differential Regulation of nramp and irt Metal Transporter Genes in Wild Type and Iron Uptake Mutants of Tomato. J. Biol. Chem. 2003, 278, 24697–24704. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Y.; Xu, W.; Chai, Y.; Li, T.; Zhang, C.; Yang, M.; He, Z.; Feng, D. Differences of Cd uptake and expression of MT family genes and NRAMP2 in two varieties of ryegrasses. Environ. Sci. Pollut. Res. 2018, 26, 13738–13745. [Google Scholar] [CrossRef]
- Thomine, S.; Lelievre, F.; Debarbieux, E.; Schroeder, J.I.; Barbier-Brygoo, H. AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J. 2003, 34, 685–695. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhu, X.; Shen, J.; Xing, H.; Zou, Z.; Ma, Y.; Wang, Y.; Fang, W. Genome-wide identification, characterization and expression analysis of the amino acid permease gene family in tea plants (Camellia sinensis). Genomics 2020, 112, 2866–2874. [Google Scholar] [CrossRef]
- Shen, J.; Zou, Z.; Xing, H.; Duan, Y.; Zhu, X.; Ma, Y.; Wang, Y.; Fang, W. Genome-Wide Analysis Reveals Stress and Hormone Responsive Patterns of JAZ Family Genes in Camellia Sinensis. Int. J. Mol. Sci. 2020, 21, 2433. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Nelson, B.K.; Cai, X.; Nebenführ, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007, 51, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ma, Y.; Zhao, Y.; Qin, H.; Li, J.; Zhou, L.; Zhu, X.; Wang, Y.; Fang, W. Identification analysis of Plantacyanin (PLC) genes in Camellia sinensis and functional identification of CsPLC-3 in yeast. J. Hortic. Sci. Biotechnol. 2020, 95, 578–589. [Google Scholar] [CrossRef]
| Name | Gene ID | Number of Amino Acids | Molecular Weight | Theoretical pI | Exon Number | Transmembrane Number | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| CsNRAMP1 | TEA000600.1 | 525 | 57,407.13 | 5.23 | 4 | 11 | Membrane bound Vacuolar |
| CsNRAMP2 | TEA000624.1 | 497 | 54,044.99 | 4.94 | 5 | 9 | Membrane bound Vacuolar |
| CsNRAMP3 | TEA009385.1 | 589 | 64,295.88 | 8.75 | 13 | 10 | Plasma membrane |
| CsNRAMP4 | TEA002435.1 | 485 | 52,482.32 | 8.81 | 12 | 11 | Plasma membrane |
| CsNRAMP5 | TEA017264.1 | 512 | 56,070.19 | 8.05 | 12 | 11 | Plasma membrane |
| CsNRAMP6 | TEA012361.1 | 721 | 80,236.18 | 8.55 | 10 | 11 | Membrane bound Vacuolar |
| CsNRAMP7 | TEA012584.1 | 279 | 30,575.14 | 9.18 | 3 | 6 | Membrane bound Vacuolar |
| CsNRAMP8 | TEA022476.1 | 902 | 98,625.55 | 9.01 | 17 | 14 | Plasma membrane |
| CsNRAMP9 | TEA025235.1 | 527 | 58,851.23 | 8.17 | 5 | 6 | Membrane bound Vacuolar |
| CsNRAMP10 | TEA032256.1 | 375 | 42,411.81 | 8.36 | 5 | 3 | Membrane bound Vacuolar |
| CsNRAMP11 | TEA011223.1 | 1373 | 150,318.02 | 5.81 | 10 | 12 | Membrane bound Vacuolar * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Duan, Y.; Han, Z.; Shang, X.; Zhang, K.; Zou, Z.; Ma, Y.; Li, F.; Fang, W.; Zhu, X. Genome-Wide Identification and Expression Analysis of the NRAMP Family Genes in Tea Plant (Camellia sinensis). Plants 2021, 10, 1055. https://doi.org/10.3390/plants10061055
Li J, Duan Y, Han Z, Shang X, Zhang K, Zou Z, Ma Y, Li F, Fang W, Zhu X. Genome-Wide Identification and Expression Analysis of the NRAMP Family Genes in Tea Plant (Camellia sinensis). Plants. 2021; 10(6):1055. https://doi.org/10.3390/plants10061055
Chicago/Turabian StyleLi, Jinqiu, Yu Duan, Zhaolan Han, Xiaowen Shang, Kexin Zhang, Zhongwei Zou, Yuanchun Ma, Fang Li, Wanping Fang, and Xujun Zhu. 2021. "Genome-Wide Identification and Expression Analysis of the NRAMP Family Genes in Tea Plant (Camellia sinensis)" Plants 10, no. 6: 1055. https://doi.org/10.3390/plants10061055
APA StyleLi, J., Duan, Y., Han, Z., Shang, X., Zhang, K., Zou, Z., Ma, Y., Li, F., Fang, W., & Zhu, X. (2021). Genome-Wide Identification and Expression Analysis of the NRAMP Family Genes in Tea Plant (Camellia sinensis). Plants, 10(6), 1055. https://doi.org/10.3390/plants10061055






