Genotypic Variability on Grain Yield and Grain Nutritional Quality Characteristics of Wheat Grown under Elevated CO2 and High Temperature
Abstract
1. Introduction
2. Results
2.1. Wheat Production and Grain Yield
2.2. Wheat Grain Nutritional Quality
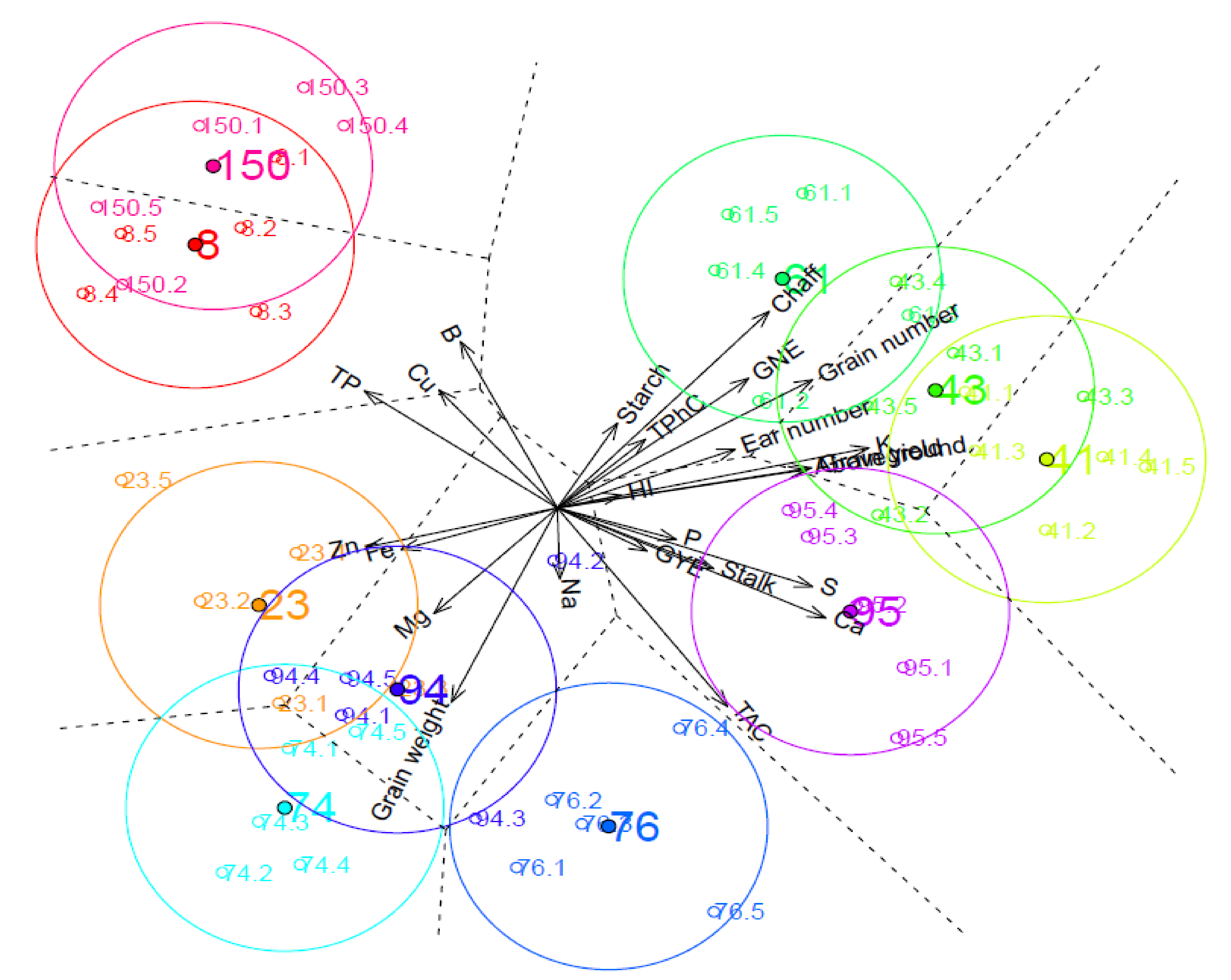
2.3. Genotypic Characterization
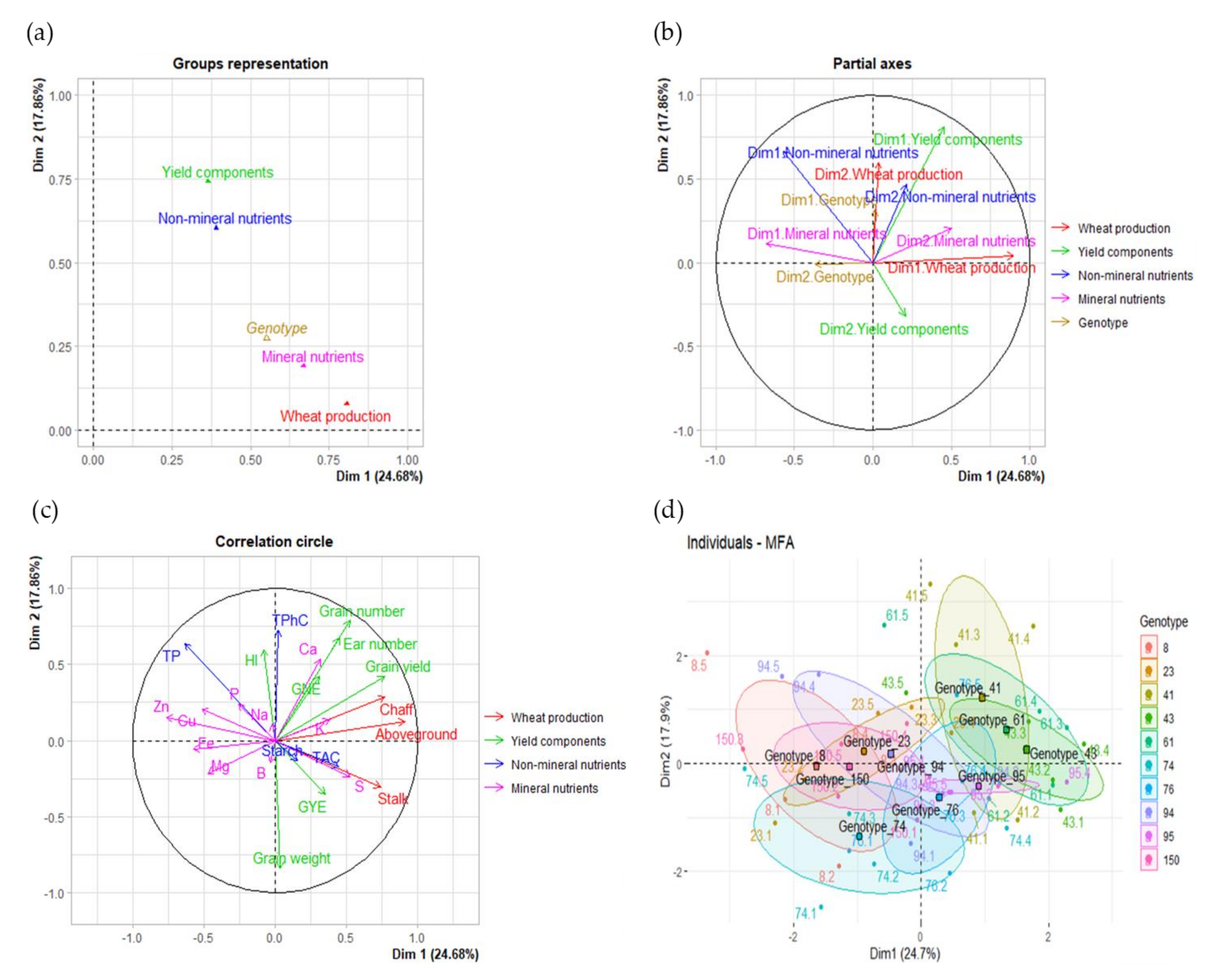
2.4. Wheat Production, Grain Yield, and Nutritional Quality Traits
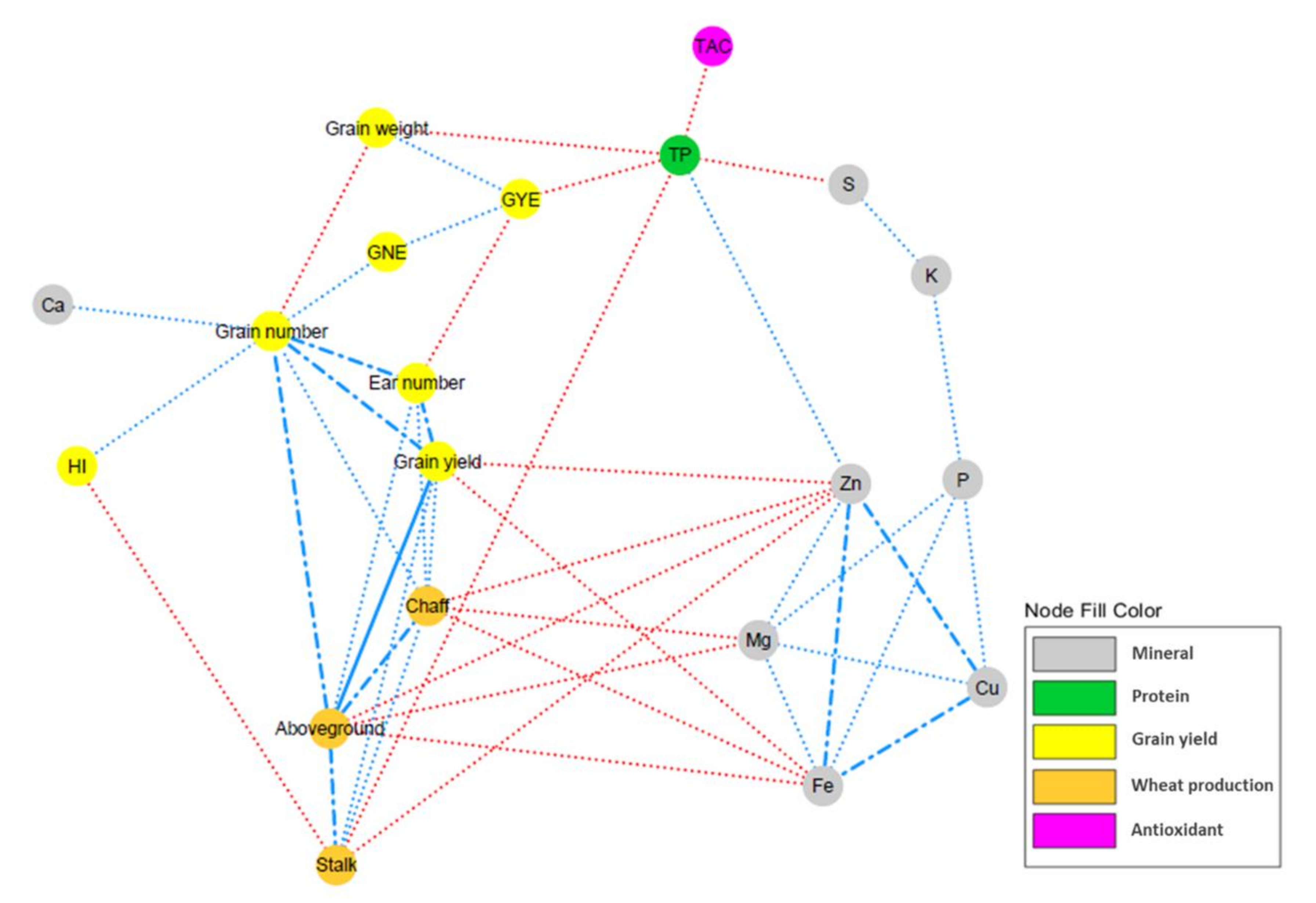
2.5. Grain Nutrient Content
3. Discussion
3.1. Grain Yield and Related Traits
3.2. Grain Nutritional Quality Traits
3.3. Grain Yield and Quality Trade-Off
4. Materials and Methods
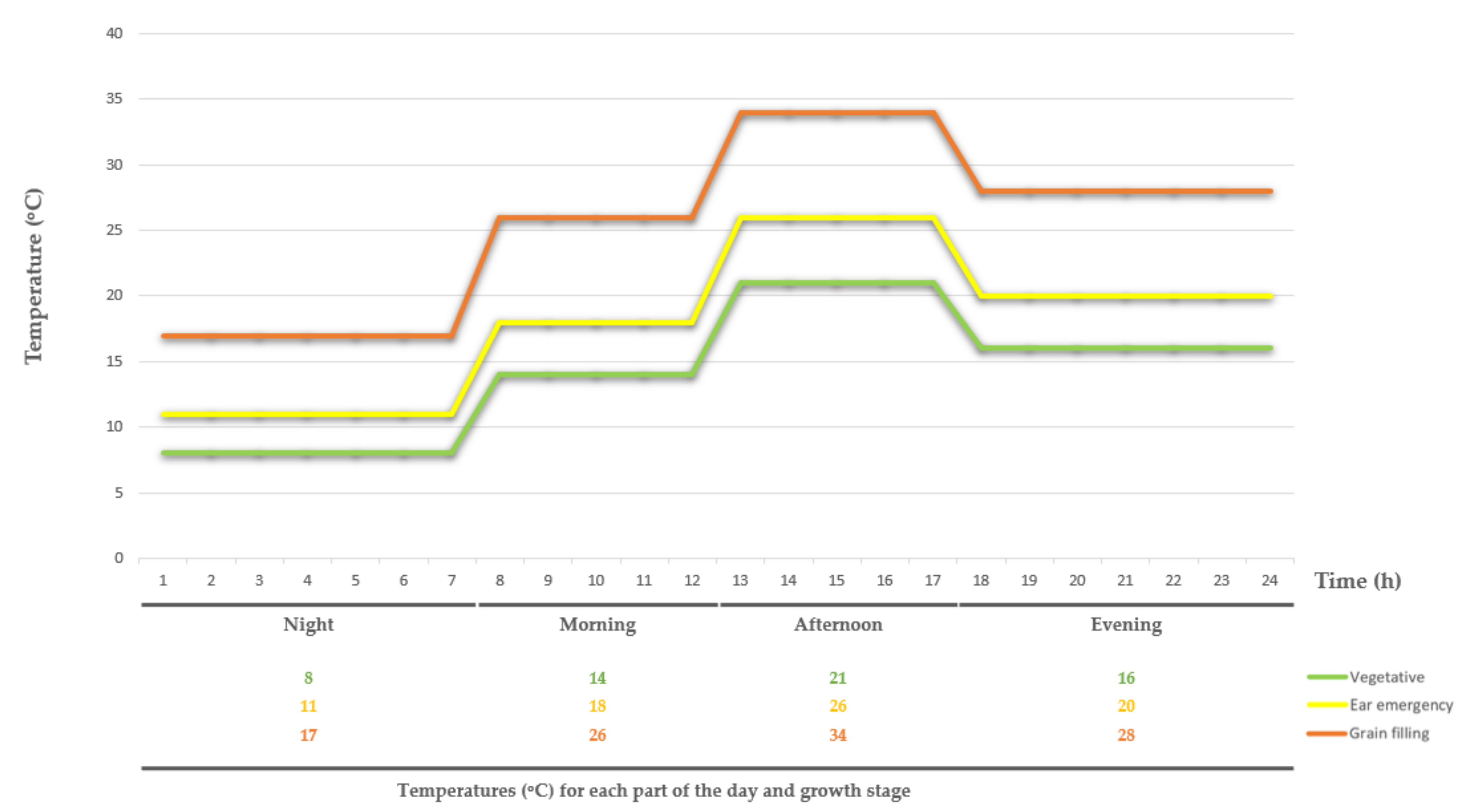
4.1. Plant Material and Growth Conditions
4.2. Harvesting and Yield Parameter Measurements
4.3. Sample Preparation and Analysis of Starch
4.4. Total N and Protein Concentration
4.5. Total Antioxidant Capacity and Total Phenolic Compound Measurements
4.6. Determination of Mineral Nutrients
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Genotype | Pedigree | Accesion | Top Name |
|---|---|---|---|
| 8 | KACHU/KIRITATI | BW 49924 | CMSS07Y00127S-0B-099Y-099M-099NJ-099NJ-6WGY-0B |
| 23 | SUPER 152*2/TECUE #1 | BW 49956 | CMSS07B00614T-099TOPY-099M-099Y-099M-49WGY-0B |
| 41 | SUPER 152/BAJ #1 | BW 50048 | CMSS07Y00195S-0B-099Y-099M-099Y-5M-0WGY |
| 43 | SUPER 152//WEEBILL1*2/BRAMBLING | BW 50050 | CMSS07Y00196S-0B-099Y-099M-099Y-6M-0WGY |
| 61 | TOBARITO M 97/PASTOR*2//AKURI | BW 50122 | CMSS07Y01094T-099TOPM-099Y-099M-099NJ-099NJ-17WGY-0B |
| 74 | WEEBILL1/KUKUNA//TACUPETO F2001/3/QUAIU #2 | BW 50193 | CMSS07B00246S-099M-099Y-099M-5WGY-0B |
| 76 | WHEATEAR/KUKUNA/3/C80.1/3*BATAVIA//2*WEEBILL1/4/QUAIU | BW 50196 | CMSS07B00264S-099M-099NJ-099NJ-2WGY-0B |
| 94 | WEEBILL1*2/KURUKU*2//SUPER 152 | BW 50264 | CMSS07B00685T-099TOPY-099M-099Y-099M-17WGY-0B |
| 95 | FRET2/KUKUNA//FRET2/3/HEILO/4/BLOUK #1 | BW 50266 | CMSS07B00715T-099TOPY-099M-099Y-099M-7WGY-0B |
| 150 | Gazul |
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2019. Safeguarding against Economic Slowdowns and Downturns; FAO: Rome, Italy, 2019; ISBN 978-92-5-131570-5. [Google Scholar]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate Trends and Global Crop Production Since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Gamage, D.; Thompson, M.; Sutherland, M.; Hirotsu, N.; Makino, A.; Seneweera, S. New insights into the cellular mechanisms of plant growth at elevated atmospheric carbon dioxide concentrations. Plant Cell Environ. 2018, 41, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- NOAA-ESRL Trends in Atmospheric Carbon Dioxide. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/index.html (accessed on 1 April 2021).
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Högy, P.; Brunnbauer, M.; Koehler, P.; Schwadorf, K.; Breuer, J.; Franzaring, J.; Zhunusbayeva, D.; Fangmeier, A. Grain quality characteristics of spring wheat (Triticum aestivum) as affected by free-air CO2 enrichment. Environ. Exp. Bot. 2013, 88, 11–18. [Google Scholar] [CrossRef]
- Plessis, A.; Ravel, C.; Bordes, J.; Balfourier, F.; Martre, P. Association study of wheat grain protein composition reveals that gliadin and glutenin composition are trans-regulated by different chromosome regions. J. Exp. Bot. 2013, 64, 3627–3644. [Google Scholar] [CrossRef]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef]
- Högy, P.; Fangmeier, A. Effects of elevated atmospheric CO2 on grain quality of wheat. J. Cereal Sci. 2008, 48, 580–591. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef]
- Pérez, P.; Morcuende, R.; Martı́n del Molino, I.; Martı́nez-Carrasco, R. Diurnal changes of Rubisco in response to elevated CO2, temperature and nitrogen in wheat grown under temperature gradient tunnels. Environ. Exp. Bot. 2005, 53, 13–27. [Google Scholar] [CrossRef]
- Taub, D.R.; Wang, X. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Morcuende, R.; del Pozo, A.; Martínez-Carrasco, R.; Pérez, P. Involvement of nitrogen and cytokinins in photosynthetic acclimation to elevated CO2 of spring wheat. J. Plant Physiol. 2013, 170, 1337–1343. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, P.J. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Chang. 2014, 4, 477–480. [Google Scholar] [CrossRef]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Feil, R.; Lunn, J.E.; Watanabe, M.; Arrivault, S.; Stitt, M.; Hoefgen, R.; Morcuende, R. Metabolic and transcriptional analysis of durum wheat responses to elevated CO2 at low and high nitrate supply. Plant Cell Physiol. 2016, 57, 2133–2146. [Google Scholar] [CrossRef]
- Tausz-Posch, S.; Tausz, M.; Bourgault, M. Elevated [CO2] effects on crops: Advances in understanding acclimation, nitrogen dynamics and interactions with drought and other organisms. Plant Biol. 2020, 22, 38–51. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Högy, P.; Wieser, H.; Köhler, P.; Schwadorf, K.; Breuer, J.; Franzaring, J.; Muntifering, R.; Fangmeier, A. Effects of elevated CO2 on grain yield and quality of wheat: Results from a 3-year free-air CO2 enrichment experiment. Plant Biol. 2009, 11, 60–69. [Google Scholar] [CrossRef]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.; Fitzgerald, G.; Seneweera, S. Rising atmospheric CO2 concentration affects mineral nutrient and protein concentration of wheat grain. Food Chem. 2012, 133, 1307–1311. [Google Scholar] [CrossRef]
- Sánchez de La Puente, L.; Pérez, P.; Martínez-Carrasco, R.; Morcuende, R.; Martín del Molino, I.M. Action of elevated CO2 and high temperatures on the mineral chemical composition of two varieties of wheat. Agrochimica 2000, 44, 221–230. [Google Scholar]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Nuttall, J.G.; O’Leary, G.J.; Panozzo, J.F.; Walker, C.K.; Barlow, K.M.; Fitzgerald, G.J. Models of grain quality in wheat—A review. Filed Crop. Res. 2017, 202, 136–145. [Google Scholar] [CrossRef]
- Trethowan, R.M.; Reynolds, M.P.; Ortiz-Monasterio, J.I.; Ortiz, R. The genetic basis of the green revolution in wheat production. Plant Breed. Rev. 2007, 28, 39–58. [Google Scholar] [CrossRef]
- Fan, M.S.; Zhao, F.J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 2008, 22, 315–324. [Google Scholar] [CrossRef]
- Ercoli, L.; Lulli, L.; Arduini, I.; Mariotti, M.; Masoni, A. Durum wheat grain yield and quality as affected by S rate under Mediterranean conditions. Eur. J. Agron. 2011, 35, 63–70. [Google Scholar] [CrossRef]
- Högy, P.; Kottmann, L.; Schmid, I.; Fangmeier, A. Heat, wheat and CO2: The relevance of timing and the mode of temperature stress on biomass and yield. J. Agron. Crop Sci. 2019, 205, 608–615. [Google Scholar] [CrossRef]
- Pérez, P.; Alonso, A.; Zita, G.; Morcuende, R.; Martínez-Carrasco, R. Down-regulation of Rubisco activity under combined increases of CO2 and temperature minimized by changes in Rubisco kcat in wheat. Plant Growth Regul. 2011, 65, 439–447. [Google Scholar] [CrossRef]
- Vicente, R.; Bolger, A.M.; Martínez-Carrasco, R.; Pérez, P.; Gutiérrez, E.; Usadel, B.; Morcuende, R. De novo transcriptome analysis of durum wheat flag leaves provides new insights into the regulatory response to elevated CO2 and high temperature. Front. Plant Sci. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Cai, C.; Yin, X.; He, S.; Jiang, W.; Si, C.; Struik, P.C.; Luo, W.; Li, G.; Xie, Y.; Xiong, Y.; et al. Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Glob. Chang. Biol. 2016, 22, 856–874. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Z.; Jiang, D.; Högy, P.; Fangmeier, A. Independent and combined effects of elevated CO2 and post-anthesis heat stress on protein quantity and quality in spring wheat grains. Food Chem. 2019, 277, 524–530. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Chang. Biol. 2021, 27, 27–49. [Google Scholar] [CrossRef]
- Driever, S.M.; Lawson, T.; Andralojc, P.J.; Raines, C.A.; Parry, M.A.J. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. J. Exp. Bot. 2014, 65, 4959–4973. [Google Scholar] [CrossRef]
- Fangmeier, A.; De Temmerman, L.; Mortensen, L.; Kemp, K.; Burke, J.; Mitchell, R.; Van Oijen, M.; Weigel, H.J. Effects on nutrients and on grain quality in spring wheat crops grown under elevated CO2 concentrations and stress conditions in the European, multiple-site experiment “ESPACE-wheat”. Eur. J. Agron. 1999, 10, 215–229. [Google Scholar] [CrossRef]
- Högy, P.; Keck, M.; Niehaus, K.; Franzaring, J.; Fangmeier, A. Effects of atmospheric CO2 enrichment on biomass, yield and low molecular weight metabolites in wheat grain. J. Cereal Sci. 2010, 52, 215–220. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Araus, J.L.; Park, R.; Calderini, D.; Miralles, D.; Shen, T.; Zhang, J.; Parry, M.A.J. Prospects of doubling global wheat yields. Food Energy Secur. 2013, 2, 34–48. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.; Royo, C.; Aparicio, N.; Martín-Sánchez, J.A.; Álvaro, F. Genetic improvement of bread wheat yield and associated traits in Spain during the 20th century. J. Agric. Sci. 2013, 151, 105–118. [Google Scholar] [CrossRef]
- Sayre, K.D.; Rajaram, S.; Fischer, R.A. Yield potential progress in short bread wheats in northwest Mexico. Crop Sci. 1997, 37, 36–42. [Google Scholar] [CrossRef]
- Royo, C.; Martos, V.; Ramdani, A.; Villegas, D.; Rharrabti, Y.; García Del Moral, L.F. Changes in yield and carbon isotope discrimination of Italian and Spanish durum wheat during the 20th century. Agron. J. 2008, 100, 352–360. [Google Scholar] [CrossRef]
- Slafer, G.A. Genetic basis of yield as viewed from a crop physiologist’s perspective. Ann. Appl. Biol. 2003, 142, 117–128. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate impacts on agriculture: Implications for crop production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef]
- Weichert, H.; Högy, P.; Mora-Ramirez, I.; Fuchs, J.; Eggert, K.; Koehler, P.; Weschke, W.; Fangmeier, A.; Weber, H. Grain yield and quality responses of wheat expressing a barley sucrose transporter to combined climate change factors. J. Exp. Bot. 2017, 68, 5511–5525. [Google Scholar] [CrossRef]
- Chakrabarti, B.; Singh, S.D.; Kumar, V.; Harit, R.C.; Misra, S. Growth and yield response of wheat and chickpea crops under high temperature. Indian J. Plant Physiol. 2013, 18, 7–14. [Google Scholar] [CrossRef]
- Lizana, X.C.; Calderini, D.F. Yield and grain quality of wheat in response to increased temperatures at key periods for grain number and grain weight determination: Considerations for the climatic change scenarios of Chile. J. Agric. Sci. 2013, 151, 209–221. [Google Scholar] [CrossRef]
- Acreche, M.M.; Slafer, G.A. Grain weight response to increases in number of grains in wheat in a Mediterranean area. Field Crop. Res. 2006, 98, 52–59. [Google Scholar] [CrossRef]
- Sadras, V.O.; Slafer, G.A. Environmental modulation of yield components in cereals: Heritabilities reveal a hierarchy of phenotypic plasticities. Field Crop. Res. 2012, 127, 215–224. [Google Scholar] [CrossRef]
- Chatzav, M.; Peleg, Z.; Ozturk, L.; Yazici, A.; Fahima, T.; Cakmak, I.; Saranga, Y. Genetic diversity for grain nutrients in wild emmer wheat: Potential for wheat improvement. Ann. Bot. 2010, 105, 1211–1220. [Google Scholar] [CrossRef]
- Oury, F.X.; Leenhardt, F.; Rémésy, C.; Chanliaud, E.; Duperrier, B.; Balfourier, F.; Charmet, G. Genetic variability and stability of grain magnesium, zinc and iron concentrations in bread wheat. Eur. J. Agron. 2006, 25, 177–185. [Google Scholar] [CrossRef]
- Murphy, K.M.; Reeves, P.G.; Jones, S.S. Relationship between yield and mineral nutrient concentrations in historical and modern spring wheat cultivars. Euphytica 2008, 163, 381–390. [Google Scholar] [CrossRef]
- Pandey, A.; Khan, M.K.; Hakki, E.E.; Thomas, G.; Hamurcu, M.; Gezgin, S.; Gizlenci, O.; Akkaya, M.S. Assessment of genetic variability for grain nutrients from diverse regions: Potential for wheat improvement. Springerplus 2016, 5. [Google Scholar] [CrossRef]
- Gomez-Becerra, H.F.; Yazici, A.; Ozturk, L.; Budak, H.; Peleg, Z.; Morgounov, A.; Fahima, T.; Saranga, Y.; Cakmak, I. Genetic variation and environmental stability of grain mineral nutrient concentrations in Triticum dicoccoides under five environments. Euphytica 2010, 171, 39–52. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC gene regulating senescence improves grain protein, Zinc, and Iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef]
- Morgounov, A.; Gómez-Becerra, H.F.; Abugalieva, A.; Dzhunusova, M.; Yessimbekova, M.; Muminjanov, H.; Zelenskiy, Y.; Ozturk, L.; Cakmak, I. Iron and zinc grain density in common wheat grown in Central Asia. Euphytica 2007, 155, 193–203. [Google Scholar] [CrossRef]
- Peleg, Z.; Cakmak, I.; Ozturk, L.; Yazici, A.; Jun, Y.; Budak, H.; Korol, A.B.; Fahima, T.; Saranga, Y. Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat × wild emmer wheat RIL population. Theor. Appl. Genet. 2009, 119, 353–369. [Google Scholar] [CrossRef]
- Shi, R.; Li, H.; Tong, Y.; Jing, R.; Zhang, F.; Zou, C. Identification of quantitative trait locus of zinc and phosphorus density in wheat (Triticum aestivum L.) grain. Plant Soil 2008, 306, 95–104. [Google Scholar] [CrossRef]
- Distelfeld, A.; Cakmak, I.; Peleg, Z.; Ozturk, L.; Yazici, A.M.; Budak, H.; Saranga, Y.; Fahima, T. Multiple QTL-effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations. Physiol. Plant. 2007, 129, 635–643. [Google Scholar] [CrossRef]
- Stangoulis, J.C.R.; Huynh, B.L.; Welch, R.M.; Choi, E.Y.; Graham, R.D. Quantitative trait loci for phytate in rice grain and their relationship with grain micronutrient content. Euphytica 2007, 154, 289–294. [Google Scholar] [CrossRef]
- Ozkan, H.; Brandolini, A.; Torun, A.; AltIntas, S.; Eker, S.; Kilian, B.; Braun, H.J.; Salamini, F.; Cakmak, I. Natural variation and identification of microelements content in seeds of einkorn wheat (Triticum Monococcum). In Wheat Production in Stressed Environments. Developments in Plant Breeding; Buck, H.T., Nisi, J.E., Salomón, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 12, pp. 455–462. ISBN 978-1-4020-5497-6. [Google Scholar]
- Vreugdenhil, D.; Aarts, M.G.M.; Koornneef, M.; Nelissen, H.; Ernst, W.H.O. Natural variation and QTL analysis for cationic mineral content in seeds of Arabidopsis thaliana. Plant Cell Environ. 2004, 27, 828–839. [Google Scholar] [CrossRef]
- Hesse, H.; Nikiforova, V.; Gakière, B.; Hoefgen, R. Molecular analysis and control of cysteine biosynthesis: Integration of nitrogen and sulphur metabolism. J. Exp. Bot. 2004, 55, 1283–1292. [Google Scholar] [CrossRef]
- Bielecka, M.; Watanabe, M.; Morcuende, R.; Scheible, W.R.; Hawkesford, M.J.; Hesse, H.; Hoefgen, R. Transcriptome and metabolome analysis of plant sulfate starvation and resupply provides novel information on transcriptional regulation of metabolism associated with sulfur, nitrogen and phosphorus nutritional responses in Arabidopsis. Front. Plant Sci. 2015, 5, 1–18. [Google Scholar] [CrossRef]
- Del Molino, I.M.M.; Rojo, B.; Martínez-Carrasco, R.; Pérez, P. Amino acid composition of wheat grain. 1: Changes during development. J. Sci. Food Agric. 1988, 42, 29–37. [Google Scholar] [CrossRef]
- Dai, Z.; Plessis, A.; Vincent, J.; Duchateau, N.; Besson, A.; Dardevet, M.; Prodhomme, D.; Gibon, Y.; Hilbert, G.; Pailloux, M.; et al. Transcriptional and metabolic alternations rebalance wheat grain storage protein accumulation under variable nitrogen and sulfur supply. Plant J. 2015, 83, 326–343. [Google Scholar] [CrossRef]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic acids in wheat varieties in the healthgrain diversity screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Orozco, R.; Li, L.; Harflett, C.; Shewry, P.R.; Ward, J.L. Effects of environment and genotype on phenolic acids in wheat in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2010, 58, 9341–9352. [Google Scholar] [CrossRef] [PubMed]
- Bencze, S.; Makádi, M.; Aranyos, T.J.; Földi, M.; Hertelendy, P.; Mikó, P.; Bosi, S.; Negri, L.; Drexler, D. Re-introduction of ancient wheat cultivars into organic agriculture-Emmer and Einkorn cultivation experiences under marginal conditions. Sustainability 2020, 12, 1584. [Google Scholar] [CrossRef]
- Calderini, D.F.; Torres-León, S.; Slafer, G.A. Consequences of wheat breeding on nitrogen and phosphorus yield, grain nitrogen and phosphorus concentration and associated traits. Ann. Bot. 1995, 76, 315–322. [Google Scholar] [CrossRef]
- Shewry, P.R.; Pellny, T.K.; Lovegrove, A. Is modern wheat bad for health? Nat. Plants 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Pleijel, H.; Uddling, J. Yield vs. Quality trade-offs for wheat in response to carbon dioxide and ozone. Glob. Chang. Biol. 2012, 18, 596–605. [Google Scholar] [CrossRef]
- Asif, M.; Yilmaz, O.; Ozturk, L. Elevated carbon dioxide ameliorates the effect of Zn deficiency and terminal drought on wheat grain yield but compromises nutritional quality. Plant Soil 2017, 411, 57–67. [Google Scholar] [CrossRef]
- Pleijel, H.; Högy, P. CO2 dose-response functions for wheat grain, protein and mineral yield based on FACE and open-top chamber experiments. Environ. Pollut. 2015, 198, 70–77. [Google Scholar] [CrossRef]
- Wieser, H.; Manderscheid, R.; Erbs, M.; Weigel, H.J. Effects of elevated atmospheric CO2 concentrations on the quantitative protein composition of wheat grain. J. Agric. Food Chem. 2008, 56, 6531–6535. [Google Scholar] [CrossRef]
- Gourdji, S.M.; Mathews, K.L.; Reynolds, M.; Crossa, J.; Lobell, D.B. An assessment of wheat yield sensitivity and breeding gains in hot environments. Proc. R. Soc. B Biol. Sci. 2013, 280. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Gutiérrez, D.; Morcuende, R.; Verdejo, A.L.; Kostadinova, S.; Martínez-Carrasco, R.; Pérez, P. Changes in leaf morphology and composition with future increases in CO2 and temperature revisited: Wheat in field chambers. J. Plant Growth Regul. 2009, 28, 349–357. [Google Scholar] [CrossRef]
- Córdoba, J.; Molina-Cano, J.L.; Pérez, P.; Morcuende, R.; Moralejo, M.; Savé, R.; Martínez-Carrasco, R. Photosynthesis-dependent/independent control of stomatal responses to CO2 in mutant barley with surplus electron transport capacity and reduced SLAH3 anion channel transcript. Plant Sci. 2015, 239, 15–25. [Google Scholar] [CrossRef][Green Version]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Gutiérrez, E.; Morcuende, R. Nitrate supply and plant development influence nitrogen uptake and allocation under elevated CO2 in durum wheat grown hydroponically. Acta Physiol. Plant. 2015, 37. [Google Scholar] [CrossRef]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Usadel, B.; Kostadinova, S.; Morcuende, R. Quantitative RT—PCR platform to measure transcript levels of c and n metabolism-related genes in durum wheat: Transcript profiles in elevated [CO2] and high temperature at different levels of N supply. Plant Cell Physiol. 2015, 56, 1556–1573. [Google Scholar] [CrossRef]
- Morcuende, R.; Kostadinova, S.; Pérez, P.; Martín Del Molino, I.M.; Martínez-Carrasco, R. Nitrate is a negative signal for fructan synthesis, and the fructosyltransferase-inducing trehalose inhibits nitrogen and carbon assimilation in excised barley leaves. New Phytol. 2004, 161, 749–759. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “antioxidant power”: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests 2021. R Package Version 0.7.0. Available online: https://cran.r-project.org/package=rstatix (accessed on 1 April 2021).
- Pohlert, T. PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended 2020. R Package Version 1.9.0. Available online: https://CRAN.R-project.org/package=PMCMRplus (accessed on 1 April 2021).
- Vicente-Villardon, J.L. MultBiplotR: MULTivariate Analysis Using Biplots 2021. R Package Version 1.3.30. Available online: https://cran.r-project.org/package=MultBiplot (accessed on 1 April 2021).
- Lê, S.; Josse, J.; Husson, F. {FactoMineR}: A package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses 2020. R Package Version 1.0.7. Available online: https://cran.r-project.org/package=factoextra (accessed on 1 April 2021).
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research 2021. R Package Version 2.1.3. Available online: https://cran.r-project.org/package=psych (accessed on 1 April 2021).
- Shannon, P. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
| Genotype | Aboveground (g plant−1) | Stalk (g plant−1) | Chaff (g plant−1) | Grain Yield (g plant−1) | Grain Number (No. plant−1) | |||||||||||||||
| 8 | 19.78 | ± | 1.77 | b | 8.39 | ± | 0.52 | ab | 3.26 | ± | 0.33 | ab | 8.13 | ± | 1.27 | ac | 207.89 | ± | 35 | a |
| 23 | 20.61 | ± | 2.52 | ab | 8.18 | ± | 0.70 | ab | 3.14 | ± | 0.68 | ab | 9.30 | ± | 1.25 | abc | 229.82 | ± | 35 | a |
| 41 | 23.12 | ± | 1.24 | ab | 8.93 | ± | 0.56 | ab | 3.92 | ± | 0.35 | ab | 10.27 | ± | 0.95 | ab | 308.75 | ± | 64 | a |
| 43 | 24.41 | ± | 2.75 | ab | 9.40 | ± | 1.59 | ab | 4.18 | ± | 0.39 | a | 10.83 | ± | 1.08 | b | 281.74 | ± | 22.1 | a |
| 61 | 24.90 | ± | 2.08 | a | 10.44 | ± | 1.16 | a | 4.47 | ± | 0.65 | ab | 9.98 | ± | 0.73 | abc | 287.37 | ± | 41.8 | a |
| 74 | 20.78 | ± | 2.97 | ab | 9.30 | ± | 1.81 | ab | 2.77 | ± | 0.48 | b | 8.71 | ± | 1.54 | abc | 202.84 | ± | 39.2 | a |
| 76 | 21.42 | ± | 2.85 | ab | 9.64 | ± | 1.19 | ab | 3.51 | ± | 0.69 | ab | 8.27 | ± | 1.26 | ac | 205.46 | ± | 37.5 | a |
| 94 | 20.53 | ± | 2.11 | ab | 8.45 | ± | 1.49 | ab | 3.53 | ± | 0.38 | ab | 8.56 | ± | 0.59 | ac | 240.53 | ± | 29.9 | a |
| 95 | 22.25 | ± | 2.25 | ab | 9.18 | ± | 1.05 | ab | 3.60 | ± | 0.39 | ab | 9.48 | ± | 1.02 | abc | 230.08 | ± | 23.1 | a |
| 150 | 19.46 | ± | 2.52 | b | 7.71 | ± | 1.32 | b | 3.79 | ± | 0.80 | ab | 7.96 | ± | 0.59 | c | 220.05 | ± | 22.4 | a |
| Mean | 21.73 | ± | 2.80 | 8.96 | ± | 1.34 | 3.62 | ± | 0.68 | 9.15 | ± | 1.35 | 241.45 | ± | 49.30 | |||||
| p value | 0.005 | 0.044 | 0.008 * | 0.001 | 0.007 * | |||||||||||||||
| Ear number (No. plant−1) | Grain weight (mg grain−1) | GYE (g ear−1) | GNE (No. ear−1) | HI | ||||||||||||||||
| 8 | 6.15 | ± | 0.65 | a | 39.33 | ± | 3.79 | ab | 1.32 | ± | 0.11 | b | 33.63 | ± | 2.4 | c | 0.41 | ± | 0.03 | ab |
| 23 | 5.90 | ± | 1.07 | a | 40.58 | ± | 1.58 | ab | 1.59 | ± | 0.12 | abc | 39.14 | ± | 1.47 | abc | 0.45 | ± | 0.01 | a |
| 41 | 7.60 | ± | 1.27 | a | 34.03 | ± | 4.73 | ab | 1.37 | ± | 0.13 | ab | 40.43 | ± | 2.86 | ab | 0.44 | ± | 0.02 | ab |
| 43 | 6.35 | ± | 0.42 | a | 38.58 | ± | 4.35 | ab | 1.71 | ± | 0.16 | c | 44.59 | ± | 5.31 | a | 0.44 | ± | 0.03 | ab |
| 61 | 7.05 | ± | 0.74 | a | 35.16 | ± | 4.11 | ab | 1.42 | ± | 0.1 | abc | 40.70 | ± | 3.38 | a | 0.40 | ± | 0.03 | ab |
| 74 | 6.00 | ± | 0.85 | a | 43.09 | ± | 1.63 | a | 1.45 | ± | 0.14 | abc | 33.78 | ± | 3.83 | bc | 0.42 | ± | 0.05 | ab |
| 76 | 6.20 | ± | 1.46 | a | 40.49 | ± | 2.7 | ab | 1.37 | ± | 0.21 | ab | 33.65 | ± | 3.44 | c | 0.39 | ± | 0.02 | b |
| 94 | 5.90 | ± | 0.76 | a | 35.88 | ± | 3.53 | ab | 1.47 | ± | 0.18 | abc | 40.92 | ± | 3.7 | a | 0.42 | ± | 0.04 | ab |
| 95 | 5.85 | ± | 0.52 | a | 41.21 | ± | 1.96 | ab | 1.62 | ± | 0.05 | ac | 39.30 | ± | 0.87 | abc | 0.43 | ± | 0.01 | ab |
| 150 | 5.65 | ± | 0.72 | a | 36.30 | ± | 2.38 | b | 1.42 | ± | 0.15 | abc | 39.07 | ± | 1.84 | abc | 0.41 | ± | 0.03 | ab |
| Mean | 6.27 | ± | 1.00 | 38.46 | ± | 4.11 | 1.47 | ± | 0.18 | 38.52 | ± | 4.55 | 0.42 | ± | 0.03 | |||||
| p value | 0.176 * | 0.004 * | 0.001 | 7.37 × 10−6 | 0.001 * | |||||||||||||||
| GNE: grain number ear−1; GYE: grain yield ear−1; HI: harvest index. Each value is the mean ± standard deviation (SD) of five replicates (n = 5) for each genotype. Mean indicates the mean ± SD for each trait with all the genotypes and replicates (N = 50). The calculation of statistical significance (p value) is based on one-way analysis of variance (ANOVA) or Welch test (*). Within columns, numbers followed by the same letter indicate non-statistically significant differences at p < 0.05 as determined by post-hoc tests. |  | |||||||||||||||||||
| Genotype | Starch (µmol g−1) | TP (mg g−1) | TAC (µmol eq Trolox g−1) | TPhC (µmol eq Galic Ac. g−1) | ||||||||||||
| 8 | 3589.29 | ± | 213.82 | a | 94.93 | ± | 13.43 | ab | 1.19 | ± | 0.21 | ab | 6.23 | ± | 0.61 | ab |
| 23 | 3272.55 | ± | 197.88 | a | 86.23 | ± | 2.66 | a | 1.35 | ± | 0.12 | a | 6.51 | ± | 0.41 | a |
| 41 | 3356.48 | ± | 123.52 | a | 80.09 | ± | 11.35 | ab | 1.40 | ± | 0.20 | a | 6.25 | ± | 0.62 | a |
| 43 | 3661.72 | ± | 330.58 | a | 77.72 | ± | 8.43 | ab | 1.35 | ± | 0.15 | a | 6.26 | ± | 0.25 | a |
| 61 | 3515.85 | ± | 170.01 | a | 83.11 | ± | 12.12 | ab | 1.25 | ± | 0.18 | ab | 6.03 | ± | 0.49 | ab |
| 74 | 3507.61 | ± | 89.15 | a | 83.00 | ± | 6.00 | ab | 1.26 | ± | 0.17 | ab | 5.27 | ± | 0.33 | b |
| 76 | 3310.92 | ± | 108.57 | a | 81.47 | ± | 7.35 | ab | 1.30 | ± | 0.11 | a | 6.03 | ± | 0.55 | ab |
| 94 | 3448.82 | ± | 263.92 | a | 85.86 | ± | 17.54 | ab | 1.39 | ± | 0.18 | a | 6.10 | ± | 0.40 | ab |
| 95 | 3690.14 | ± | 330.48 | a | 74.79 | ± | 3.79 | b | 1.43 | ± | 0.09 | a | 6.29 | ± | 0.53 | a |
| 150 | 3446.49 | ± | 233.64 | a | 90.63 | ± | 7.29 | ab | 0.97 | ± | 0.10 | b | 5.82 | ± | 0.15 | ab |
| Mean | 3479.99 | ± | 241.88 | 83.78 | ± | 10.68 | 1.29 | ± | 0.19 | 6.08 | ± | 0.53 | ||||
| p value | 0.060 | 0.013 * | 0.002 | 0.013 | ||||||||||||
| TAC: total antioxidant capacity; TP: total protein; TPhC: total phenolic compounds. Each value is the mean ± standard deviation (SD) of five replicates (n = 5) for each genotype. Mean indicates the mean ± SD for each trait with all the genotypes and replicates (N = 50). The calculation of statistical significance (p value) is based on one-way analysis of variance (ANOVA) or Welch test (*). Within columns, numbers followed by the same letter indicate non-statistically significant differences at p < 0.05 as determined by post-hoc tests. |  | |||||||||||||||
| Genotype | B (µg g−1) | Ca (µg g−1) | Cu (µg g−1) | Fe (µg g−1) | K (µg g−1) | |||||||||||||||
| 8 | 2.14 | ± | 0.53 | a | 280.37 | ± | 23.24 | c | 6.92 | ± | 0.42 | a | 22.76 | ± | 2.95 | a | 3436.69 | ± | 203.07 | a |
| 23 | 1.67 | ± | 0.42 | a | 317.57 | ± | 33.71 | abc | 6.43 | ± | 0.83 | abc | 21.77 | ± | 2.12 | a | 3410.14 | ± | 98.87 | a |
| 41 | 1.56 | ± | 0.62 | a | 388.19 | ± | 32.76 | d | 6.56 | ± | 0.55 | ab | 21.21 | ± | 1.69 | a | 4097.08 | ± | 152.49 | b |
| 43 | 1.19 | ± | 0.33 | a | 330.20 | ± | 8.44 | abcd | 5.66 | ± | 0.38 | bc | 17.98 | ± | 2.28 | a | 3644.07 | ± | 240.32 | ac |
| 61 | 1.98 | ± | 0.34 | a | 347.96 | ± | 18.93 | abd | 6.36 | ± | 0.90 | abc | 19.57 | ± | 2.21 | a | 3921.67 | ± | 188.12 | bc |
| 74 | 1.53 | ± | 0.65 | a | 309.98 | ± | 37.66 | abc | 6.50 | ± | 0.61 | abc | 24.18 | ± | 8.14 | a | 3487.24 | ± | 190.11 | a |
| 76 | 1.27 | ± | 0.24 | a | 339.24 | ± | 48.76 | abcd | 5.30 | ± | 0.47 | c | 19.34 | ± | 1.11 | a | 3681.37 | ± | 167.47 | ac |
| 94 | 1.33 | ± | 0.35 | a | 357.77 | ± | 31.20 | abd | 6.35 | ± | 0.57 | abc | 21.36 | ± | 2.17 | a | 3393.57 | ± | 148.70 | a |
| 95 | 1.33 | ± | 0.24 | a | 364.81 | ± | 14.56 | bd | 5.90 | ± | 0.49 | abc | 19.37 | ± | 1.99 | a | 3716.26 | ± | 131.68 | ac |
| 150 | 1.70 | ± | 1.01 | a | 302.97 | ± | 17.26 | ac | 6.21 | ± | 0.20 | abc | 20.92 | ± | 1.97 | a | 3484.80 | ± | 163.20 | a |
| Mean | 1.57 | ± | 0.56 | 333.91 | ± | 40.58 | 6.22 | ± | 0.69 | 20.85 | ± | 3.43 | 3627.29 | ± | 273.80 | |||||
| p value | 0.049 * | 2.11 × 10−5 | 0.004 | 0.152 | 2.01 × 10−7 | |||||||||||||||
| Mg (µg g−1) | Na (µg g−1) | P (µg g−1) | S (µg g−1) | Zn (µg g−1) | ||||||||||||||||
| 8 | 1314.49 | ± | 48.84 | a | 14.37 | ± | 5.99 | a | 5488.15 | ± | 212.79 | ab | 33.97 | ± | 31.37 | a | 35.47 | ± | 3.04 | a |
| 23 | 1322.06 | ± | 82.92 | a | 12.42 | ± | 5.82 | a | 5359.80 | ± | 160.00 | ab | 41.83 | ± | 15.25 | a | 37.67 | ± | 2.28 | a |
| 41 | 1276.31 | ± | 63.32 | ab | 14.38 | ± | 9.53 | a | 5603.18 | ± | 143.59 | a | 101.52 | ± | 33.43 | b | 35.00 | ± | 3.44 | a |
| 43 | 1194.65 | ± | 69.17 | ab | 3.74 | ± | 1.56 | a | 5309.50 | ± | 235.44 | ab | 76.01 | ± | 26.39 | ab | 31.38 | ± | 3.55 | a |
| 61 | 1152.71 | ± | 17.78 | b | 13.22 | ± | 9.19 | a | 5447.82 | ± | 97.35 | ab | 77.37 | ± | 19.35 | ab | 34.06 | ± | 3.73 | a |
| 74 | 1303.19 | ± | 79.88 | ab | 7.44 | ± | 1.09 | a | 5388.58 | ± | 353.24 | ab | 74.24 | ± | 21.05 | ab | 38.67 | ± | 5.72 | a |
| 76 | 1176.51 | ± | 96.62 | ab | 18.06 | ± | 12.50 | a | 5312.23 | ± | 141.24 | ab | 76.60 | ± | 13.21 | ab | 32.68 | ± | 2.70 | a |
| 94 | 1295.18 | ± | 69.67 | ab | 12.44 | ± | 5.27 | a | 5284.17 | ± | 256.79 | ab | 66.56 | ± | 9.92 | ab | 37.79 | ± | 5.32 | a |
| 95 | 1232.46 | ± | 69.16 | ab | 9.94 | ± | 6.24 | a | 5424.47 | ± | 195.71 | ab | 90.37 | ± | 15.18 | b | 33.51 | ± | 2.27 | a |
| 150 | 1172.26 | ± | 110.51 | ab | 6.38 | ± | 1.90 | a | 5045.06 | ± | 305.33 | b | 64.56 | ± | 15.59 | ab | 37.31 | ± | 2.76 | a |
| Mean | 1243.98 | ± | 92.35 | 11.24 | ± | 7.48 | 5366.30 | ± | 246.71 | 70.30 | ± | 27.42 | 35.35 | ± | 4.06 | |||||
| p value | 0.001 | 0.006 * | 0.042 | 0.001 | 0.038 | |||||||||||||||
| Each value is the mean ± standard deviation (SD) of five replicates (n = 5) for each genotype. Mean indicates the mean ± SD for each trait with all the genotypes and replicates (N = 50). The calculation of statistical significance (p value) is based on one-way analysis of variance (ANOVA) or Welch test (*). Within columns, numbers followed by the same letter indicate non-statistically significant differences at p < 0.05 as determined by post-hoc tests. |  | |||||||||||||||||||
| Dim.1 | Dim.2 | ||||
|---|---|---|---|---|---|
| Traits | Corr. | Cos2 | Traits | Corr. | Cos2 |
| K | 0.72 | 0.52 | Chaff | 0.41 | 0.17 |
| Ca | 0.62 | 0.39 | B | 0.35 | 0.12 |
| Grain number | 0.59 | 0.35 | GNE | 0.27 | 0.07 |
| S | 0.59 | 0.35 | Grain number | 0.27 | 0.07 |
| Grain yield | 0.59 | 0.35 | Cu | 0.25 | 0.06 |
| Aboveground | 0.58 | 0.34 | TP | 0.25 | 0.06 |
| Chaff | 0.49 | 0.24 | Starch | 0.18 | 0.03 |
| GNE | 0.44 | 0.20 | TPhC | 0.14 | 0.02 |
| Ear number | 0.41 | 0.17 | K | 0.13 | 0.02 |
| TAC | 0.40 | 0.16 | Ear number | 0.12 | 0.02 |
| Stalk | 0.36 | 0.13 | Grain yield | 0.08 | 0.01 |
| P | 0.28 | 0.08 | Aboveground | 0.08 | 0.01 |
| GYE | 0.21 | 0.04 | HI | 0.03 | 0.00 |
| TPhC | 0.21 | 0.04 | P | −0.07 | 0.00 |
| HI | 0.15 | 0.02 | Zn | −0.08 | 0.01 |
| Starch | 0.14 | 0.02 | Fe | −0.09 | 0.01 |
| Na | 0.01 | 0.00 | GYE | −0.09 | 0.01 |
| B | −0.23 | 0.05 | Stalk | −0.13 | 0.02 |
| Grain weight | −0.25 | 0.06 | Na | −0.15 | 0.02 |
| Cu | −0.28 | 0.08 | S | −0.17 | 0.03 |
| Mg | −0.29 | 0.08 | Mg | −0.22 | 0.05 |
| Fe | −0.36 | 0.13 | Ca | −0.23 | 0.05 |
| Zn | −0.44 | 0.20 | Grain weight | −0.41 | 0.17 |
| TP | −0.45 | 0.20 | TAC | −0.42 | 0.17 |
| Corr.: correlation; Dim.: Dimension; GNE: grain number ear−1; GYE: grain yield ear−1; HI: harvest index; TAC: total antioxidant capacity; TP: total protein; TPhC: total phenolic compounds. Corr. indicates the correlation between the variable and the dimension. The squared correlation (Cos2) values between the variables and the dimensions are used to estimate the quality of the representation. | |||||
 | |||||
| Dim. 1 | Dim. 2 | ||||||
|---|---|---|---|---|---|---|---|
| Variable Groups | Corr. | Cos2 | Contr. | Variable Groups | Corr. | Cos2 | Contr. |
| Wheat production | 0.81 | 0.63 | 36.17 | Wheat production | 0.08 | 0.01 | 4.79 |
| Yield components | 0.37 | 0.10 | 16.35 | Yield components | 0.74 | 0.40 | 46.01 |
| Non-mineral nutrients | 0.39 | 0.08 | 17.49 | Non-mineral nutrients | 0.60 | 0.20 | 37.41 |
| Mineral nutrients | 0.67 | 0.22 | 29.99 | Mineral nutrients | 0.19 | 0.02 | 11.80 |
| Supplementary group | Supplementary group | ||||||
| Genotype | 0.55 | 0.03 | Genotype | 0.27 | 0.01 | ||
| Continuous variables | Continuous variables | ||||||
| Aboveground | 0.91 | 0.83 | 15.26 | Grain number | 0.79 | 0.62 | 10.91 |
| Chaff | 0.77 | 0.59 | 10.83 | TPhC | 0.72 | 0.52 | 20.05 |
| Grain yield | 0.77 | 0.59 | 7.43 | Ear number | 0.68 | 0.46 | 8.01 |
| Stalk | 0.74 | 0.55 | 10.07 | TP | 0.64 | 0.41 | 15.55 |
| Grain number | 0.53 | 0.28 | 3.49 | HI | 0.60 | 0.36 | 6.26 |
| S | 0.53 | 0.28 | 4.15 | Ca | 0.54 | 0.29 | 5.97 |
| Ear number | 0.45 | 0.20 | 2.59 | GNE | 0.42 | 0.18 | 3.15 |
| TAC | 0.45 | 0.20 | 5.57 | Grain yield | 0.42 | 0.18 | 3.11 |
| K | 0.38 | 0.15 | 2.20 | Chaff | 0.28 | 0.08 | 2.04 |
| GYE | 0.35 | 0.12 | 1.54 | P | 0.24 | 0.06 | 1.19 |
| Ca | 0.32 | 0.10 | 1.53 | Cu | 0.21 | 0.04 | 0.89 |
| GNE | 0.31 | 0.10 | 1.21 | Zn | 0.15 | 0.02 | 0.49 |
| Starch | 0.16 | 0.02 | 0.67 | K | 0.14 | 0.02 | 0.40 |
| Grain weight | 0.04 | 0.00 | 0.02 | Aboveground | 0.13 | 0.02 | 0.42 |
| TPhC | 0.02 | 0.00 | 0.01 | Na | 0.11 | 0.01 | 0.25 |
| Na | −0.02 | 0.00 | 0.01 | Fe | −0.05 | 0.00 | 0.06 |
| B | −0.03 | 0.00 | 0.01 | Starch | −0.13 | 0.02 | 0.65 |
| HI | −0.08 | 0.01 | 0.08 | B | −0.15 | 0.02 | 0.45 |
| P | −0.26 | 0.07 | 1.00 | TAC | −0.17 | 0.03 | 1.17 |
| Mg | −0.48 | 0.23 | 3.41 | Mg | −0.22 | 0.05 | 0.97 |
| Cu | −0.52 | 0.27 | 4.01 | S | −0.24 | 0.06 | 1.16 |
| Fe | −0.57 | 0.33 | 4.92 | Stalk | −0.30 | 0.09 | 2.33 |
| TP | −0.64 | 0.41 | 11.25 | GYE | −0.35 | 0.13 | 2.20 |
| Zn | −0.76 | 0.58 | 8.75 | Grain weight | −0.84 | 0.71 | 12.37 |
| Dim.: dimension; Contr.: contribution; Corr.: correlation; GNE: grain number ear−1; GYE: grain yield ear−1; HI: harvest index; TAC: total antioxidant capacity; TP: total protein; TPhC: total phenolic compounds. Genotype is the group based on a categorical variable specifying the genotypic identity of each sample. The vegetative biomass, grain yield and nutritional quality traits were split up into four groups: Wheat production (aboveground, stalk and chaff biomasses), Yield components (grain yield, grain number, ear number, grain weight, grain yield ear−1, grain number ear−1, and harvest index), Non-mineral nutrients (starch, total protein, total phenolic compound concentrations and total antioxidant capacity) and Mineral nutrients (B, Ca, Cu, Fe, K, Mg, Na, P, S, and Zn mineral concentrations). Corr. indicates the correlation between the variable and the dimension. The values for the squared correlation (Cos2) between the variables and the dimensions are used to estimate the quality of the representation. Contr. expresses the contributions, in percentage, of each variable in accounting for the variability in the dimension. | |||||||
 | |||||||
| Stalk | Chaff | Grain Yield | Grain Number | Ear Number | Grain Weight | GYE | GNE | HI | Starch | TP | TAC | TPhC | B | Ca | Cu | Fe | K | Mg | Na | P | S | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aboveground | 0.81 | 0.75 | 0.86 | 0.66 | 0.63 | 0.02 | 0.20 | 0.22 | −0.03 | 0.09 | −0.39 | 0.24 | 0.07 | 0.05 | 0.29 | −0.35 | −0.47 | 0.26 | −0.45 | −0.19 | −0.20 | 0.37 | −0.60 |
| Stalk | 0.50 | 0.46 | 0.19 | 0.33 | 0.34 | 0.19 | −0.08 | −0.50 | 0.13 | −0.62 | 0.29 | −0.21 | 0.13 | 0.06 | −0.31 | −0.32 | 0.32 | −0.28 | −0.13 | −0.13 | 0.44 | −0.54 | |
| Chaff | 0.61 | 0.64 | 0.55 | −0.34 | 0.01 | 0.30 | −0.13 | −0.09 | −0.26 | 0.05 | 0.16 | 0.04 | 0.24 | −0.30 | −0.50 | 0.28 | −0.59 | −0.14 | −0.26 | 0.38 | −0.50 | ||
| Grain yield | 0.84 | 0.69 | −0.11 | 0.24 | 0.37 | 0.44 | 0.08 | −0.15 | 0.27 | 0.26 | −0.06 | 0.42 | −0.30 | −0.47 | 0.11 | −0.34 | −0.16 | −0.14 | 0.19 | −0.49 | |||
| Grain number | 0.75 | −0.56 | −0.05 | 0.55 | 0.50 | 0.01 | 0.14 | 0.05 | 0.40 | −0.09 | 0.48 | −0.18 | −0.38 | 0.14 | −0.42 | −0.16 | −0.07 | 0.07 | −0.31 | ||||
| Ear number | −0.41 | −0.48 | −0.05 | 0.22 | −0.13 | 0.26 | −0.01 | 0.28 | 0.03 | 0.31 | −0.14 | −0.36 | 0.16 | −0.35 | 0.05 | 0.05 | 0.13 | −0.29 | |||||
| Grain weight | 0.51 | −0.42 | −0.28 | 0.13 | −0.51 | 0.24 | −0.41 | 0.05 | −0.33 | −0.13 | 0.06 | −0.15 | 0.32 | 0.04 | −0.09 | 0.17 | −0.11 | ||||||
| GYE | 0.46 | 0.13 | 0.22 | −0.59 | 0.37 | −0.06 | −0.04 | −0.02 | −0.27 | −0.16 | −0.12 | 0.01 | −0.26 | −0.31 | 0.10 | −0.25 | |||||||
| GNE | 0.43 | 0.09 | −0.08 | 0.13 | 0.30 | −0.08 | 0.33 | −0.05 | −0.08 | 0.14 | −0.20 | −0.32 | −0.10 | 0.00 | 0.00 | ||||||||
| HI | −0.01 | 0.42 | 0.10 | 0.35 | −0.27 | 0.31 | 0.07 | −0.03 | −0.21 | 0.13 | −0.11 | 0.09 | −0.27 | 0.14 | |||||||||
| Starch | −0.10 | 0.05 | −0.05 | −0.05 | −0.25 | −0.03 | −0.19 | −0.07 | −0.06 | −0.12 | −0.17 | −0.13 | −0.32 | ||||||||||
| TP | −0.55 | 0.30 | −0.03 | −0.10 | 0.32 | 0.25 | −0.35 | 0.01 | 0.12 | 0.11 | −0.56 | 0.46 | |||||||||||
| TAC | 0.02 | −0.04 | 0.28 | −0.16 | −0.28 | 0.17 | 0.21 | 0.07 | 0.01 | 0.35 | −0.35 | ||||||||||||
| TPhC | −0.08 | 0.27 | 0.15 | 0.02 | 0.04 | −0.12 | 0.07 | 0.15 | −0.26 | 0.01 | |||||||||||||
| B | −0.25 | 0.31 | 0.21 | 0.18 | −0.03 | 0.27 | 0.18 | 0.19 | 0.09 | ||||||||||||||
| Ca | −0.16 | −0.14 | 0.37 | −0.08 | −0.01 | 0.24 | 0.26 | −0.07 | |||||||||||||||
| Cu | 0.76 | 0.08 | 0.47 | 0.24 | 0.48 | −0.20 | 0.65 | ||||||||||||||||
| Fe | 0.11 | 0.50 | 0.25 | 0.53 | −0.19 | 0.78 | |||||||||||||||||
| K | −0.16 | 0.05 | 0.59 | 0.57 | −0.05 | ||||||||||||||||||
| Mg | 0.10 | 0.46 | −0.18 | 0.45 | |||||||||||||||||||
| Na | 0.13 | 0.02 | 0.13 | ||||||||||||||||||||
| P | 0.14 | 0.44 | |||||||||||||||||||||
| S | −0.24 | ||||||||||||||||||||||
| GNE: grain number ear−1; GYE: grain yield ear−1; HI: harvest index; TAC: total antioxidant capacity; TP: total protein; TPhC: total phenolic compounds. Data were generated from Spearman correlation analysis. Values in bold represent signification (p < 0.05). |  | ||||||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos-Barbero, E.L.; Pérez, P.; Martínez-Carrasco, R.; Arellano, J.B.; Morcuende, R. Genotypic Variability on Grain Yield and Grain Nutritional Quality Characteristics of Wheat Grown under Elevated CO2 and High Temperature. Plants 2021, 10, 1043. https://doi.org/10.3390/plants10061043
Marcos-Barbero EL, Pérez P, Martínez-Carrasco R, Arellano JB, Morcuende R. Genotypic Variability on Grain Yield and Grain Nutritional Quality Characteristics of Wheat Grown under Elevated CO2 and High Temperature. Plants. 2021; 10(6):1043. https://doi.org/10.3390/plants10061043
Chicago/Turabian StyleMarcos-Barbero, Emilio L., Pilar Pérez, Rafael Martínez-Carrasco, Juan B. Arellano, and Rosa Morcuende. 2021. "Genotypic Variability on Grain Yield and Grain Nutritional Quality Characteristics of Wheat Grown under Elevated CO2 and High Temperature" Plants 10, no. 6: 1043. https://doi.org/10.3390/plants10061043
APA StyleMarcos-Barbero, E. L., Pérez, P., Martínez-Carrasco, R., Arellano, J. B., & Morcuende, R. (2021). Genotypic Variability on Grain Yield and Grain Nutritional Quality Characteristics of Wheat Grown under Elevated CO2 and High Temperature. Plants, 10(6), 1043. https://doi.org/10.3390/plants10061043







