Combining Genetic and Multidimensional Analyses to Identify Interpretive Traits Related to Water Shortage Tolerance as an Indirect Selection Tool for Detecting Genotypes of Drought Tolerance in Wheat Breeding
Abstract
1. Introduction
2. Material and Methods
2.1. Plants and Experimental Design
2.2. Measurements of Morpho-Physiological and Agronomic Traits and Data Collection
2.2.1. Leaf Water Status Parameters
2.2.2. Photosynthetic Parameters
2.2.3. Morphological Parameters
2.2.4. Agronomic and Yield Traits Measurements
2.2.5. Statistical Analysis
3. Results
3.1. Phenotypic Variability of Measured Traits Across Seasons and Genotypes
3.2. Estimation of Genetic Parameters for Measured Traits
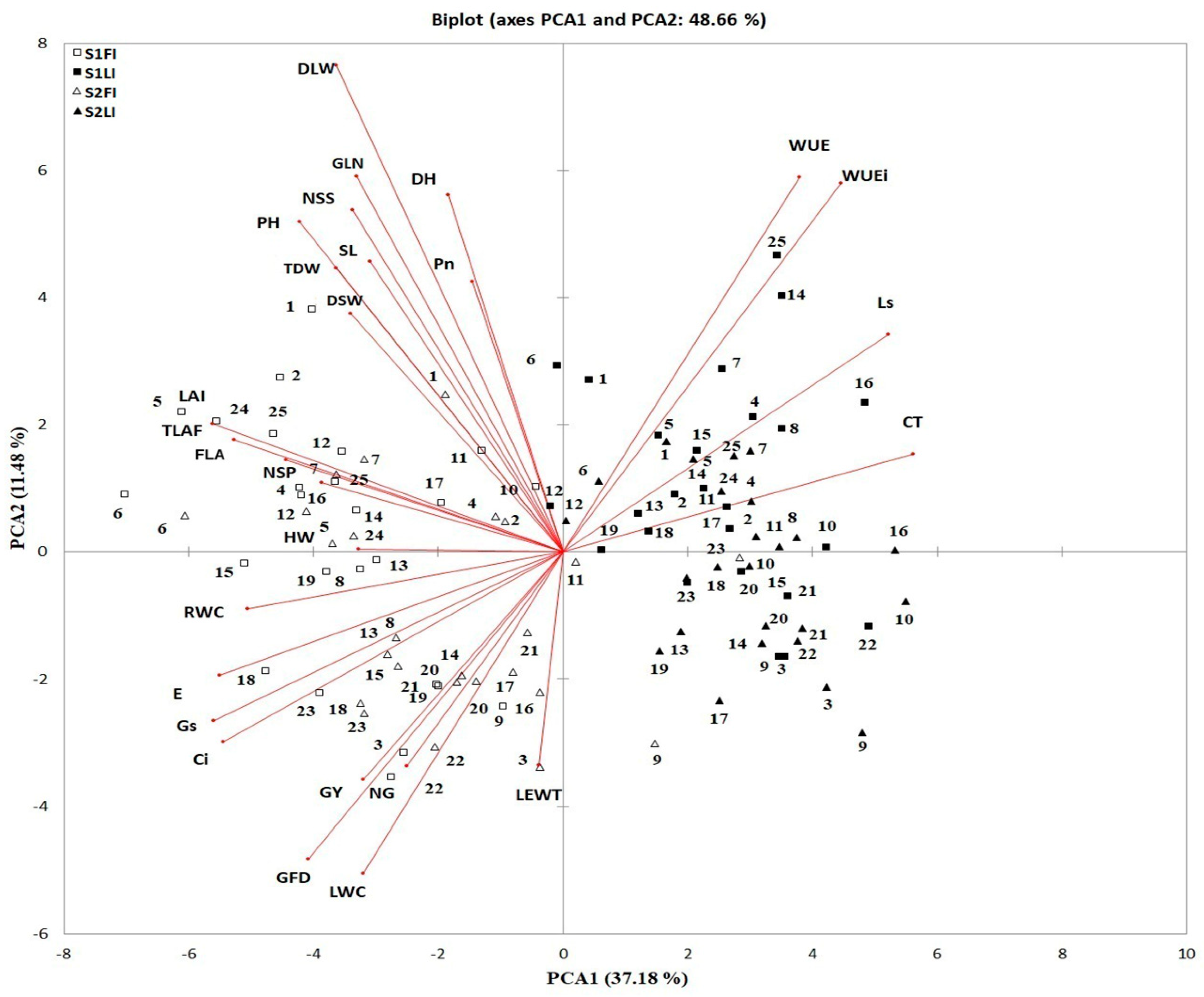
3.3. Multicolinearity and Principal Component Analysis
3.4. Identification of Traits Related to Yield Performance
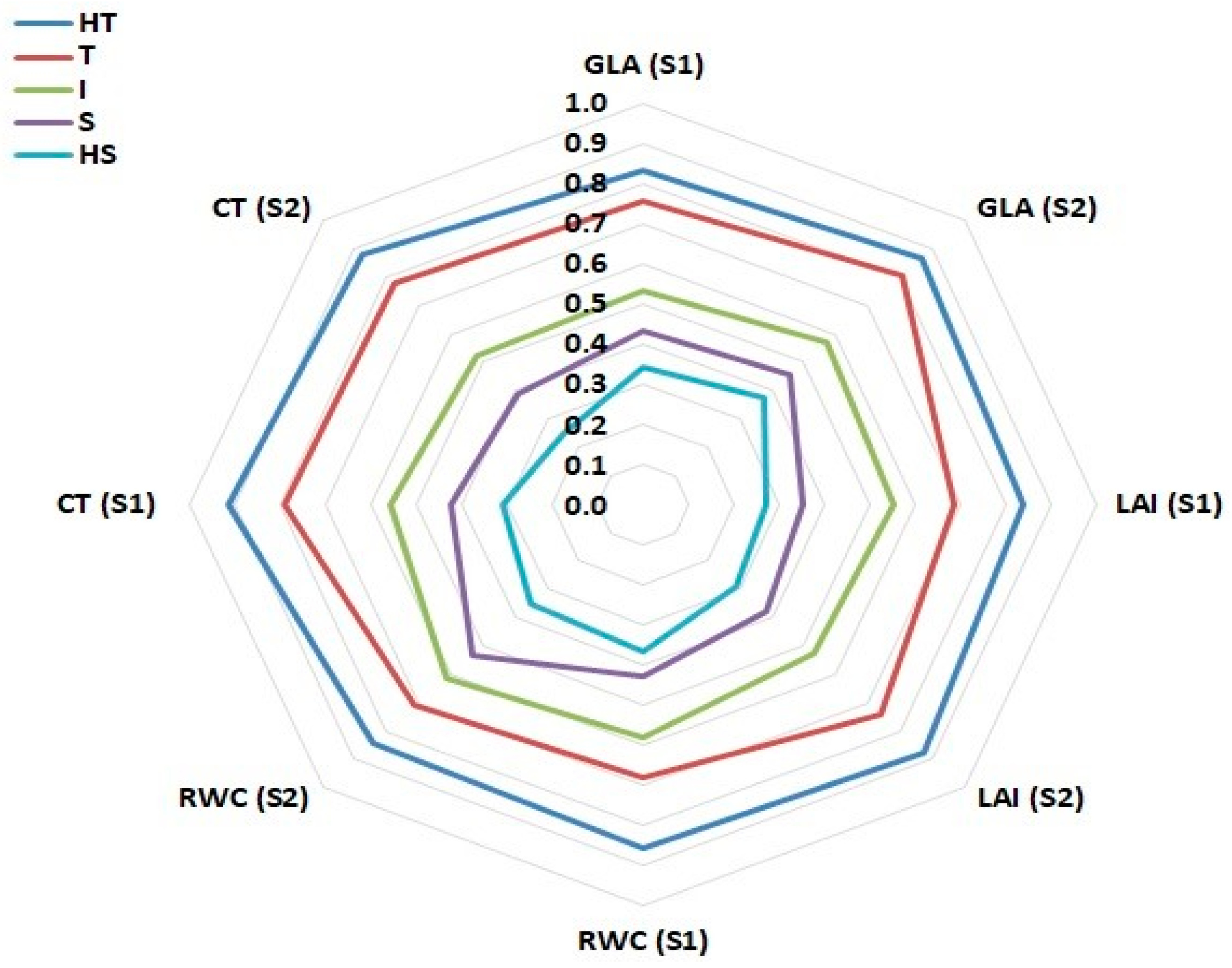
3.5. Classification of Drought-Tolerance of Twenty-Five Wheat Genotypes
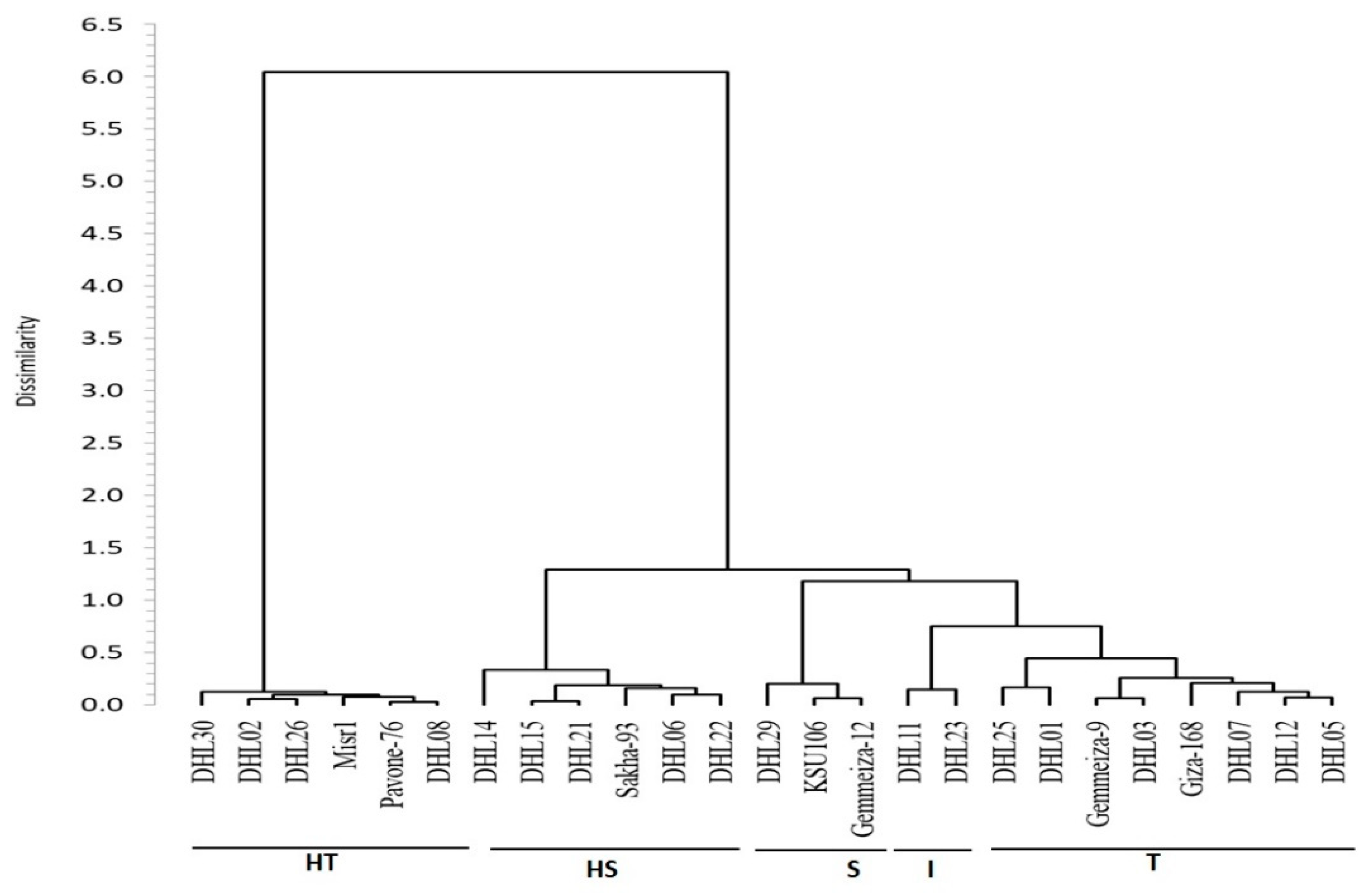
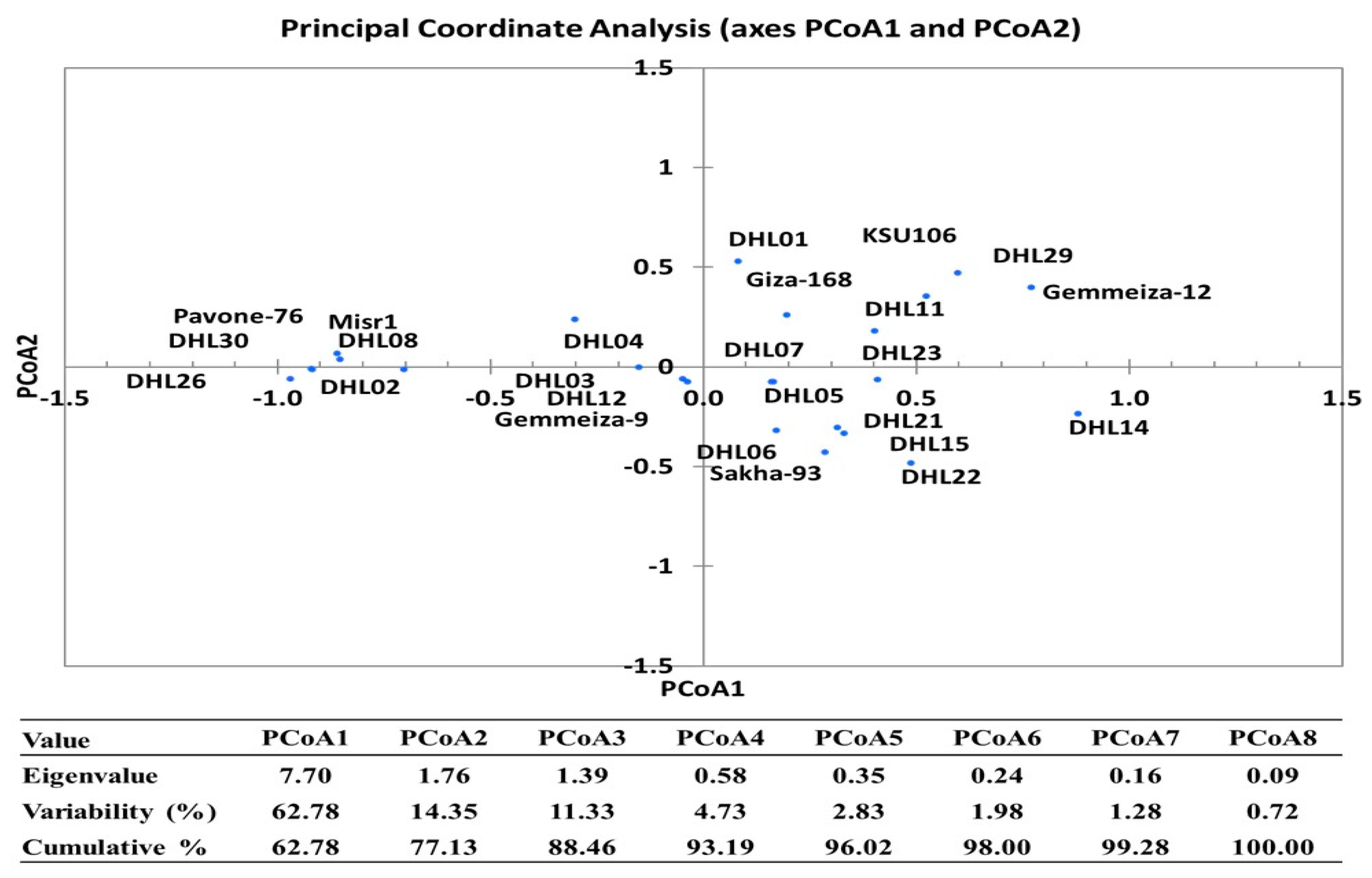
3.6. Clustering and Genetic Relationships between the Genotypes for Drought Tolerance
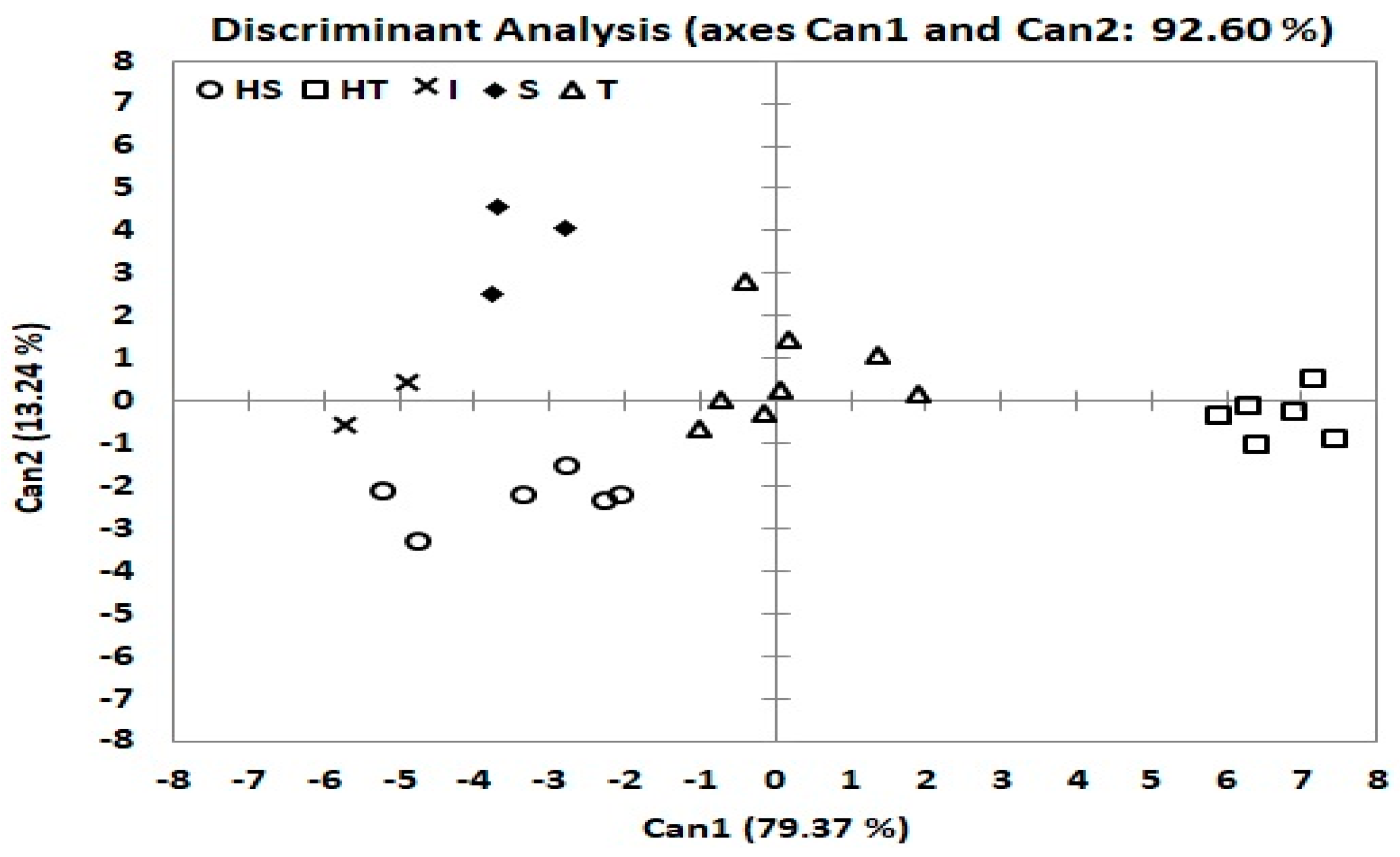
3.7. Differentiation of Drought Groups by Discriminant Function Analysis and MANOVA
3.8. Phenotypic Variation Among Drought Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hafez, E.M.; Seleiman, M.F. Response of barley quality traits, yield and antioxidant enzymes to water-stress and chemical inducers. Int. J. Plant. Prod. 2017, 11, 477–490. [Google Scholar]
- Ding, Z.; Ali, E.F.; Elmahdy, A.M.; Ragab, K.E.; Seleiman, M.F.; Kheir, A.M. Modeling the combined impacts of deficit irrigation, rising temperature and compost application on wheat yield and water productivity. Agric. Water Manag. 2021, 244, 106626. [Google Scholar] [CrossRef]
- Seleiman, M.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.; Battaglia, M. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2007, 58, 147–159. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Al-Suhaibani, N.; Al-Ashkar, I.; Alotaibi, M.; Tahir, M.U.; Solieman, T.; Hassan, W.M. Combining genetic analysis and multivariate modeling to evaluate spectral reflectance indices as indirect selection tools in wheat breeding under water deficit stress Conditions. Remote Sens. 2020, 12, 1480. [Google Scholar] [CrossRef]
- Abdolshahi, R.; Nazari, M.; Safarian, A.; Sadathossini, T.; Salarpour, M.; Amiri, H. Integrated selection criteria for drought tolerance in wheat (Triticum aestivum L.) breeding programs using discriminant analysis. Field Crop. Res. 2015, 174, 20–29. [Google Scholar] [CrossRef]
- Cattivelli, L.; Rizza, F.; Badeck, F.-W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Marè, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crop. Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Golabadi, M.; Arzani, A.; Maibody, S.M. Assessment of drought tolerance in segregating populations in durum wheat. Afr. J. Agric. Res. 2006, 1, 162–171. [Google Scholar]
- Mollasadeghi, V. Evaluation of drought tolerance indices bread wheat genotypes in end-season drought stress conditions. J. Biol. 2012, 1, 99–107. [Google Scholar] [CrossRef]
- Abdolshahi, R.; Safarian, A.; Nazari, M.; Pourseyedi, S.; Mohamadi-Nejad, G. Screening drought-tolerant genotypes in bread wheat (Triticum aestivum L.) using different multivariate methods. Arch. Agron. Soil Sci. 2013, 59, 685–704. [Google Scholar] [CrossRef]
- Barakat, M.; Al-Doss, A.; El-Hendawy, S.; Al-Suhaibani, N.; Abdella, K.; Al-Ashkar, I. Deciphering novel QTL for spectral reflectance indices in spring wheat. Cereal Res. Commun. 2021, 1–13. [Google Scholar] [CrossRef]
- Araus, J.; Slafer, G.; Reynolds, M.; Royo, C. Plant breeding and drought in C3 cereals: What should we breed for? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Edmeades, G.; McMaster, G.; White, J.; Campos, H. Genomics and the physiologist: Bridging the gap between genes and crop response. Field Crop. Res. 2004, 90, 5–18. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. Field Crop. Res. 1996, 48, 65–80. [Google Scholar] [CrossRef]
- Bänzinger, M. Breeding for Drought and Nitrogen Stress Tolerance in Maize: From Theory to Practice; CIMMYT: Mexico City, Mexico, 2000. [Google Scholar]
- Condon, A.G.; Richards, R.; Rebetzke, G.; Farquhar, G. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef]
- Reynolds, M.; Manes, Y.; Izanloo, A.; Langridge, P. Phenotyping approaches for physiological breeding and gene discovery in wheat. Ann. Appl. Biol. 2009, 155, 309–320. [Google Scholar] [CrossRef]
- Evans, R.; Skaggs, R.; Sneed, R. Stress day index models to predict corn and soybean relative yield under high water table conditions. Trans. ASAE 1991, 34, 1997–2005. [Google Scholar] [CrossRef]
- Grzesiak, S.; Hordyńska, N.; Szczyrek, P.; Grzesiak, M.T.; Noga, A.; Szechyńska-Hebda, M. Variation among wheat (Triticum easativum L.) genotypes in response to the drought stress: I–selection approaches. J. Plant Interact. 2019, 14, 30–44. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alderfasi, A.; Ben Romdhane, W.; Seleiman, M.F.; El-Said, R.A.; Al-Doss, A. Morphological and genetic diversity within salt tolerance detection in eighteen wheat genotypes. Plants 2020, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Manickavelu, A.; Nadarajan, N.; Ganesh, S.; Gnanamalar, R.; Babu, R.C. Drought tolerance in rice: Morphological and molecular genetic consideration. Plant Growth Regul. 2006, 50, 121–138. [Google Scholar] [CrossRef]
- Al-Ashkar, I.M.; Zaazaa, E.I.; El Sabagh, A.; Barutcular, C. Physio-Biochemical and molecular characterization for drought tolerance in rice genotypes at early seedling stage. J. Exp. Biol. Agric. Sci. 2016, 4, 675–687. [Google Scholar] [CrossRef]
- Barakat, M.; El-Hendawy, S.; Al-Suhaibani, N.; Elshafei, A.; Al-Doss, A.; Al-Ashkar, I.; Ahmed, E.; Al-Gaadi, K. The genetic basis of spectral reflectance indices in drought-stressed wheat. Acta Physiol. Plant. 2016, 38, 1–11. [Google Scholar] [CrossRef]
- Cano, F.J.; López, R.; Warren, C.R. Implications of the mesophyll conductance to CO2 for photosynthesis and water-use efficiency during long-term water stress and recovery in two contrasting E ucalyptus species. Plant Cell Environ. 2014, 37, 2470–2490. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.-B.; Chu, L.-Y.; Jaleel, C.A.; Manivannan, P.; Panneerselvam, R.; Shao, M.-A. Understanding water deficit stress-induced changes in the basic metabolism of higher plants–biotechnologically and sustainably improving agriculture and the ecoenvironment in arid regions of the globe. Crit. Rev. Biotechnol. 2009, 29, 131–151. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- Olsovska, K.; Kovar, M.; Brestic, M.; Zivcak, M.; Slamka, P.; Shao, H.B. Genotypically identifying wheat mesophyll conductance regulation under progressive drought stress. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Aranda, I.; Gil-Pelegrín, E.; Gascó, A.; Guevara, M.; Cano, J.; De Miguel, M.; Ramírez-Valiente, J.; Peguero-Pina, J.; Perdiguero, P.; Soto, A. Drought response in forest trees: From the species to the gene. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 293–333. [Google Scholar]
- Mason, R.E.; Singh, R.P. Considerations when deploying canopy temperature to select high yielding wheat breeding lines under drought and heat stress. Agronomy 2014, 4, 191–201. [Google Scholar] [CrossRef]
- Pask, A.; Pietragalla, J.; Mullan, D.; Reynolds, M. Physiological Breeding II: A Field Guide to Wheat Phenotyping; CIMMYT: Mexico City, Mexico, 2012. [Google Scholar]
- Rashid, A.; Stark, J.; Tanveer, A.; Mustafa, T. Use of canopy temperature measurements as a screening tool for drought tolerance in spring wheat. J. Agron. Crop Sci. 1999, 182, 231–238. [Google Scholar] [CrossRef]
- Bahar, B.; Yildirim, M.; Barutcular, C.; Ibrahim, G. Effect of canopy temperature depression on grain yield and yield components in bread and durum wheat. Not. Bot. Horti Agrobot. Cluj Napoca 2008, 36, 34–37. [Google Scholar]
- Al-Ashkar, I.; Romdhane, W.B.; El-Said, R.A.; Ghazy, A.; Attia, K.; Al-Doss, A. Agro-Physiologic responses and stress-related gene expression of four doubled haploid wheat lines under salinity stress conditions. Biology 2021, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K. Breeding Rice for Drought-Prone Environments; International Rice Research Institute: Los Baños, Philippines, 2003; Volume 1. [Google Scholar]
- Dadshani, S.; Sharma, R.C.; Baum, M.; Ogbonnaya, F.C.; Léon, J.; Ballvora, A. Multi-dimensional evaluation of response to salt stress in wheat. PLoS ONE 2019, 14, e0222659. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alderfasi, A.; El-Hendawy, S.; Al-Suhaibani, N.; El-Kafafi, S.; Seleiman, M.F. Detecting salt tolerance in doubled haploid wheat lines. Agronomy 2019, 9, 211. [Google Scholar] [CrossRef]
- Chakraborty, K.; Mondal, S.; Ray, S.; Samal, P.; Pradhan, B.; Chattopadhyay, K.; Kar, M.K.; Swain, P.; Sarkar, R.K. Tissue tolerance coupled with ionic discrimination can potentially minimize the energy cost of salinity tolerance in rice. Front. Plant Sci. 2020, 11, 265. [Google Scholar] [CrossRef]
- El-Hennawy, M.A.; Abdalla, A.F.; Shafey, S.A.; Al-Ashkar, I.M. Production of doubled haploid wheat lines (Triticum aestivum L.) using anther culture technique. Ann. Agric. Sci. 2011, 56, 63–72. [Google Scholar] [CrossRef]
- Clarke, J.M.; McCaig, T.N. Excised-leaf water retention capability as an indicator of drought resistance of Triticum genotypes. Can. J. Plant Sci. 1982, 62, 571–578. [Google Scholar] [CrossRef]
- Barrs, H. Determination of water deficits in plant tissues. Water Deficit Plant Growth 1968, 1, 235–368. [Google Scholar]
- Bowyer, P.; Danson, F.M. Sensitivity of spectral reflectance to variation in live fuel moisture content at leaf and canopy level. Remote Sens. Environ. 2004, 92, 297–308. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Shangguan, Z. Leaf gas exchange and fluorescence of two winter wheat varieties in response to drought stress and nitrogen supply. PLoS ONE 2016, 11, e0165733. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Fehr, W. Principles of Cultivar Development: Theory and Technique; Macmillian Publishing Company: New York, NY, USA, 1991. [Google Scholar]
- Liu, G.; Gai, J.; Ma, Y. Evaluation of drought tolerance of soybean germplasm from lower Yangtze and Huai Valleys. J. Nanjing Agric. Univ. 1989, 12, 15–21. [Google Scholar]
- Truxillo, C. Multivariate Statistical Methods: Practical Research Applications; SAS Institute: Cary, NC, USA, 2003; ISBN 159994586X. [Google Scholar]
- Edmeades, G.O.; Bolanos, J.; Chapman, S.C.; Lafitte, H.R.; Banziger, M. Selection improves drought tolerance in tropical maize populations: I. Gains in biomass, grain yield, and harvest index. Crop Sci. 1999, 39, 1306–1315. [Google Scholar] [CrossRef]
- Bustos-Korts, D.; Boer, M.P.; Malosetti, M.; Chapman, S.; Chenu, K.; Zheng, B.; van Eeuwijk, F.A. Combining crop growth modeling and statistical genetic modeling to evaluate phenotyping strategies. Front. Plant Sci. 2019, 10, 1491. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P. Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. J. Exp. Bot. 2012, 63, 3789–3798. [Google Scholar] [CrossRef] [PubMed]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Falconer, D.; Mackay, T.; Bulmer, M. Introduction to Quantitative Genetics. Genet. Res. 1996, 68, 183. [Google Scholar]
- Obala, J.; Saxena, R.; Singh, V.; Vechalapu, S.; Das, R.R.; Rathore, A.; Kumar, C.S.; Saxena, K.; Tongoona, P.; Sibiya, J.; et al. Genetic variation and relationships of total seed protein content with some agronomic traits in pigeonpea (Cajanus cajan (L.) Millsp.). Aust. J. Crop Sci. 2018, 12, 1859–1865. [Google Scholar] [CrossRef]
- Bidhan, R.; Bhadra, S. Effects of toxic levels of aluminium on seedling parameters of rice under hydroponic culture. Rice Sci. 2014, 21, 217–223. [Google Scholar]
- Masole, H.; Gumbo, M. Performance of early to medium maturity maize genotypes during the 1991–1992 drought in Zambia. In Proceedings of the Maize Research for Stress Environments: Fourth Eastern and Southern African Regional Maize Conference, Harare, Zimbabwe, 28 March 1994; pp. 117–121. [Google Scholar]
- Cruz, C.; Regazzi, A.; Carneiro, P.J.V.U. Modelos Biométricos Aplicados ao Melhoramento Genético, 5th ed.; UFV Francisco São José: Viçosa, Brazil, 2012. [Google Scholar]
- Olivoto, T.; de Souza, V.Q.; Nardino, M.; Carvalho, I.R.; Ferrari, M.; de Pelegrin, A.J.; Szareski, V.J.; Schmidt, D. Multicollinearity in path analysis: A simple method to reduce its effects. Agron. J. 2017, 109, 131–142. [Google Scholar] [CrossRef]
- Khan, A.S.; Ashfaq, M.; Asad, M.A. A correlation and path coefficient analysis for some yield components in bread wheat. Asian J. Plant Sci. 2003, 2, 582–584. [Google Scholar]
- Al-Ashkar, I.; Alotaibi, M.; Refay, Y.; Ghazy, A.; Zakri, A.; Al-Doss, A. Selection criteria for high-yielding and early-flowering bread wheat hybrids under heat stress. PLoS ONE 2020, 15, e0236351. [Google Scholar] [CrossRef]
- Del Moral, L.; Rharrabti, Y.; Villegas, D.; Royo, C. Evaluation of grain yield and its components in durum wheat under Mediterranean conditions. Agron. J. 2003, 95, 266–274. [Google Scholar] [CrossRef]
- Pržulj, N.; Momcilovic, V. Characterization of vegetative and grain filling periods of winter wheat by stepwise regression procedure. I. Vegetative period. Genetika 2011, 43, 349–359. [Google Scholar] [CrossRef]
- Soleymanifard, A.; Naseri, R.; Meysam, M. The study genetic variation and factor analysis for agronomic traits of Durum wheat genotypes using cluster analysis and path analysis under drought stress condition in western of Iran. Int. Res. J. Appl. Basic Sci. 2012, 3, 479–485. [Google Scholar]
- Bojarian, M.; Asadi-Gharneh, H.A.; Golabadi, M. Factor analysis, stepwise regression and path coefficient analyses of yield, yield-associated traits, and fruit quality in tomato. Int. J. Veg. Sci. 2019, 25, 542–553. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Rattey, A.R.; Farquhar, G.D.; Richards, R.A.; Condon, A.T.G. Genomic regions for canopy temperature and their genetic association with stomatal conductance and grain yield in wheat. Funct. Plant Biol. 2013, 40, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.P.; Pierre, C.S.; Saad, A.S.; Vargas, M.; Condon, A.G. Evaluating potential genetic gains in wheat associated with stress-adaptive trait expression in elite genetic resources under drought and heat stress. Crop Sci. 2007, 47, S-172–S-189. [Google Scholar] [CrossRef]
- Gautam, A.; Prasad, S.S.; Jajoo, A.; Ambati, D. Canopy temperature as a selection parameter for grain yield and its components in durum wheat under terminal heat stress in late sown conditions. Agric. Res. 2015, 4, 238–244. [Google Scholar] [CrossRef]
- Reynolds, M.; Pask, A.; Mullan, D. Physiological Breeding I: Interdisciplinary Approaches to Improve Crop Adaptation; CIMMYT: Mexico City, Mexico, 2012. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.L.; Moore, P.P.; Shanks, C.H.; Gil, F.F.; Chandler, C.K. Genotype × environment interaction for resistance to spider mites in Fragaria. J. Am. Soc. Hortic. Sci. 1999, 124, 353–357. [Google Scholar] [CrossRef]
- Sabaghnia, N.; Mohammadi, M.; Karimizadeh, R. Principal coordinate analysis of genotype × environment interaction for grain yield of bread wheat in the semi-arid regions. Genetika 2013, 45, 691–701. [Google Scholar] [CrossRef][Green Version]
| Source | DF | LWC | RWC | CT | LEWT | Pn | Gs | Ci | E | WUE | |
| S | 1 | 302.284 ** | 68.392* | 0.589 ** | 0.00010 ** | 37.002 ** | 0.0047 ** | 16593.14 ** | 2.869 ** | 5.763 ** | |
| rep(S) | 4 | 7.271 | 15.656 | 0.121 | 0 | 0.098 | 0.0001 | 1739.11 ** | 0.087 * | 0.049 | |
| I | 1 | 1095.88 ** | 3711.21 ** | 516.17 ** | 0.00007 ** | 2.869* | 0.1647 ** | 125277.99 ** | 30.033 ** | 30.630 ** | |
| S*I | 1 | 0.301 | 184.836 ** | 5.057 ** | 0.00013 ** | 0.029 | 0.0007 ** | 320.974 | 0.019 | 1.109 ** | |
| rep(I*S) | 4 | 2.992 | 6.914 | 0.107 | 0 | 0.287 | 0.0005 | 794.38 | 0.035 | 0.018 | |
| G | 24 | 82.303 ** | 124.998 ** | 2.985 ** | 0.00026 ** | 24.174 ** | 0.0074 ** | 4028.503 ** | 1.400 ** | 2.856 ** | |
| S*G | 24 | 87.854 ** | 89.304 ** | 0.793 ** | 0.00002 ** | 0.414 | 0.0003 ** | 402.104 * | 0.072 ** | 0.769 ** | |
| I*G | 24 | 19.435 ** | 20.881 ** | 1.692 ** | 0.00003 ** | 4.500 ** | 0.0000 ** | 2244.551 ** | 0.835 ** | 1.163 ** | |
| S*I*G | 24 | 23.334 ** | 38.558 ** | 1.014 ** | 0.00002 ** | 0.443 | 0.0049 ** | 212.418 | 0.052 | 0.655 ** | |
| Error | 192 | 9.543 | 12.659 | 0.078 | 0 | 0.627 | 0.0001 | 294.977 | 0.038 | 0.06 | |
| Source | DF | WUEi | LS | GLN | FLA | GLA | LAI | DSW | DLW | TDW | DH |
| S | 1 | 3923.432 ** | 0.108 ** | 4.844 ** | 2.519 | 4633.31 ** | 11.13 ** | 1.371 * | 0.019 ** | 1.72 ** | 8.670 ** |
| rep(S) | 4 | 13.004 | 0.0008 | 0.185 ** | 1.827 | 85.068 ** | 0.035 | 0.518 | 0.008 * | 0.421 | 0.227 |
| I | 1 | 17840.78 ** | 0.478 ** | 5.206 ** | 701.02 ** | 45628.4 ** | 300.42 ** | 40.85 ** | 0.710 ** | 52.35 ** | 145.60 ** |
| S*I | 1 | 2952.544 ** | 0.0001 | 0.113 | 8.653 ** | 1757.88 ** | 16.55 ** | 0.59 | 0.008 | 0.73 | 0.03 |
| rep(I*S) | 4 | 8.258 | 0 | 0.05 | 1.997 | 28.21 | 0.054 | 0.324 | 0.011 | 0.306 | 3.147 |
| G | 24 | 1089.461 ** | 0.019 ** | 1.457 ** | 190.58 ** | 2308.83 ** | 9.163 ** | 48.01 ** | 0.537 ** | 52.62 ** | 164.83 ** |
| S*G | 24 | 287.937 ** | 0.002 ** | 0.487 ** | 9.922 ** | 544.106 ** | 2.863 ** | 0.023 | 0 | 0.026 | 0.121 |
| I*G | 24 | 649.685 ** | 0.015 ** | 0.135 ** | 16.613 ** | 205.589 ** | 1.771 ** | 2.416 ** | 0.071 ** | 2.753 ** | 2.721 ** |
| S*I*G | 24 | 174.860 ** | 0.001 ** | 0.151 ** | 4.897 ** | 133.676 ** | 1.216 ** | 0.035 | 0.001 | 0.041 | 0.079 |
| Error | 192 | 16.693 | 0.0004 | 0.039 | 1.184 | 14.539 | 0.034 | 0.298 | 0.004 | 0.321 | 0.926 |
| Source | DF | DM | GFD | NSP | PH | SL | NSS | NG | HW | GY | |
| S | 1 | 6.750 ** | 5.880 * | 590875.3 ** | 21.87 | 137.783 ** | 173.95 ** | 367.37 ** | 2363.38 ** | 3.272 ** | |
| rep(S) | 4 | 3.307 * | 0.807 | 411.987 | 17.667 | 0.126 | 1.346 ** | 4.756 | 5.208 | 0.13 | |
| I | 1 | 1352.6 ** | 622.1 ** | 933645.6 ** | 2296.3 ** | 27.731 ** | 64.255 ** | 1578.9 ** | 1700.23 ** | 92.596 ** | |
| S*I | 1 | 0.27 | 0.48 | 58408.65 ** | 383.07 ** | 0.431 | 1.952* | 1309.0 ** | 362.362 ** | 0.536 ** | |
| rep(I*S) | 4 | 3.277 | 0.764 | 849.35 | 12.719 | 0.188 | 0.533 | 2.252 | 0.797 | 0.029 | |
| G | 24 | 150.73 ** | 38.06 ** | 53328.17 ** | 440.15 ** | 7.170 ** | 18.001 ** | 227.47 ** | 314.603 ** | 11.965 ** | |
| S*G | 24 | 0.076 | 0.144 | 18636.06 ** | 83.814 ** | 7.029 ** | 13.620 ** | 45.081 ** | 53.422 ** | 0.713 ** | |
| I*G | 24 | 5.501 ** | 5.844 ** | 10219.66 ** | 33.791 ** | 0.861 ** | 0.597 | 38.455 ** | 29.064 ** | 1.714 ** | |
| S*I*G | 24 | 0.194 | 0.341 | 10869.40 ** | 20.360 ** | 0.394 ** | 1.348 ** | 41.090 ** | 20.545 ** | 0.302 ** | |
| Error | 192 | 1.341 | 1.692 | 680.423 | 10.701 | 0.196 | 0.379 | 4.409 | 4.39 | 0.078 |
| Traits | Seasons | Full | Limited | Combined Data | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | ||
| LWC | Season1 | 69.18 | 85.52 | 74.48 ± 4.02 ** | 61.39 | 79.27 | 70.66 ± 3.52 ** | 68.89 | 78.69 | 73.55 ± 2.08 ** |
| Season2 | 68.59 | 82.62 | 76.44 ± 3.31 ** | 67.42 | 76.12 | 72.64 ± 2.29 ** | ||||
| RWC | Season1 | 77.35 | 89.42 | 84.18 ± 3.03 ** | 69.96 | 83.01 | 78.83 ± 3.13 ** | 73.11 | 85.62 | 81.01 ± 2.53 ** |
| Season2 | 71.34 | 91.71 | 84.82 ± 4.76 ** | 68.44 | 81.78 | 76.21 ± 3.98 ** | ||||
| CT | Season1 | 16.29 | 18.58 | 17.38 ± 0.55 ** | 18.88 | 20.40 | 19.73 ± 0.43 ** | 17.78 | 19.08 | 18.51 ± 0.42 ** |
| Season2 | 15.47 | 18.25 | 17.02 ± 0.79 ** | 18.75 | 21.17 | 19.91 ± 0.61 ** | ||||
| LEWT | Season1 | 0.011 | 0.028 | 0.025 ± 0.00 ** | 0.013 | 0.025 | 0.024 ± 0.00 ** | 0.011 | 0.025 | 0.022 ± 0.00 ** |
| Season2 | 0.010 | 0.026 | 0.024 ± 0.00 ** | 0.011 | 0.021 | 0.018 ± 0.00 ** | ||||
| Pn | Season1 | 8.67 | 12.93 | 11.39 ± 1.00 ** | 8.24 | 13.27 | 11.17 ± 1.37 ** | 8.44 | 12.49 | 10.93 ± 1.05 ** |
| Season2 | 8.24 | 11.97 | 10.67 ± 1.00 ** | 8.15 | 12.84 | 10.49 ± 1.35 ** | ||||
| Gs | Season1 | 0.15 | 0.24 | 0.19 ± 0.03 ** | 0.09 | 0.18 | 0.14 ± 0.02 ** | 0.13 | 0.20 | 0.16 ± 0.02 ** |
| Season2 | 0.12 | 0.24 | 0.18 ± 0.03 ** | 0.08 | 0.18 | 0.13 ± 0.02 ** | ||||
| Ci | Season1 | 281.51 | 378.75 | 330.98 ± 19.35 ** | 247.41 | 325.01 | 287.9 ± 21.3 ** | 276.80 | 331.33 | 302.0 ± 13.4 ** |
| Season2 | 284.10 | 343.27 | 314.03 ± 13.16** | 228.71 | 305.86 | 275.1 ± 19.9** | ||||
| E | Season1 | 2.55 | 3.95 | 3.27 ± 0.39 ** | 1.96 | 3.30 | 2.62 ± 0.28 ** | 2.31 | 3.52 | 2.85 ± 0.27 ** |
| Season2 | 2.26 | 3.79 | 3.07 ± 0.36 ** | 1.75 | 3.13 | 2.45 ± 0.34 ** | ||||
| WUE | Season1 | 2.90 | 4.50 | 3.57 ± 0.41 ** | 3.44 | 5.47 | 4.33 ± 0.51 ** | 3.12 | 4.58 | 3.81 ± 0.35 ** |
| Season2 | 2.67 | 4.56 | 3.41 ± 0.44 ** | 2.50 | 4.75 | 3.94 ± 0.59 ** | ||||
| WUEi | Season1 | 50.53 | 78.66 | 61.92 ± 8.11 ** | 63.87 | 107.11 | 83.8 ± 12.60 ** | 59.29 | 81.23 | 69.21 ± 6.67 ** |
| Season2 | 46.83 | 85.33 | 61.04 ± 9.95 ** | 55.56 | 86.80 | 70.10 ± 8.51 ** | ||||
| Ls | Season1 | 0.19 | 0.36 | 0.26 ± 0.04 ** | 0.26 | 0.48 | 0.34 ± 0.05 ** | 0.28 | 0.39 | 0.32 ± 0.03 ** |
| Season2 | 0.22 | 0.39 | 0.30 ± 0.04 ** | 0.29 | 0.45 | 0.38 ± 0.04 ** | ||||
| GLN | Season1 | 32.07 | 45.67 | 38.53 ± 3.69 ** | 18.03 | 44.00 | 29.78 ± 5.04 ** | 29.71 | 44.82 | 35.27 ± 3.27 ** |
| Season2 | 30.65 | 43.94 | 36.57 ± 3.34 ** | 26.84 | 45.92 | 36.20 ± 4.12 ** | ||||
| FLA | Season1 | 17.82 | 33.06 | 23.54 ± 3.50 ** | 15.93 | 25.42 | 20.14 ± 2.34 ** | 16.39 | 29.36 | 21.76 ± 2.91 ** |
| Season2 | 16.17 | 32.73 | 23.03 ± 3.72 ** | 14.09 | 27.61 | 20.35 ± 3.05 ** | ||||
| GLA | Season1 | 66.00 | 113.83 | 88.14 ± 12.22 ** | 40.06 | 83.98 | 58.52 ± 10.81 ** | 54.36 | 102.68 | 77.24 ± 10.72 ** |
| Season2 | 56.75 | 131.18 | 91.08 ± 15.64 ** | 47.16 | 96.10 | 71.22 ± 10.96 ** | ||||
| LAI | Season1 | 2.45 | 5.40 | 3.80 ± 0.94 ** | 1.17 | 3.10 | 2.12 ± 0.64 ** | 2.13 | 3.65 | 2.96 ± 0.60 ** |
| Season2 | 2.11 | 5.11 | 3.45 ± 0.83 ** | 1.29 | 3.23 | 2.30 ± 0.49 ** | ||||
| DSW | Season1 | 6.18 | 11.37 | 8.44 ± 1.37 ** | 5.48 | 9.57 | 7.62 ± 1.16 ** | 6.01 | 10.41 | 7.97 ± 1.22 ** |
| Season2 | 6.18 | 10.95 | 8.23 ± 1.31 ** | 5.27 | 9.74 | 7.58 ± 1.24 ** | ||||
| DLW | Season1 | 0.64 | 1.34 | 1.01 ± 0.20 ** | 0.70 | 1.16 | 0.90 ± 0.14 ** | 0.67 | 1.23 | 0.95 ± 0.16 ** |
| Season2 | 0.64 | 1.32 | 0.99 ± 0.19 ** | 0.69 | 1.20 | 0.90 ± 0.15 ** | ||||
| TDW | Season1 | 6.82 | 12.49 | 9.46 ± 1.45 ** | 6.26 | 10.73 | 8.52 ± 1.24 ** | 6.68 | 11.55 | 8.92 ± 1.30 ** |
| Season2 | 6.82 | 12.03 | 9.21 ± 1.39 ** | 6.03 | 10.95 | 8.48 ± 1.32 ** | ||||
| DH | Season1 | 71.00 | 80.33 | 75.40 ± 2.73 ** | 70.00 | 78.33 | 74.04 ± 2.64 ** | 70.67 | 79.50 | 74.88 ± 2.67 ** |
| Season2 | 71.33 | 80.67 | 75.71 ± 2.79 ** | 70.33 | 78.67 | 74.39 ± 2.63 ** | ||||
| MD | Season1 | 118.33 | 127.00 | 121.61 ± 2.71 ** | 114.67 | 121.67 | 117.28 ± 2.16 ** | 117.00 | 123.83 | 119.59 ± 2.36 ** |
| Season2 | 118.67 | 126.33 | 121.84 ± 2.69 ** | 115.33 | 122.00 | 117.63 ± 2.12 ** | ||||
| GFD | Season1 | 42.67 | 49.67 | 45.95 ± 1.52 ** | 40.00 | 45.33 | 43.00 ± 1.60 ** | 41.50 | 47.50 | 44.61 ± 1.41 ** |
| Season2 | 43.00 | 50.00 | 46.13 ± 1.47 ** | 40.33 | 45.67 | 43.35 ± 1.55 ** | ||||
| NSP | Season1 | 433.33 | 770.00 | 579.07 ± 93.35 ** | 349.67 | 570.00 | 440.39 ± 54.31 ** | 384.08 | 544.17 | 465.55 ± 43.54 ** |
| Season2 | 393.33 | 573.33 | 462.93 ± 48.38 ** | 286.67 | 496.67 | 379.80 ± 44.76 ** | ||||
| PH | Season1 | 73.00 | 97.00 | 83.45 ± 7.04 ** | 67.92 | 84.67 | 75.56 ± 4.46 ** | 71.71 | 87.47 | 79.25 ± 4.75 ** |
| Season2 | 71.33 | 91.00 | 80.67 ± 5.34 ** | 66.67 | 85.67 | 77.31 ± 5.21 ** | ||||
| SL | Season1 | 8.73 | 11.27 | 10.06 ± 0.75 ** | 8.17 | 10.83 | 9.38 ± 0.70 ** | 8.01 | 9.84 | 9.04 ± 0.50 ** |
| Season2 | 6.67 | 10.60 | 8.63 ± 0.93 ** | 6.33 | 9.53 | 8.10 ± 0.84 ** | ||||
| NSS | Season1 | 13.60 | 17.56 | 16.01 ± 1.06 ** | 13.61 | 16.83 | 15.25 ± 0.85 ** | 13.09 | 16.32 | 14.88 ± 0.94 ** |
| Season2 | 11.67 | 17.11 | 14.66 ± 1.56 ** | 10.80 | 15.90 | 13.59 ± 1.40 ** | ||||
| NG | Season1 | 32.07 | 45.67 | 38.53 ± 3.69 ** | 18.03 | 44.00 | 29.78 ± 5.04 ** | 29.71 | 44.82 | 35.27 ± 3.27 ** |
| Season2 | 30.65 | 43.94 | 36.57 ± 3.34 ** | 26.84 | 45.92 | 36.20 ± 4.12 ** | ||||
| HW | Season1 | 42.32 | 61.97 | 51.72 ± 5.26 ** | 35.81 | 52.38 | 44.88 ± 4.70 ** | 37.24 | 52.68 | 45.53 ± 3.97 ** |
| Season2 | 32.89 | 50.57 | 44.01 ± 4.45 ** | 32.72 | 46.91 | 41.51 ± 3.98 ** | ||||
| GY | Season1 | 3.71 | 7.24 | 5.15 ± 0.86 ** | 2.90 | 5.99 | 3.99 ± 0.68 ** | 3.50 | 6.49 | 4.68 ± 0.66 ** |
| Season2 | 3.80 | 7.19 | 5.31 ± 0.83 ** | 3.27 | 6.20 | 4.25 ± 0.61 ** | ||||
| Traits | h2 | GCV | PCV | GA | GG |
|---|---|---|---|---|---|
| LWC | 36.06 | 2.76 | 4.60 | 2.51 | 3.14 |
| RWC | 86.36 | 10.73 | 11.55 | 16.64 | 20.55 |
| CT | 88.39 | 10.33 | 10.99 | 3.70 | 20.01 |
| LEWT | 88.30 | 26.71 | 28.42 | 0.01 | 51.71 |
| Pn | 80.81 | 11.73 | 13.05 | 2.37 | 21.73 |
| Gs | 30.37 | 8.53 | 15.48 | 0.02 | 9.69 |
| Ci | 38.81 | 3.82 | 6.13 | 14.79 | 4.90 |
| E | 38.92 | 7.48 | 11.99 | 0.27 | 9.61 |
| WUE | 55.30 | 9.52 | 12.80 | 0.56 | 14.58 |
| WUEi | 29.99 | 7.54 | 13.78 | 5.89 | 8.51 |
| Ls | 41.37 | 9.80 | 15.24 | 0.04 | 12.99 |
| GLN | 66.92 | 7.40 | 9.05 | 0.48 | 12.47 |
| FLA | 88.65 | 17.23 | 18.30 | 7.28 | 33.42 |
| GLA | 73.32 | 15.39 | 17.97 | 20.95 | 27.14 |
| LAI | 62.70 | 18.90 | 23.87 | 1.13 | 30.83 |
| DSW | 94.46 | 24.43 | 25.13 | 3.90 | 48.91 |
| DLW | 86.26 | 20.72 | 22.31 | 0.38 | 39.65 |
| TDW | 94.27 | 22.83 | 23.51 | 4.08 | 45.66 |
| DH | 97.82 | 4.91 | 4.96 | 7.49 | 10.00 |
| MD | 95.63 | 2.91 | 2.98 | 7.01 | 5.86 |
| GFD | 81.86 | 3.69 | 4.08 | 3.06 | 6.87 |
| NSP | 65.48 | 11.65 | 14.39 | 90.46 | 19.41 |
| PH | 77.91 | 6.75 | 7.65 | 9.72 | 12.27 |
| SL | 39.48 | 7.06 | 11.23 | 0.82 | 9.14 |
| NSS | 27.37 | 4.39 | 8.40 | 0.70 | 4.74 |
| NG | 80.41 | 11.12 | 12.40 | 7.25 | 20.55 |
| HW | 80.31 | 10.08 | 11.24 | 8.47 | 18.60 |
| GY | 57.60 | 10.80 | 14.24 | 0.79 | 16.89 |
| Traits | Before Excluding Traits | After Excluding Traits | Traits | Before Excluding Traits | After Excluding Traits | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tolerance | VIF | Tolerance | VIF | Tolerance | VIF | Tolerance | VIF | ||
| DH | 0.036 | 28.13 | 0.205 | 4.89 | LWC | 0.465 | 2.15 | 0.465 | 2.15 |
| DM | 0.025 | 40.34 | -- | -- | RWC | 0.300 | 3.33 | 0.301 | 3.33 |
| GFD | 0.053 | 19.02 | 0.209 | 4.77 | NG | 0.402 | 2.49 | 0.417 | 2.40 |
| DSW | 0.000 | 0.00 | 0.000 | 0.00 | HW | 0.221 | 4.54 | 0.221 | 4.53 |
| DLW | 0.000 | 0.00 | 0.000 | 0.00 | CT | 0.176 | 5.67 | 0.178 | 5.62 |
| TDW | 0.000 | 0.00 | 0.000 | 0.00 | LEWT | 0.463 | 2.16 | 0.470 | 2.13 |
| NSP | 0.151 | 6.61 | 0.152 | 6.59 | Pn | 0.184 | 5.43 | 0.186 | 5.39 |
| PH | 0.255 | 3.92 | 0.260 | 3.85 | Gs | 0.146 | 6.86 | 0.159 | 6.28 |
| NL | 0.234 | 4.28 | 0.234 | 4.28 | Ci | 0.109 | 9.15 | 0.111 | 9.02 |
| LAF | 0.130 | 7.70 | 0.142 | 7.07 | E | 0.100 | 10.02 | 0.111 | 9.03 |
| TLAF | 0.129 | 7.74 | 0.133 | 7.52 | WUE | 0.146 | 6.83 | 0.147 | 6.82 |
| LAI | 0.120 | 8.33 | 0.122 | 8.20 | WUEi | 0.103 | 9.67 | 0.104 | 9.62 |
| SL | 0.182 | 5.51 | 0.185 | 5.41 | Ls | 0.117 | 8.54 | 0.127 | 7.85 |
| NSS | 0.233 | 4.29 | 0.236 | 4.23 | GY | 0.291 | 3.44 | 0.311 | 3.22 |
| Source | Stepwise Regression | Path Coefficient | ||||||
|---|---|---|---|---|---|---|---|---|
| Partitioning the Correlation | R2 | |||||||
| Regression Coefficient | p Value * | R2 Par. | R2 Com. | Direct Effect | Indirect Effect | Correlation Value | Direct Effect | |
| Intercept | 16.232 | <0.0001 | ||||||
| GLA | 0.492 | 0.010 | 0.104 | 0.104 | −0.065 | 0.511 | 0.446 | 0.004 |
| LAI | 0.373 | 0.001 | 0.141 | 0.245 | 0.264 | 0.230 | 0.493 | 0.069 |
| RWC | 0.772 | 0.030 | 0.171 | 0.416 | 0.189 | 0.201 | 0.390 | 0.036 |
| CT | −0.497 | < 0.0001 | 0.400 | 0.816 | −0.653 | 0.020 | −0.633 | 0.426 |
| Gs | 0.326 | 0.040 | 0.070 | 0.886 | −0.173 | 0.479 | 0.306 | 0.030 |
| Indirect effect | 0.321 | |||||||
| Total R2 | 0.886 | 0.886 | ||||||
| Residual | 0.338 | 0.338 | ||||||
| No. | Genotypes | GLA | LAI | RWC | CT | Over All data | Over All Data | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | Class | S2 | Class | Combined | Class | ||
| 1 | DHL12 | 0.785 | 0.865 | 0.709 | 0.786 | 0.726 | 0.738 | 0.820 | 0.836 | 0.760 | T | 0.806 | HT | 0.783 | T |
| 2 | DHL02 | 0.792 | 0.821 | 0.779 | 0.844 | 0.820 | 0.792 | 0.851 | 0.857 | 0.811 | HT | 0.829 | HT | 0.820 | HT |
| 3 | DHL25 | 0.710 | 0.782 | 0.642 | 0.712 | 0.657 | 0.668 | 0.742 | 0.756 | 0.688 | T | 0.730 | T | 0.709 | T |
| 4 | DHL30 | 0.876 | 0.907 | 0.862 | 0.933 | 0.907 | 0.876 | 0.988 | 0.900 | 0.908 | HT | 0.904 | HT | 0.906 | HT |
| 5 | DHL07 | 0.688 | 0.758 | 0.621 | 0.689 | 0.636 | 0.647 | 0.754 | 0.696 | 0.675 | T | 0.697 | T | 0.686 | T |
| 6 | DHL26 | 0.834 | 0.864 | 0.820 | 0.889 | 0.863 | 0.834 | 0.941 | 0.857 | 0.865 | HT | 0.861 | HT | 0.863 | HT |
| 7 | Gemmeiza-9 | 0.850 | 0.847 | 0.729 | 0.809 | 0.710 | 0.797 | 0.885 | 0.817 | 0.793 | T | 0.818 | HT | 0.805 | HT |
| 8 | DHL11 | 0.494 | 0.532 | 0.525 | 0.476 | 0.528 | 0.589 | 0.532 | 0.471 | 0.520 | I | 0.517 | I | 0.518 | I |
| 9 | KSU106 | 0.400 | 0.406 | 0.311 | 0.360 | 0.392 | 0.487 | 0.380 | 0.372 | 0.371 | S | 0.406 | I | 0.389 | S |
| 10 | Gemmeiza-12 | 0.473 | 0.479 | 0.379 | 0.399 | 0.462 | 0.567 | 0.454 | 0.409 | 0.442 | I | 0.464 | I | 0.453 | I |
| 11 | DHL01 | 0.708 | 0.706 | 0.640 | 0.672 | 0.591 | 0.664 | 0.738 | 0.681 | 0.669 | T | 0.681 | T | 0.675 | T |
| 12 | DHL14 | 0.330 | 0.352 | 0.274 | 0.198 | 0.340 | 0.343 | 0.320 | 0.196 | 0.316 | S | 0.272 | S | 0.294 | S |
| 13 | DHL29 | 0.428 | 0.479 | 0.361 | 0.380 | 0.440 | 0.540 | 0.433 | 0.390 | 0.415 | I | 0.447 | I | 0.431 | I |
| 14 | DHL15 | 0.347 | 0.408 | 0.303 | 0.310 | 0.376 | 0.379 | 0.330 | 0.275 | 0.339 | S | 0.343 | S | 0.341 | S |
| 15 | DHL06 | 0.330 | 0.389 | 0.274 | 0.310 | 0.358 | 0.361 | 0.315 | 0.199 | 0.319 | S | 0.315 | S | 0.317 | S |
| 16 | Misr1 | 0.892 | 0.925 | 0.878 | 0.951 | 0.878 | 0.937 | 0.980 | 0.917 | 0.907 | HT | 0.932 | HT | 0.920 | HT |
| 17 | DHL05 | 0.822 | 0.906 | 0.743 | 0.824 | 0.761 | 0.773 | 0.852 | 0.832 | 0.794 | T | 0.834 | HT | 0.814 | HT |
| 18 | Giza-168 | 0.695 | 0.766 | 0.628 | 0.697 | 0.643 | 0.654 | 0.726 | 0.741 | 0.673 | T | 0.714 | T | 0.694 | T |
| 19 | DHL23 | 0.571 | 0.611 | 0.573 | 0.580 | 0.636 | 0.641 | 0.584 | 0.573 | 0.591 | I | 0.601 | T | 0.596 | I |
| 20 | Sakha-93 | 0.372 | 0.396 | 0.294 | 0.332 | 0.404 | 0.368 | 0.321 | 0.295 | 0.347 | S | 0.348 | S | 0.348 | S |
| 21 | DHL21 | 0.323 | 0.344 | 0.195 | 0.288 | 0.351 | 0.320 | 0.279 | 0.256 | 0.287 | S | 0.302 | S | 0.295 | S |
| 22 | DHL22 | 0.347 | 0.370 | 0.289 | 0.295 | 0.377 | 0.344 | 0.300 | 0.276 | 0.328 | S | 0.321 | S | 0.325 | S |
| 23 | DHL03 | 0.800 | 0.841 | 0.761 | 0.723 | 0.740 | 0.752 | 0.815 | 0.852 | 0.779 | T | 0.792 | T | 0.786 | T |
| 24 | Pavone-76 | 0.776 | 0.804 | 0.803 | 0.787 | 0.803 | 0.776 | 0.833 | 0.839 | 0.804 | HT | 0.801 | HT | 0.803 | HT |
| 25 | DHL08 | 0.834 | 0.864 | 0.864 | 0.846 | 0.863 | 0.834 | 0.896 | 0.903 | 0.864 | HT | 0.862 | HT | 0.863 | HT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ashkar, I.; Al-Suhaibani, N.; Abdella, K.; Sallam, M.; Alotaibi, M.; Seleiman, M.F. Combining Genetic and Multidimensional Analyses to Identify Interpretive Traits Related to Water Shortage Tolerance as an Indirect Selection Tool for Detecting Genotypes of Drought Tolerance in Wheat Breeding. Plants 2021, 10, 931. https://doi.org/10.3390/plants10050931
Al-Ashkar I, Al-Suhaibani N, Abdella K, Sallam M, Alotaibi M, Seleiman MF. Combining Genetic and Multidimensional Analyses to Identify Interpretive Traits Related to Water Shortage Tolerance as an Indirect Selection Tool for Detecting Genotypes of Drought Tolerance in Wheat Breeding. Plants. 2021; 10(5):931. https://doi.org/10.3390/plants10050931
Chicago/Turabian StyleAl-Ashkar, Ibrahim, Nasser Al-Suhaibani, Kamel Abdella, Mohammed Sallam, Majed Alotaibi, and Mahmoud F. Seleiman. 2021. "Combining Genetic and Multidimensional Analyses to Identify Interpretive Traits Related to Water Shortage Tolerance as an Indirect Selection Tool for Detecting Genotypes of Drought Tolerance in Wheat Breeding" Plants 10, no. 5: 931. https://doi.org/10.3390/plants10050931
APA StyleAl-Ashkar, I., Al-Suhaibani, N., Abdella, K., Sallam, M., Alotaibi, M., & Seleiman, M. F. (2021). Combining Genetic and Multidimensional Analyses to Identify Interpretive Traits Related to Water Shortage Tolerance as an Indirect Selection Tool for Detecting Genotypes of Drought Tolerance in Wheat Breeding. Plants, 10(5), 931. https://doi.org/10.3390/plants10050931







