Abstract
Photorespiration, or C2 photosynthesis, is generally considered a futile cycle that potentially decreases photosynthetic carbon fixation by more than 25%. Nonetheless, many essential processes, such as nitrogen assimilation, C1 metabolism, and sulfur assimilation, depend on photorespiration. Most studies of photosynthetic and photorespiratory reactions are conducted with magnesium as the sole metal cofactor despite many of the enzymes involved in these reactions readily associating with manganese. Indeed, when manganese is present, the energy efficiency of these reactions may improve. This review summarizes some commonly used methods to quantify photorespiration, outlines the influence of metal cofactors on photorespiratory enzymes, and discusses why photorespiration may not be as wasteful as previously believed.
1. Introduction
Photorespiration involves the oxygenation of ribulose-1,5-bisphosphate (RuBP) to form 3-phosphoglycerate (3PGA) and 2-phosphoglycolate (2PG) and the subsequent carbon oxidation pathways that release CO2 under light conditions [1,2,3,4,5]. Because it produces 2PG, a compound “toxic” to many enzymes in photosynthetic metabolism, and oxidizes organic carbon without seemingly generating ATP, photorespiration is generally considered a wasteful process. The following sections examines how the photorespiratory pathway converts 2PG into glycolate, the only carbon source for the photosynthetic carbon oxidation cycle [6], a cycle that together with nitrogen assimilation, C1 metabolism, and sulfur assimilation generates essential amino acids and intermediate compounds [7]. Moreover, the three enzymes involved in the initial photorespiratory steps within chloroplasts—Rubisco, malic enzyme, and phosphoglycolate phosphatase—have metal binding sites that accommodate either Mg2+ or Mn2+, and balance between the binding of these enzymes to Mg2+ or Mn2+ may shift the relative rates and energy efficiencies of photosynthesis and photorespiration [8].
2. Photosynthesis vs. Photorespiration
2.1. Rubisco
Atmospheric CO2 concentration has increased more than 20% during the past 35 years [9]. The major sink for this CO2 is the approximately 258 billion tons per year that photosynthetic organisms convert into organic carbon compounds through carbon fixation via the Calvin–Benson pathway [10]. This pathway begins with Rubisco (Ribulose 1,5-bisphosphate carboxylase–oxygenase), the most abundant protein on the planet [11].
Rubisco comes in three forms [12]:
Form I, which is found in cyanobacteria, proteobacteria, chlorophyte algae, heterokont algae, and haptophyte algae, and higher plants, is the most common [13,14]. It is a hexadecamer containing eight identical large subunits (~55,000 Mr), each with a metal-binding site, and eight small subunits (~15,000 Mr). The large subunits are coded by a single plastomic gene, whereas the small subunits are coded by a nuclear multigene family that consists of 2 to 22 members, depending on the species [15]. Complex cellular machinery is required to assemble this form of Rubisco and to maintain its activity [16]. Form I Rubisco, until recently, had resisted all efforts to generate a functional holoenzyme in vitro or upon recombinant expression in E. coli [17].
Form II Rubisco, found in proteobacteria, archaea, and dinoflagellate algae, contains one or more isodimers with subunits that share about 30% identity to the large subunit of Form I Rubisco [8].
Form III Rubisco, found in archaea, has one or five isodimers composed of subunits homologous to the large subunit of Form I Rubisco [8].
Form II and Form III Rubisco show greater similarity in their primary sequence to one another than either do to the large subunit of Form I Rubisco [8].
All three forms of Rubisco catalyze not only the reaction in which the carboxylation of the five-carbon sugar RuBP generates two molecules of the three-carbon organic acid 3-phosphoglycerate (3PGA), but also an alternative reaction in which oxidation of RuBP generates one molecule of 3PGA and one of 2PG (Figure 1) [8]. The carboxylation pathway of photosynthesis expends 3 ATP and 2 NADPH per RuBP regenerated and produces a carbon in hexose [18], whereas the oxygenation pathway of photorespiration reportedly expends 3.5 ATP and 2 NADPH per RuBP regenerated but produces no additional organic carbon [19,20].
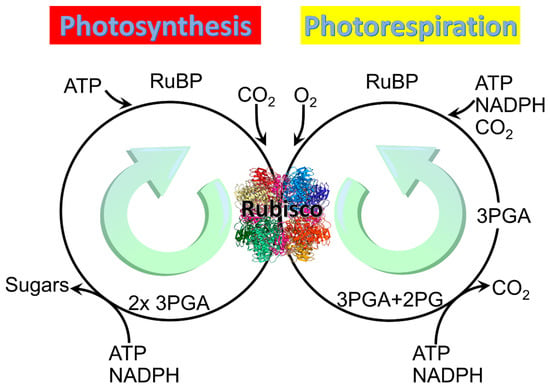
Figure 1.
Two main reactions of Rubisco: Photosynthesis and photorespiration. Rubisco structure picture credit: Laguna design / science photo library.
Rubisco must be activated before it can carboxylate or oxygenate RuBP. Activation of the three forms of Rubisco involves binding of Mn2+ or Mg2+ [21,22]. Binding of Mg2+ requires carbamylation of Rubisco by the addition of CO2. One histidine at the active site of Rubisco rotates into an alternate conformation, resulting in a transient binding site where Mg2+ is partially neutralized by the conversion of two water molecules to hydroxide ions and coordinated indirectly by three histidine residues through the water molecules. Subsequently, the hydroxide ions cause a lysine residue at the active site to become deprotonated and rotate 120 degrees into the trans conformer, which brings its nitrogen into close proximity to the carbon of CO2, allowing for the formation of a covalent bond that produces a carbamyl group. This carbamyl group causes the Mg2+ ion to transfer to a second binding site, after which the histidine that first rotated returns to its original conformation [23]. It is unclear whether binding Mn2+ follows a similar mechanism and whether it requires an activator CO2 to be bound first [21,22]; hence, understanding the mechanism of Mn2+ binding to Rubisco is important to future research on Rubisco kinetics. During in vitro studies, Rubisco is often activated at pH 8.0 in the presence of CO2 and either Mg2+ or Mn2+.
Rubisco can also bind to other metals. When bound to Fe2+, Ni2+, Cu2+, Ca2+, or Co2+, Rubisco may exhibit some carboxylase and oxygenase activity [24]. For example, one study found that Rubisco from R. rubrum, when bound to Co2+, was incapable of carboxylation but still capable of oxygenation [24]. Another study found that Rubisco from spinach performed both carboxylation and oxygenation when bound to Ni2+ or Co2+ [25]. When bound to some other metal ions, including Cd2+, Cr2+, and Ga2+, Rubisco cannot catalyze either carboxylation or oxygenation [24]. Although it is known that the metal ion plays a role in stabilizing the activator carbamate and determining the active site’s structure, its effect upon the reactions catalyzed by Rubisco is still not completely understood. One hypothesis is that Mg2+, because of its electron-withdrawing properties, polarizes the C2 carbonyl of RuBP, which favors the removal of the C3 proton and thereby contributes to enolization [21].
NADPH complexes strongly with Rubisco and acts as an effector molecule to maintain the Rubisco catalytic pocket in an open confirmation that more rapidly facilitates CO2-Mg2+ activation when CO2 and Mg2+ are present in suboptimal concentrations [26,27,28,29]. The crystal structure of Rubisco with both Mg2+ and NADPH as ligands indicates that NADPH binds to the catalytic site of Rubisco through metal-coordinated water molecules [26]. The activated enzyme catalyzes either carboxylation or oxygenation of the enediol form of the five-carbon sugar ribulose-1,5-bisphosphate (RuBP) [14,21,22,30,31].
2.2. Balance between Carboxylation and Oxygenation and Metal Cofactors
Several factors alter the balance between Rubisco carboxylation and oxygenation and, thereby, alter the relative rates of photosynthesis and photorespiration. These include the concentrations of CO2 and O2 at the active site of Rubisco, the specificity of the enzyme for each gas, and whether the enzyme is associated with Mg2+ or Mn2+ [32]. These divalent cations share the same binding site in Rubisco [14,22,33], and in tobacco, Rubisco associates with both metals and rapidly exchanges one metal for the other [32]. Nonetheless, nearly all recent studies on the photosynthetic and photorespiratory pathways have been conducted in the presence of Mg2+ and absence of Mn2+ [8]. Rubisco binding of Mg2+ accelerates carboxylation, whereas binding of Mn2+ slows carboxylation [25,34,35,36,37,38]. Chloroplastic Mg2+ and Mn2+ activities seem to be regulated at the cellular level because in isolated tobacco chloroplasts, activities of the metals were directly proportional to their concentrations in the medium [32]. The thermodynamics of binding Mg2+ to Rubisco were similar for enzymes isolated from a Form I and a Form II species [32]. By contrast, the thermodynamics of binding differed greatly between the two Rubisco forms when the enzymes were associated with Mn2+ [32].
Mg2+ and Mn2+ have nearly identical ionic radii but highly disparate electron configurations: Mg2+ (1s22s22p6 or [Ne]) has a very stable outer shell [8], whereas Mn2+ has five unpaired d electrons (1s22s22p63s23p63d5 or [Ar]3d5) that are susceptible to many redox reactions. An aerated solution of activated Mn2+-Rubisco exhibits a long-lived chemiluminescence when RuBP is added [39,40]. This chemiluminescence was attributed to a spin-flip within the Mn2+ 3d manifold, leading to an excited quartet (S = 3/2) d5 electronic configuration that decays over the course of 1 to 5 min back to the sextet (S = 5/2) ground state electronic configuration [39]. Excited states are intrinsically better oxidants and reductants (larger reduction/oxidation potentials) than their corresponding ground states [41,42,43]; thus, the observed chemiluminescence opens the possibility that the RuBP-O2-Mn2+—Rubisco excited state may be quenched via electron transfer. Consequently, the liberated reducing equivalent could participate in the reduction of NADP+ to NADPH (Figure 2, blue pathway). In this way, oxidation of RuBP via O2 may proceed in a spin-allowed manner, while the Mn2+ remains “innocent” in the generation of the oxygenated RuBP precursor. Mn2+-centered redox may still proceed, with oxidation of excited Mn2+ to Mn3+ occurring in a manner independent of, but parallel to, substrate oxidation.
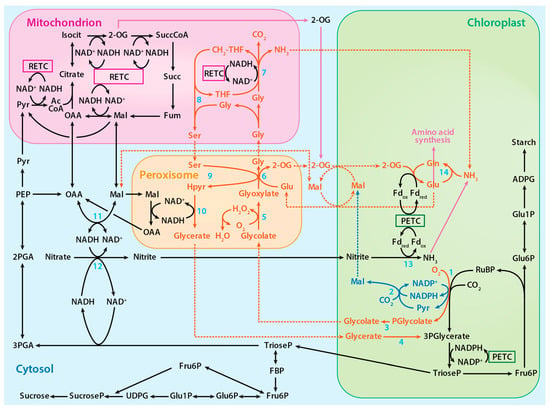
Figure 2.
The proposed photorespiratory pathway within the context of photosynthetic carbon and nitrogen metabolism. The solid red lines represent reactions of the photorespiratory pathway, the solid blue lines represent reactions of the proposed alternative photorespiratory pathway, the solid purple lines represent reactions of amino acid synthesis, and the dotted lines represent associated transport processes. Numbered reactions are catalyzed by the following enzymes: 1. Rubisco, 2. Malic enzyme, 3. Phosphoglycolate phosphatase, 4. Glycerate kinase, 5. Glycolate oxidase, 6. Glutamate:glyoxylate aminotransferase, 7. Glycine decarboxylase complex, 8. Serine hydroxymethyltransferase-1, 9. Serine:glyoxylate aminotransferase, 10. Hydroxypyruvate reductase-1, 11. Malate dehydrogenase, 12, Nitrate reductase, 13 Nitrite reductase, and 14. Glutamine synthetase. PETC designates photosynthetic electron transport chain and RETC, respiratory electron transport chain. Adapted from ref. [8]. Copyright 2018 Springer Nature Ltd.
In wheat leaves, the ratio of Mn2+ to Mg2+ contents increased as the CO2 levels increased and when the nitrogen source was nitrate rather than ammonium [32]. Nitrate assimilation into amino acids in shoots is heavily dependent on photorespiration, whereas ammonium assimilation is much less so. This indicates that plants shifted to Rubisco Mn2+ binding in order to compensate for the slower photorespiration rates and slower amino acid production that would otherwise occur under elevated CO2 and nitrate nutrition.
2.3. The Photorespiratory Pathway
The 3-phosphoglycerate produced during photorespiration, like that produced during photosynthesis, is converted to triose phosphate and used to regenerate RuBP. On the other hand, 2-phosphoglycolate is converted to glycolate by phosphoglycolate phosphatase. In the peroxisome and mitochondrion, a series of reactions converts glycolate to glycerate, which is ultimately returned to the chloroplast to regenerate RuBP (Figure 2) [8]. In addition to Rubisco, several other chloroplast enzymes in the photorespiratory pathway, including malic enzyme and phosphoglycolate phosphatase, bind either Mg2+ or Mn2+ [8]. The plastid isoform of malic enzyme in Arabidopsis and tobacco catalyzes the reverse pyruvate synthesis reaction (pyruvate + CO2 + NADPH → malate + NADP) [44,45]. Phosphoglycolate phosphatase, which is responsible for the hydrolysis of 2-phosphoglycolate to glycolate, binds to and is activated by either metal [46]. Hypothesized is an alternative photorespiratory pathway that increases photorespiration energy efficiency by generating malate (RuBP + O2 + CO2 + H2O → glycolate + malate + 2Pi) when Mn2+ binds to these enzymes (Figure 2) [8].
3. Estimating Rates of Photorespiration
Many different methods have been employed for estimating rates of photorespiration. The following sections outline the general approach of each method and highlights the assumptions and potential errors in each. The hope is that certain methods might be better suited for assessing the influence of Mn2+ vs. Mg2+ on the relative rates of oxygenation and carboxylation in situ.
3.1. Traditional Methods for Estimating Photorespiration
3.1.1. Post Illumination CO2 Burst
This method measures the evolution of CO2 from a leaf for 1 to 4 min after turning off the light because glycine metabolism continues longer in the dark than CO2 assimilation [47]. The rate of CO2 generation is measured by a transient CO2 analyzer [48] when the light has just been turned off or at the maximum rate of CO2 evolution observed. CO2 assimilation, however, does not stop immediately after the light is off. Separating CO2 assimilation from the CO2 burst effects during this time is difficult, and hence this method underestimates photorespiratory rates [49,50]. This method also fails to consider variations in mitochondrial respiration, leading to overestimates of photorespiratory rates [51].
3.1.2. O2 Inhibition of Net CO2 Assimilation
This method aims to assess the photorespiration rate from the increase in the CO2 assimilation rate after switching from normal to low O2 concentrations. Yet, changes in CO2 assimilation with O2 concentration may derive from components of the photosynthetic pathway other than photorespiration [4]. For example, when starch and sucrose synthesis limit photosynthesis, increasing or decreasing the photorespiration does not affect net CO2 assimilation [52].
3.1.3. Photorespiration CO2 Efflux into CO2-Free Air
This method estimates photorespiration from the CO2 efflux rate in CO2-free air. A high-O2 and low-CO2 environment, however, promotes photorespiration [4]. Additionally, a CO2-free atmosphere inhibits both the activity of Rubisco [53] and the regeneration of its substrate RuBP [54], leading to underestimates of photorespiration.
3.1.4. Ratio of 14CO2 to 12CO2 Uptake
In this method, 14CO2 and 12CO2 fluxes are measured after feeding a leaf with 14CO2 for a short period of time. Gross photosynthesis is estimated from 14CO2 uptake measured using an ionization chamber attached to an electrometer, while net photosynthesis is estimated from 12CO2 measured using an infrared gas analyzer. Photorespiration is estimated as the difference between gross and net photosynthesis [55] (Figure 3).
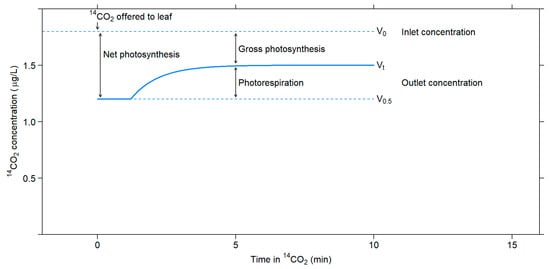
Figure 3.
Changes in 14CO2 concentration that occur upon exposing a leaf in the light to 14CO2. Adapted with permission from ref. [55]. Copyright 1971 Canadian Science Publishing.
There are several uncertainties associated with this method. The recycling effect on the specific activity of CO2 inside the leaf can cause about a 20% error. One must consider the specific activity of CO2 inside the leaf to obtain an accurate estimate of the gross photosynthesis rate because CO2 efflux through photorespiration dilutes the 14C label in the intercellular spaces, decreasing the specific activity of CO2. The activity might be even lower at the actual carboxylation site than in the interleaf spaces because of photorespiratory CO2 loss [56]. Moreover, Rubisco carboxylation discriminates about 2.9% against 13C [57,58] and about 5.5% against 14C [4], resulting in errors in estimations of photorespiration rates that exceed 25% [4,57].
3.2. Recent Methods for Estimating Photorespiration
3.2.1. Calculation from Kinetics Models
Rubisco reaction kinetics can provide an estimate of the photorespiration rate [4,59]. This method can provide accurate estimates of photorespiration rates if the CO2 compensation point in the absence of mitochondrial respiration (Γ*) being known for a given plant species. The rate of oxygenation, which is assumed to be twice the rate of photorespiration, is given by:
where A is the rate of photosynthetic CO2 assimilation, Rd is the rate of respiration other than photorespiration, and:
where C is the CO2 concentration.
The principal drawbacks of this method are that it does not directly measure photorespiration and depends on estimates of C and Γ*. There are several techniques for estimating C at the site of Rubisco activity, but estimating Γ* is more difficult. Values for Γ* are known for only a few species, and depend on estimates of kinetic parameters, which themselves rely on estimates of photorespiration [59].
3.2.2. CO2 Efflux into 13CO2-Air
The gas exchange method is based on the FvCB (Farquhar, van Caemmerer, and Berry) model [4,59]. First, ambient air is rapidly replaced with air containing 13CO2 and no 12CO2. The levels of released 12CO2 can be measured either using an infrared gas analyzer or a membrane inlet mass spectrometer. Because the rate of 12CO2 release includes both photorespiration and mitochondrial respiration, additional effort is needed to separate these effects. For example, in one approach, the rate of 12CO2 release is calculated as:
where F is the gas flow rate, 12CR and 12CS are the mole fractions of 12CO2 in the chamber without and with a leaf, WR and WS are the corresponding water mole fractions, and a is the illuminated leaf area in the chamber [60]. To divide this quantity into photorespiration and mitochondrial respiration, the air is replaced with air containing 10,000 ppm 13CO2 and the concentration of 13CO2 over 2 min is fitted to an exponential curve. The mitochondrial respiration Rd is taken to be the rate of 12CO2 release after 2 min.
This method can provide estimates of both carboxylation and oxygenation if one assumes that the rate of mitochondrial respiration (Rd) is not affected by the sudden high CO2 concentration, that 0.5 CO2 is generated per oxygenation reaction when the CO2 released per oxygenation varies widely with temperature and light level and among species [7], and that leaves do not naturally contain any 13C [59]. If intracellular reassimilation is significant and it often is [60], substantial errors in the estimate can result. These errors can be accounted for by monitoring the release of 12CO2 after switching from ambient air to air with a high concentration of 13CO2; however, high CO2 concentrations could affect mitochondrial respiration and thus produce error in the estimate of photorespiration. The presence of naturally occurring 13C also generates additional errors [59].
3.2.3. Labelling of Photosynthates with 14C
Leaves at a photosynthetic steady state are exposed to 14CO2 for different lengths to label primary and stored photosynthates. Exposing the leaf to an ambient concentration of 14CO2 for 10 to 15 min will label primary photosynthates, such as the metabolites from the Calvin cycle, glycolate cycle, and intermediates of starch and sucrose synthesis and of glycolysis [44]. Longer exposures (2 to 3 h) will label stored photosynthates, such as starch, sucrose, fructans, and vacuolar acids. 14CO2 efflux into different backgrounds containing various combinations of O2 and CO2 concentrations provides an estimate of photorespiration [61,62]. Four different backgrounds are used: first, 21% O2 and ambient CO2 to measure the steady-state release of CO2 from both photosynthesis and photorespiration; second, 1.5% O2 and ambient CO2 to measure the rate of photorespiration only; third, 21% O2 and 30,000 μmol/mol CO2 to limit CO2 reassimilation; and fourth, 21% O2 with no CO2 to measure the specific radioactivity of CO2 efflux [63].
The assumptions for this method are that all photosynthates must be labeled during the labeling time frames and that Rd is not affected by the percentage of O2 in the air. A recent report indicated that Rd was actually lower at a lower O2 concentration (2%) than at an ambient concentration (21%) [64]. One also has to assume that the mitochondrial respiration (Rd) value does not change upon transient exposure to high CO2 levels.
3.2.4. Measuring Photorespiratory Ammonia
Photorespiration generates NH3 in addition to CO2 during the conversion from glycine to serine in mitochondria [65]. Adding glutamine synthetase (GS) inhibitors methionine sulphoximine [35] or phosphinothricin [66] prevents ammonia reassimilation in chloroplasts, and NH3 subsequently accumulates in the leaf. The advantages of this method also include the prevention of CO2 refixation and uncertainties in Rd values under the experimental conditions [35,66]. This approach, however, depends on several assumptions: (1) The GS inhibitors do not inhibit photorespiration, and (2) they can prevent NH3 refixation completely.
Other factors might limit the diffusion of NH3 out of the leaves, leading to an underestimation of photorespiration [59]. GS inhibitors will disrupt the C2 cycle under photorespiratory conditions, and glycolate will rapidly accumulate, which in turn will inhibit photosynthesis. Feeding the plant an amino acid donor, such as glutamine, together with GS inhibitors will help minimize this inhibition effect [66,67].
Quantification of ammonia poses some challenges. The commonly used ion chromatography method to quantify NH4+ may overestimate the amount of NH4+ because methylamine, ethylamine, ethanolamine, and some non-protein amino acids co-elute with NH4+. Degradation of labile nitrogen metabolites in leaf extract, xylem sap, and apoplastic fluid to NH4+ during extraction will cause further overestimation of NH4+ levels [68].
3.2.5. Measuring 18O2 Consumption and Labeled Metabolites
Replacing ambient air in a chamber containing a leaf with air containing 18O2 provides another estimate of the photorespiration rate. A mass spectrometer measures levels of 16O2 and 18O2. The rate of oxygenation is estimated as:
and carboxylation as:
Ref. [69,70].
Unfortunately, this method cannot separate photorespiration from other light-dependent O2-consuming processes, such as light-dependent differences in the rate of mitochondrial respiration [46,48]. To diminish these errors, the mass spectrometer can quantify 18O-labeled metabolites, such as glycolate, glycine, and serine; with several assumptions about the photorespiratory pathway, such as the pool sizes of the labeled metabolites [49], one can then use the amounts of labeled metabolites to calculate the photorespiration rate [71,72].
3.2.6. NMR Measurements on 13C-Labeled Metabolites
This method requires that plants receive fertilizer labeled with 15N and that leaves subsequently be exposed to 13CO2. Rotational-echo double resonance (REDOR) detects 13C within two covalent bonds of 15N and thus assesses the formation of organic nitrogen metabolites labeled with 13C [59,73]. The ratio of 13C-labeled to unlabeled phosphorylated Calvin–Benson cycle metabolites between 2 and 4 min after exposure to 13CO2 indicates the ratio of photosynthesis to photorespiration [50]. This assumes that metabolites produced from photosynthesis are fully labeled in less than 2 min after being exposed to 13CO2 and that those produced from photorespiration do not become labeled until after 4 min. These assumptions may lead to errors because photosynthesis may re-assimilate some of the 12CO2 generated by photorespiration and because photorespiration may produce intermediates labeled with 13C in less than 2 min [60]. Furthermore, this method is based on the premise that photorespiration releases one CO2 for every two oxygenations, when the CO2 released per oxygenation varies widely with temperature and light level and among species [7].
3.2.7. Quantification of 2-Phosphoglycolate (2PG) and Photorespiratory Metabolites by Mass Spectrometry
This method uses LC-MS/MS to measure directly the first intermediate, 2PG, of photorespiration when Rubisco oxygenates RuBP, and GC-MS to measure other photorespiratory metabolites. In the LC-MS/MS portion, 2PG is separated from other molecules in three steps: First, liquid chromatography separates 2PG based on its physiochemical properties; second, mass spectrometry separates 2PG based on its m/z ratio; and third, mass spectrometry separates 2PG based on its m/z ratio after being fragmented [74]. Readings from the LC-MS/MS samples are compared with 2PG standard solutions [75]. Additionally, GC-MS is used to quantify additional photorespiratory metabolites, such as glycolate, glyoxylate, glycine, serine, hydroxypyruvate, and glycerate [74,76].
This approach has estimated photorespiratory rates in plant mutants deficient in expression of genes coding for photorespiratory enzymes. The gaseous environment of the aerial part of the plant, but not the root, was altered before experimentally determining the changes in the metabolite (2PG) content [77,78,79,80,81].
This method has several problems [74,82,83,84,85]. First, non-volatile salts and metabolites were deposited at the inlet of MS/MS after eluting from the LC step, which is very common when using anion-exchange chromatography [84]. Second, numerous metabolites eluted from the LC step had overlapping and asymmetrical peaks resulting from the matrix effect (interference in the ionization between compounds with similar elution times) [82,85], which significantly affects the sensitivity and accuracy of the measurements on a specific metabolite, such as 2PG. Third, post-harvest changes in metabolite concentrations can severely affect the quantification of 2PG [74,83]. Fourth, the GC-MS step is not targeted and therefore is potentially prone to error if other compounds with a similar molar mass as the photorespiratory metabolites are present [76].
3.2.8. CO2 Labeling and MS Analysis
Isotopically nonstationary metabolic flux analysis (INST-MFA) can trace 13C-labeled photorespiratory metabolites in plants exposed to 13CO2 to assess the photorespiration rate [86,87,88,89,90]. Monitoring the isotope incorporation in downstream metabolites over time assesses the relative contributions of different pathways after administration of the tracer. The turnover rates of each enzyme determine the labeling dynamics (Figure 4). Mathematical metabolic models specific for each pathway are often used to enumerate mass and isotopomer balances and ensure atoms’ conservation within the system. The models’ proposed metabolic fluxes are compared with those measured experimentally, and differences are minimized with each subsequent iteration.
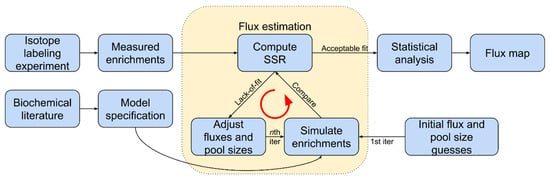
Figure 4.
Simplified INST-MFA workflow to estimate flux. Software, such as INCA and OpenMebius, is used in several steps. Fluxes and pool sizes are initially guessed and then adjusted in each iteration, converging upon a flux map that fits measurements of metabolism during the ILE. Adapted with permission from ref. [92]. Copyright 2018 Elsevier Ltd.
The INST-MFA approach presents several challenges. A minimum of three sample time points is needed for precise measurements of metabolic fluxes [91]. This makes experimental design more complex and time-consuming. To ensure accurate and precise measurements, the pool size for each component of a metabolic pathway has to be very specific. Absolute quantification of intracellular pool sizes, however, is not yet possible even with pool size measurements made with optimized mathematical modeling [91]. A second challenge of this approach is isotopic transients. Some intracellular metabolites can exhibit short isotopic transients that last only for a few minutes or seconds. Rapid sampling and quenching have to be achieved to obtain precise and meaningful INST-MFA measurements [92].
3.2.9. Micro-Optode Measurement of O2 Consumption
We have been conducting direct oxygenation rate measurements using a needle-type O2 micro-optode to examine the effects of metal cofactors on Rubisco photorespiration reactions. In this instrument, a polymer optical fiber transmits the excitation wavelength to the tip of the sensor and at the same time transmits the fluorescence response of an oxygen-sensitive dye that is immobilized in a polymer matrix at the tip. The rate of oxygenation can be calculated easily by comparing the amount of quenching of the excitation light by dissolved O2. The micro-optode has a 50–70-µm tip diameter, which makes it possible for a micro-scale setup, such as in a micro-cuvette or plate. The most important advantages for this type of sensor are that the micro-optode does not consume O2 in contrast to the other commonly used O2 sensors, such as a Clark electrode [93,94]; it has no stirring sensitivity; and it is resistant to most corrosive environments. The micro-optode also works in both gas (%O2) and liquid phases (DO), which makes it possible to measure O2 exchanges accurately up to 250% air O2 saturation in intact plant leaves, bioreactors, cell cultivation, microtiter plates, and many general oxygen measurements in liquids [95,96,97,98,99,100].
4. Photorespiration and Other Metabolic Pathways
4.1. NO3− Assimilation
Multiple lines of evidence link shoot NO3− assimilation to photorespiration:
- (a)
- Elevated CO2 or low O2 levels inhibited shoot NO3− reduction [101].
- (b)
- In independent 14N and 15N labeling experiments, assimilation of either 14N–NO3− or 15N–NO3− decreased under CO2 enrichment [102].
- (c)
- Under elevated CO2 conditions, NO3– nutrient absorption and organic N accumulation levels in various plant species declined when plants received NO3− as a sole N source [102,103,104,105,106].
- (d)
- C3 plants receiving NO3− as their sole N source experienced slower growth under CO2 enrichment than those receiving NH4+ [9,107,108].
In wheat and Arabidopsis plants grown under CO2 enrichment and receiving NO3− containing 15N at natural abundance levels, shoot tissues became less enriched with 15N organic compounds [102,109]: elevated CO2 inhibited shoot NO3− reduction so it was less limited by nitrate availability, and NO3− reductase discriminated more strongly against 15N–NO3− [110].
The assimilatory quotient (AQ) is the ratio of net CO2 consumption to net O2 evolution in plant shoots [111]. During shoot NO3− assimilation, ferredoxin generated from the photosynthetic electron chain reduces NO2− to NH4+ rather than producing NADPH, and so net O2 evolution increases without a change in net CO2 consumption. Therefore, the change in assimilatory quotient (∆AQ) when a plant receives NH4+ instead of NO3− as a sole N source provides an estimate of shoot NO3− assimilation [106]. ∆AQ decreased as the shoot internal CO2 concentration increased in C3 plants (Figure 5) [9,104,112,113].
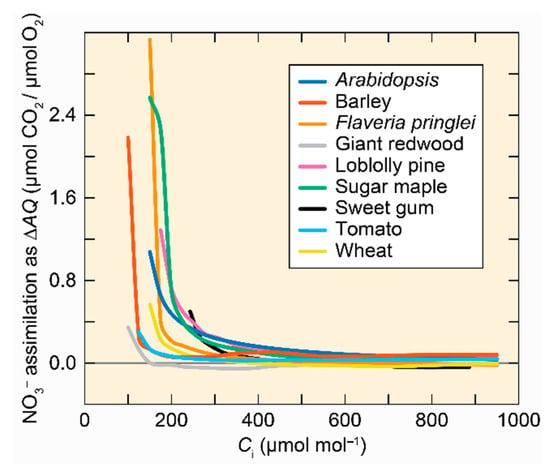
Figure 5.
Shoot NO3– assimilation as a function of shoot internal CO2 concentration (Ci) for 9 C3 species. Adapted with permission from ref. [101]. Copyright 2014 Springer Science Business Media Dordrecht.
Shoot CO2 and O2 fluxes at ambient and elevated CO2 were contrasted between stages of plant development or genotypes that have significantly different NO3− reductase activities in situ (i.e., 36- vs. 48-day-old wild-type Arabidopsis, Arabidopsis NO3− reductase knockout mutants vs. transgenic Arabidopsis overexpressing NO3− reductase, and NO3– reductase-deficient barley mutants vs. wild-type barley) [104,112]. ∆AQ, a measure of shoot NO3– assimilation, differed between these stages of development and genotypes under ambient CO2 but not under elevated CO2. This indicates that none of the stages of development or genotypes were assimilating NO3− under elevated CO2 [104,112].
Maximum NO3− reductase activity in vitro generally declined under CO2 enrichment [105,114]. Nonetheless, shoot NO3− reductase activity seldom limits NO3− assimilation in planta [115,116]. Accordingly, NO3− assimilation significantly declined only in genotypes with mutations that nearly eliminated enzyme activities [104,117,118], and genotypes with 50% higher NO3− reductase activities did not assimilate more NO3− [119]. Studies that have confused rates of enzyme activities with those of NO3− assimilation as a whole have drawn false conclusions [120,121].
One physiological mechanism that may be responsible for the interdependency of photorespiration and shoot NO3– assimilation involves the reduction of the Mn2+-RuBP complex during oxidation of RuBP. This increases the redox potential of the chloroplast [101], thereby stimulating the production of malate [122,123] and promoting its export from chloroplasts to the cytoplasm. Malate dehydrogenase in the cytoplasm converts malate to oxaloacetate, generating NADH [124,125,126] to empower the initial step of NO3− assimilation [127]. Consequently, mutations that alter malate transport or metabolism influence both photorespiration and NO3− assimilation [122,128,129].
4.2. C1 Metabolism
The photorespiratory pathway within mitochondria involve reactions with glycine. In one reaction, serine hydroxymethyltransferase 1 (SHMT1) converts glycine to serine and converts CH2-THF (5,10-methylene-tetrahydrofolate) to THF (Figure 2). In the other reaction, the glycine decarboxylase complex reduces NAD+ to NADH and catabolizes glycine to CO2, NH3, and CH2-THF (Figure 2). These C1 units, in the form of CH2-THF, serve as precursors in the synthesis of tetrahydrofuran (THF) derivatives [130,131,132,133]. One derivative of CH2-THF, 5-CH3-THF, is used to produce methionine, an essential amino acid. Methionine is a powerful antioxidant and is involved in protein synthesis and methylation of DNA, RNA, proteins, phospholipids, and other substrates [132]. In addition, about 5% of the total assimilated carbon in many secondary metabolites, such as glycine betaine, nicotine, and lignin, derive from C1 metabolism [131].
4.3. Sulfur Assimilation
Photorespiration stimulates sulfur assimilation, although the effects are relatively small. By tracing 33S in reactions involved in sulfur assimilation (such as sulfate reduction and synthesis of cysteine), and 13C in glycine and serine, a positive linear relationship was derived between relative photorespiration and sulfur assimilation. Sulfur assimilation decreases as photorespiration declines and photosynthesis increases [134].
Cysteine, the major product from sulfur assimilation, uses the sulfur element converted from serine generated from photorespiratory pathways [134,135]. H2S, produced from sulfite reduced by sulfite reductase, is incorporated into O-acetylserine (OAS) via a protein complex consisting of serine acetyl transferase and OAS thiol-lyase to form cysteine [135,136]. Cysteine is essential in methionine synthesis, glutathione metabolism, sulfur-rich protein synthesis, glucosinolate biosynthesis, and the synthesis of phytoalexins (Figure 6) [137]. Cysteine is the precursor of methionine through o-phosphohomo-serine and homocysteine. Using methyl tetrahydrofolate as a cofactor, homocysteine is methylated by methionine synthase to yield methionine. Cysteine and methionine are the major sulfur contributors found in downstream metabolites, the most important of which is S-adenosyl methionine (SAM), which is a donor in methyl group transfers, transsulfuration, and aminopropylation [135,138].
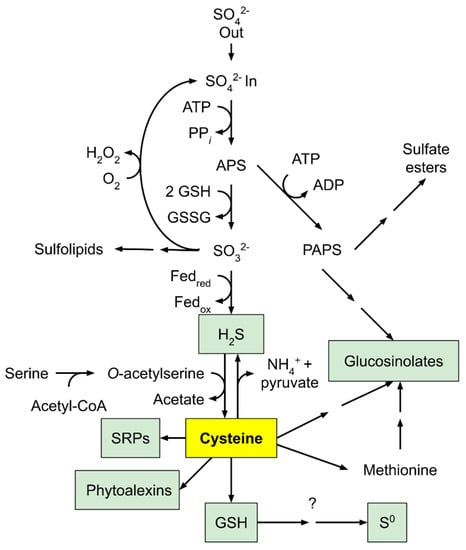
Figure 6.
An outline of sulfur assimilation and its role in producing sulfur-containing defense compounds. Adapted with permission from ref. [137]. Copyright 2005 Elsevier Ltd.
5. Conclusions
Is photorespiration simply a futile cycle? The answer is “no”. Multiple lines of evidence show its crucial role in many plant processes. Despite heroic efforts to suppress photorespiration, disrupting any photorespiratory reaction usually proves detrimental to plants [139,140]. The reassimilation of CO2 from photorespiration [60] and the important role played by photorespiration in the acclimation of plants to conditions, such as salinity [141] and elevated CO2 [142], are topics that are beyond the scope of this review but nevertheless provide important evidence showing that photorespiration is not a wasteful process. There are many promising directions for further studies on photorespiration; for example, examining Mn2+ interactions with Rubisco, further exploring the reassimilation of photorespired CO2, and exploring how the biochemical processes related to photorespiration contribute to its role in adaptation to various conditions will probably reveal that plant carbon fixation and respiration is more energy efficient than what has been previously assumed.
Author Contributions
Conceptualization, X.S. and A.B.; X.S.; writing—original draft preparation, X.S.; writing—review and editing, A.B.; visualization, X.S. and A.B.; supervision, A.B.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded in part by USDA-IWYP-16-06702, NSF grants IOS-16-55810 and CHE-19- 04310, and the John B. Orr Endowment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zelitch, I. Photorespiration: Studies with Whole Tissues. Photosynthesis II 1979, 28, 353–354. [Google Scholar]
- Canvin, D.T. Photorespiration: Comparison Between C3 and C4 Plants. Photosynthesis II 1979, 29, 368–396. [Google Scholar]
- Husic, D.W.; Husic, H.D.; Tolbert, N.E.; Black, C.C. The oxidative photosynthetic carbon cycle or C2 cycle. Crit. Rev. Plant Sci. 1987, 5, 45–99. [Google Scholar] [CrossRef]
- Sharkey, T.D. Estimating the rate of photorespiration in leaves. Physiol. Plant 1988, 73, 147–152. [Google Scholar] [CrossRef]
- Ogren, W.L. Photorespiration: Pathways, Regulation, and Modification. Annu. Rev. Plant Physiol. 1984, 35, 415–442. [Google Scholar] [CrossRef]
- Somerville, C.R.; Ogren, W.L. A phosphoglycolate phosphatase-deficient mutant of Arabidopsis. Nature 1979, 280, 833–836. [Google Scholar] [CrossRef]
- Busch, F.A. Photorespiration in the context of Rubisco biochemistry, CO2 diffusion and metabolism. Plant J. 2020, 101, 919–939. [Google Scholar] [CrossRef]
- Bloom, A.J.; Lancaster, K.M. Manganese binding to Rubisco could drive a photorespiratory pathway that increases the energy efficiency of photosynthesis. Nat. Plants 2018, 4, 414–422. [Google Scholar] [CrossRef]
- Bloom, A.J.; Smart, D.R.; Nguyen, D.T.; Searles, P.S. Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proc. Natl. Acad. Sci. USA 2002, 99, 1730–1735. [Google Scholar] [CrossRef]
- McFadden, G.I. Origin and Evolution of Plastids and Photosynthesis in Eukaryotes. Cold Spring Harb. Perspect. Biol. 2014, 6, a016105. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Rubisco: Still the most abundant protein of Earth? New Phytol. 2013, 198, 1–3. [Google Scholar] [CrossRef]
- Badger, M.R.; Bek, E.J. Multiple Rubisco forms in proteobacteria: Their functional significance in relation to CO2 acquisition by the CBB cycle. J. Exp. Bot. 2008, 59, 1525–1541. [Google Scholar] [CrossRef]
- Kono, T.; Mehrotra, S.; Endo, C.; Kizu, N.; Matusda, M.; Kimura, H.; Mizohata, E.; Inoue, T.; Hasunuma, T.; Yokota, A.; et al. A RuBisCO-mediated carbon metabolic pathway in methanogenic archaea. Nat. Commun. 2017, 8, 14007. [Google Scholar] [CrossRef]
- Tabita, F.R.; Satagopan, S.; Hanson, T.E.; Kreel, N.E.; Scott, S.S. Distinct form I, II, III, and IV Rubisco proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. J. Exp. Bot. 2007, 59, 1515–1524. [Google Scholar] [CrossRef]
- Ogawa, S.; Suzuki, Y.; Yoshizawa, R.; Kanno, K.; Makino, A. Effect of individual suppression of RBCS multigene family on Rubisco contents in rice leaves. Plant Cell Environ. 2011, 35, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Bracher, A.; Whitney, S.M.; Hartl, F.U.; Hayer-Hartl, M. Biogenesis and Metabolic Maintenance of Rubisco. Annu. Rev. Plant Biol. 2007, 68, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Aigner, H.; Wilson, R.H.; Bracher, A.; Calisse, L.; Bhat, J.Y.; Hartl, F.U.; Hayer-Hartl, M. Plant RuBisCo assembly in E. coli with five chloroplast chaperones including BSD2. Science 2017, 358, 1272–1278. [Google Scholar] [CrossRef]
- Foyer, C.H.; Bloom, A.J.; Queval, G.; Noctor, G. Photorespiratory metabolism: Genes, mutants, energetics, and redox signaling. Annu. Rev. Plant Biol. 2009, 60, 455–484. [Google Scholar] [CrossRef]
- Betti, M.; Bauwe, H.; Busch, F.A.; Fernie, A.R.; Keech, O.; Levey, M.; Ort, D.R.; Parry MA, J.; Sage, R.; Timm, S.; et al. Manipulating photorespiration to increase plant productivity: Recent advances and perspectives for crop improvement. J. Exp. Bot. 2016, 67, 2977–2988. [Google Scholar] [CrossRef]
- Walker, B.J.; VanLoocke, A.; Bernacchi, C.J.; Ort, D.R. The Costs of Photorespiration to Food Production Now and in the Future. Annu. Rev. Plant Biol. 2016, 67, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.; Andrews, T.J.; Lorimer, G.H. Reaction intermediate partitioning by ribulose-bisphosphate carboxylases with differing substrate specificities. J. Biol. Chem. 1986, 261, 10248–10256. [Google Scholar] [CrossRef]
- Miziorko, H.M.; Sealy, R.C. Characterization of the ribulosebisphosphate carboxylase-carbon dioxide-divalent cation-carboxypentitol bisphosphate complex. Biochemistry 1980, 19, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Stec, B. Structural mechanism of RuBisCO activation by carbamylation of the active site lysine. Proc. Natl. Acad. Sci. USA 2012, 109, 18785–18790. [Google Scholar] [CrossRef] [PubMed]
- Andrews, T.J.; Lorimer, G.H. The Biochemistry of Plants: A Comprehensive Treatise; Photosynthesis; Academic Press: San Diego, CA, USA, 1987; Volume 10. [Google Scholar]
- Wildner, G.F.; Henkel, J. The effect of divalent metal ions on the activity of Mg(++) depleted ribulose-1,5-bisphosphate oxygenase. Planta 1979, 146, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Mizohata, E.; Ishida, H.; Kogami, A.; Ueno, T.; Makino, A.; Inoue, T.; Yokota, A.; Mae, T.; Kai, Y. Crystal structure of rice Rubisco and implications for activation induced by positive effectors NADPH and 6-phosphogluconate. Curr. Opin. Plant Biol. 2012, 422, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Chollet, R.; Anderson, L.L. Regulation of ribulose 1,5-bisphosphate carboxylase-oxygenase activities by temperature pretreatment and chloroplast metabolites. Arch. Biochem. Biophys. 1976, 176, 344–351. [Google Scholar] [CrossRef]
- McCurry, S.D.; Pierce, J.; Tolbert, N.E.; Orme-Johnson, W.H. On the mechanism of effector-mediated activation of ribulose bisphosphate carboxylase/oxygenase. J. Biol. Chem. 1981, 256, 6623–6628. [Google Scholar] [CrossRef]
- Chu, D.K.; Bassham, J.A. Activation of ribulose 1,5-diphosphate carboxylase by nicotinamide adenine dinucleotide phosphate and other chloroplast metabolites. Plant Physiol. 1974, 54, 556–559. [Google Scholar] [CrossRef][Green Version]
- Hanson, T.E.; Satagopan, S.; Witte, B.H.; Kreel, N.E. Phylogenetic and evolutionary relationships of RubisCO and the RubisCO-like proteins and the functional lessons provided by diverse molecular forms. Philos. Trans R. Soc. Lond. B Biol. Sci. 2008, 363, 2629–2640. [Google Scholar]
- Tcherkez GG, B.; Farquhar, G.D.; Andrews, T.J. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc. Natl. Acad. Sci. USA 2006, 103, 7246–7251. [Google Scholar] [CrossRef]
- Bloom, A.J.; Kameritsch, P. Relative association of Rubisco with manganese and magnesium as a regulatory mechanism in plants. Physiol. Plant 2017, 161, 545–559. [Google Scholar] [CrossRef]
- Pierce, J.; Reddy, G.S. The sites for catalysis and activation of ribulosebisphosphate carboxylase share a common domain. Arch. Biochem. Biophys. 1986, 245, 483–493. [Google Scholar] [CrossRef]
- Jordan, D.B.; Ogren, W.L. Species variation in kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase. Arch. Biochem. Biophys. 1983, 227, 425–433. [Google Scholar] [CrossRef]
- Martin, F.; Winspear, M.J.; MacFarlane, J.D.; Oaks, A. Effect of Methionine Sulfoximine on the Accumulation of Ammonia in C3 and C4 Leaves. Plant Physiol. 1983, 71, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Christeller, J.T. The effects of bivalent cations on ribulose bisphosphate carboxylase/oxygenase. Biochem. J. 1981, 193, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Christeller, J.T.; Laing, W.A. Effects of manganese ions and magnesium ions on the activity of soya-bean ribulose bisphosphate carboxylase/oxygenase. Biochem. J. 1979, 183, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.B.; Ogren, W.L. A Sensitive Assay Procedure for Simultaneous Determination of Ribulose-1,5-bisphosphate Carboxylase and Oxygenase Activities. Plant Physiol. 1981, 67, 237–245. [Google Scholar] [CrossRef]
- Lilley, R.M.; Wang, X.; Krausz, E.; Andrews, T.J. Complete spectra of the far-red chemiluminescence of the oxygenase reaction of Mn2+-activated ribulose-bisphosphate carboxylase/oxygenase establish excited Mn2+ as the source. J. Biol. Chem. 2003, 278, 16488–16493. [Google Scholar] [CrossRef] [PubMed]
- Mogel, S.N.; McFadden, B.A. Chemiluminescence of the Mn2+-activated ribulose-1,5-bisphosphate oxygenase reaction: Evidence for singlet oxygen production. Biochemistry 1990, 29, 8333–8337. [Google Scholar] [CrossRef]
- Bock, C.R.; Connor, J.A.; Gutierrez, A.R. Estimation of excited-state redox potentials by electron-transfer quenching. Application of electron-transfer theory to excited-state redox processes. J. Am. Chem. Soc. 1979, 101, 4815–4824. [Google Scholar] [CrossRef]
- Creutz, C.; Sutin, N. Reaction of tris(bipyridine)ruthenium(III) with hydroxide and its application in a solar energy storage system. Proc. Natl. Acad. Sci. USA 1975, 72, 2858–2862. [Google Scholar] [CrossRef]
- Sattler, W.; Ener, M.E.; Blakemore, J.D. Generation of powerful tungsten reductants by visible light excitation. J. Am. Chem. Soc. 2013, 135, 10614–10617. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.L.; Drincovich, M.F.; Andreo, C.S.; Lara, M.V. Nicotiana tabacum NADP-malic enzyme: Cloning, characterization and analysis of biological role. Plant Cell Physiol. 2008, 49, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Wheeler MC, G.; Arias, C.L.; Tronconi, M.A.; Maurino, V.G.; Andreo, C.S.; Drincovitch, M.F. Arabidopsis thaliana NADP-malic enzyme isoforms: High degree of identity but clearly distinct properties. Plant Mol. Biol. 2008, 67, 231–242. [Google Scholar] [CrossRef]
- Husic, H.D.; Tolbert, N.E. Anion and divalent cation activation of phosphoglycolate phosphatase from leaves. Arch. Biochem. Biophys. 1984, 229, 64–72. [Google Scholar] [CrossRef]
- Decker, J.P. A Rapid, Postillumination Deceleration of Respiration in Green Leaves. Plant Physiol. 1955, 30, 82–84. [Google Scholar] [CrossRef]
- Peterson, R.B. Estimation of Photorespiration Based on the Initial Rate of Postillumination CO2 Release: I. A Nonsteady State Model for Measurement of CO2 Exchange Transients. Plant Physiol. 1983, 73, 978–982. [Google Scholar] [CrossRef]
- Laisk, A.; Kiirats, O.; Oja, V. Assimilatory Power (Postillumination CO2 Uptake) in Leaves. Plant Physiol. 1984, 76, 723–729. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Seemann, J.R.; Pearcy, R.W. Contribution of Metabolites of Photosynthesis to Postillumination CO2 Assimilation in Response to Lightflects. Plant Physiol. 1986, 82, 1063–1068. [Google Scholar] [CrossRef]
- Azcón-Bieto, J.; Osmond, C.B. Relationship between Photosynthesis and Respiration: The Effect of Carbohydrate Status on the Rate of CO2 Production by Respiration in Darkened and Illuminated Wheat Leaves. Plant Physiol. 1983, 71, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. O2-Insensitive Photosynthesis in C3 Plants. Plant Physiol. 1985, 78, 71–75. [Google Scholar] [CrossRef]
- Caemmerer, S.V.; Edmondson, D.L. Relationship Between Steady-State Gas Exchange, in vivo Ribulose Bisphosphate Carboxylase Activity and Some Carbon Reduction Cycle Intermediates in Raphanus sativus. Funct. Plant Biol. 1986, 13, 669–688. [Google Scholar] [CrossRef]
- Badger, M.R.; Sharkey, T.D.; von Caemmerer, S. The relationship between steady-state gas exchange of bean leaves and the levels of carbon-reduction-cycle intermediates. Planta 1984, 160, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, L.J.; Canvin, D.T. An open gas-exchange system for the simultaneous measurement of the CO2 and 14CO2 fluxes from leaves. Can. J. Bot. 1971, 49, 1299–1313. [Google Scholar] [CrossRef]
- Gerbaud, A.; Andre, M. An Evaluation of the Recycling in Measurements of Photorespiration. Plant Physiol. 1987, 83, 933–937. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.H. Carbon isotope fractionation in plants. Phytochemistry 1981, 20, 553–567. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S. Modelling of Photosynthetic Response to Environmental Conditions. Physiol. Plant Ecol. II 1982, 12, 549–587. [Google Scholar]
- Busch, F.A. Current methods for estimating the rate of photorespiration in leaves. Plant Biol. 2012, 15, 648–655. [Google Scholar] [CrossRef]
- Busch, F.A.; Sage, T.L.; Cousins, A.B.; Sage, R.F. C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ. 2012, 36, 200–212. [Google Scholar] [CrossRef]
- Parnik, T.; Keerberg, O. Decarboxylation of primary and end products of photosynthesis at different oxygen concentrations. J. Exp. Bot. 1995, 46, 1439–1477. [Google Scholar] [CrossRef]
- Pärnik, T.; Keerberg, O. Advanced radiogasometric method for the determination of the rates of photorespiratory and respiratory decarboxylations of primary and stored photosynthates under steady-state photosynthesis. Physiol. Plant 2007, 129, 34–44. [Google Scholar] [CrossRef]
- Ivanova, H.; Keerberg, O. Photorespiratory and respiratory decarboxylations in leaves of C3 plants under different CO2 concentrations and irradiances. Plant Cell Environ. 2007, 30, 1535–1544. [Google Scholar]
- Tcherkez, G.; Mahé, A.; Guérard, F.; Boex-Fontvieille, E.R.A.; Gout, E.; Lamothe, M.; Barbour, M.M.; Bligny, R. Short-term effects of CO2 and O2 on citrate metabolism in illuminated leaves. Plant Cell Environ. 2012, 35, 2208–2220. [Google Scholar] [CrossRef] [PubMed]
- Bauwe, H.; Hagemann, M.; Fernie, A.R. Photorespiration: Players, partners and origin. Trends Plant Sci. 2010, 15, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Lacuesta, M.; Dever, L.V.; Munoz-Rueda, A.; Lea, P.J. A study of photorespiratory ammonia production in the C4 plant Amaranthus edulis, using mutants with altered photosynthetic capacities. Physiol. Plant 1997, 99, 447–455. [Google Scholar] [CrossRef]
- Wendler, C.; Barniske, M.; Wild, A. Effect of phosphinothricin (glufosinate) on photosynthesis and photorespiration of C3 and C4 plants. Planta 1990, 24, 55–61. [Google Scholar] [CrossRef]
- Husted, S.; Hebbern, C.A.; Mattsson, M. A critical experimental evaluation of methods for determination of NH4+ in plant tissue, xylem sap and apoplastic fluid. Physiol. Plant 2000, 109, 167–179. [Google Scholar] [CrossRef]
- Ruuska, S.A.; Badger, M.R.; Andrews, T.J.; Von Caemmerer, S. Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: Little evidence for significant Mehler reaction. J. Exp. Bot. 2000, 51, 357–368. [Google Scholar] [CrossRef]
- Canvin, D.T.; Berry, J.A.; Badger, M.R.; Fock, H.; Osmond, C.B. Oxygen Exchange in Leaves in the Light. Plant Physiol. 1980, 66, 302–307. [Google Scholar] [CrossRef]
- De Veau, E.J.; Burris, J.E. Photorespiratory rates in wheat and maize as determined by o-labeling. Chem. Rev. 1989, 90, 500–511. [Google Scholar] [CrossRef]
- Berry, J.A.; Osmond, C.B.; Lorimer, G.H. Fixation of O2 during Photorespiration: Kinetic and Steady-State Studies of the Photorespiratory Carbon Oxidation Cycle with Intact Leaves and Isolated Chloroplasts of C3 Plants. Plant Physiol. 1978, 62, 954–967. [Google Scholar] [CrossRef]
- Cegelski, L.; Schaefer, J. Glycine Metabolism in Intact Leaves by in Vivo 13C and 15N Labeling. J. Biol. Chem. 2005, 280, 39238–39245. [Google Scholar] [CrossRef]
- Arrivault, S.; Guenther, M.; Fry, S.C.; Fuenfgeld, M.M.; Veyel, D.; Mettler-Altmann, T.; Stitt, M.; Lunn, J.E. Synthesis and Use of Stable-Isotope-Labeled Internal Standards for Quantification of Phosphorylated Metabolites by LC–MS/MS. Biochemistry 2015, 87, 6896–6904. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Bauwe, H. Photorespiration: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2017. [Google Scholar]
- Arrivault, S.; Guenther, M.; Ivakov, A.; Feil, R.; Vosloh, D.; van Dongen, J.T.; Sulpice, R.; Stitt, M. Use of reverse-phase liquid chromatography, linked to tandem mass spectrometry, to profile the Calvin cycle and other metabolic intermediates in Arabidopsis rosettes at different carbon dioxide concentrations. Plant J. 2009, 59, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A. Is there a metabolic requirement for photorespiratory enzyme activities in heterotrophic tissues? Mol. Plant 2014, 7, 248–251. [Google Scholar] [CrossRef]
- Mintz-Oron, S.; Meir, S.; Malitsky, S.; Ruppin, E.; Aharoni, A.; Shlomi, T. Reconstruction of Arabidopsis metabolic network models accounting for subcellular compartmentalization and tissue-specificity. Proc. Natl. Acad. Sci. USA 2011, 109, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Lernmark, U.; Henricson, D.; Wigge, B.; Gardestrom, P. Glycine oxidation in mitochondria isolated from light grown and etiolated plant tissue. Physiol. Plant 1991, 82, 339–344. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Application of selected reaction monitoring mass spectrometry to field-grown crop plants to allow dissection of the molecular mechanisms of abiotic stress tolerance. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Bykova, N.V. Involvement of cyanide-resistant and rotenone-insensitive pathways of mitochondrial electron transport during oxidation of glycine in higher plants. FEBS Lett. 1997, 412, 265–269. [Google Scholar] [CrossRef]
- Trufelli, H.; Palma, P.; Famiglini, G. An overview of matrix effects in liquid chromatography–mass spectrometry. Mass Spectrom. Rev. 2011, 30, 491–509. [Google Scholar] [CrossRef]
- Tohge, T.; Mettler, T.; Arrivault, S.; Carroll, A.J. From models to crop species: Caveats and solutions for translational metabolomics. Front. Plant Sci. 2011, 2, 61. [Google Scholar] [CrossRef] [PubMed]
- King, R.; Bonfiglio, R.; Fernandez, C. Mechanistic investigation of ionization suppression in electrospray ionization. J. Am. Soc. Mass Spectrom. 2000, 11, 942–950. [Google Scholar] [CrossRef]
- Bonfiglio, R.; King, R.C.; Olah, T.V. The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun. Mass Spectrom. 1999, 13, 1175–1185. [Google Scholar] [CrossRef]
- Römisch-Margl, W.; Schramek, N.; Radykewicz, T.; Ettenhuber, C.; Eylert, E.; Huber, C.; Römisch-Margl, L.; Schwarz, C.; Dobner, M.; Demmel, N.; et al. 13CO2 as a universal metabolic tracer in isotopologue perturbation experiments. Phytochemistry 2007, 68, 2273–2289. [Google Scholar] [CrossRef]
- Eisenreich, W.; Bacher, A. Advances of high-resolution NMR techniques in the structural and metabolic analysis of plant biochemistry. Phytochemistry 2007, 68, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Heise, R.; Arrivault, S.; Szecowka, M.; Tohge, T.; Nunes-Nesi, A.; Stitt, M.; Nikoloski, Z.; Fernie, A.R. Flux profiling of photosynthetic carbon metabolism in intact plants. Nat. Protoc. 2014, 9, 1803–1824. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Jazmin, L.J.; Young, J.D.; Allen, D.K. Isotopically nonstationary 13C flux analysis of changes in Arabidopsis thaliana leaf metabolism due to high light acclimation. Proc. Natl. Acad. Sci. USA 2014, 111, 16967–16972. [Google Scholar] [CrossRef] [PubMed]
- Szecowka, M.; Heise, R.; Tohge, T.; Nunes-Nesi, A.; Vosloh, D.; Huege, J.; Feil, R.; Lunn, J.; Nikoloski, Z.; Stitt, M.; et al. Metabolic Fluxes in an Illuminated Arabidopsis Rosette. Plant Cell 2013, 25, 694–714. [Google Scholar] [CrossRef]
- Adebiyi, A.O.; Jazmin, L.J.; Young, J.D. 13C flux analysis of cyanobacterial metabolism. Photosyn. Res. 2014, 126, 19–32. [Google Scholar] [CrossRef]
- Cheah, Y.E.; Young, J.D. Isotopically nonstationary metabolic flux analysis (INST-MFA): Putting theory into practice. Curr. Opin. Biotechnol. 2018, 54, 80–87. [Google Scholar] [CrossRef]
- Clark, L.C.; Wolf, R.; Granger, D.; Taylor, Z. Continuous recording of blood oxygen tensions by polarography. J. Appl. Physiol. 1953, 6, 189–193. [Google Scholar] [CrossRef]
- Severinghaus, J.W.; Astrup, P.B. History of blood gas analysis. IV. Leland Clark’s oxygen electrode. J. Clin. Monit. 1986, 2, 125–139. [Google Scholar] [CrossRef]
- Bittig, H.C.; Körtzinger, A.; Neill, C.; van Ooijen, E.; Plant, J.N.; Hahn, J.; Johnson, K.S.; Yang, B.; Emerson, S.R. Oxygen Optode Sensors: Principle, Characterization, Calibration, and Application in the Ocean. Front. Mar. Sci. 2018, 4, 429. [Google Scholar] [CrossRef]
- Fischer, M.; Falke, D.; Pawlik, T.; Sawers, R.G. Oxygen-dependent control of respiratory nitrate reduction in mycelium of Streptomyces coelicolor A3(2). J. Bacteriol. 2014, 196, 4152–4162. [Google Scholar] [CrossRef]
- Flitsch, D.; Ladner, T.; Lukacs, M.; Büchs, J. Easy to use and reliable technique for online dissolved oxygen tension measurement in shake flasks using infrared fluorescent oxygen-sensitive nanoparticles. Microb. Cell Fact. 2016, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Helm, I.; Karina, G.; Jalukse, L.; Pagano, T.; Leito, I. Comparative validation of amperometric and optical analyzers of dissolved oxygen: A case study. Environ. Monit. Assess 2018, 190, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bentzon-Tilia, M.; Severin, I.; Hansen, L.H.; Riemann, L. Genomics and Ecophysiology of Heterotrophic Nitrogen-Fixing Bacteria Isolated from Estuarine Surface Water. mBio 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Ortner, A.; Huber, D.; Haske, O. Laccase mediated oxidation of industrial lignins: Is oxygen limiting? Process Biochem. 2015, 50, 1277–1283. [Google Scholar] [CrossRef]
- Bloom, A.J. Photorespiration and nitrate assimilation: A major intersection between plant carbon and nitrogen. Photosyn. Res. 2014, 123, 117–128. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Asensio, J.S.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Cabrerizo, P.M.; Arrese, C. Pea plant responsiveness under elevated [CO2] is conditioned by the N source (N2 fixation versus NO3− fertilization). Environ. Exp. Bot. 2013, 95, 34–40. [Google Scholar] [CrossRef]
- Rachmilevitch, S.; Cousins, A.B.; Bloom, A.J. Nitrate assimilation in plant shoots depends on photorespiration. Proc. Natl. Acad. Sci. USA 2004, 101, 11506–11510. [Google Scholar] [CrossRef]
- Lekshmy, S.; Jain, V.; Khetarpal, S.; Pandey, R. Inhibition of nitrate uptake and assimilation in wheat seedlings grown under elevated CO2. Indian J. Plant Physiol. 2013, 18, 23–29. [Google Scholar] [CrossRef]
- Pleijel, H.; Uddling, J. Yield vs. quality trade-offs for wheat in response to carbon dioxide and ozone. Glob. Chang. Biol. 2011, 18, 596–605. [Google Scholar] [CrossRef]
- Bloom, A.J.; Asensio JS, R.; Randall, L.; Rachmilevitch, S.; Cousins, A.B.; Carlisle, E.A. CO2 enrichment inhibits shoot nitrate assimilation in C3 but not C4 plants and slows growth under nitrate in C3 plants. Ecology 2012, 93, 355–367. [Google Scholar] [CrossRef]
- Carlisle, E.; Myers, S.S.; Raboy, V.; Bloom, A.J. The effects of inorganic nitrogen form and CO2 concentration on wheat yield and nutrient accumulation and distribution. Front. Plant Sci. 2012, 3, 195. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, P.J., Jr. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Chang. 2014, 4, 477–480. [Google Scholar] [CrossRef]
- Carlisle, E.; Yarnes, C.; Toney, M.D.; Bloom, A.J. Nitrate reductase 15N discrimination in Arabidopsis thaliana, Zea mays, Aspergillus niger, Pichea angusta, and Escherichia coli. Front. Plant Sci. 2014, 5, 317. [Google Scholar] [CrossRef]
- Warburg, O. Assimilatory quotient and photochemical yield. Am. J. Bot. 1948, 35, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Caldwell, R.M.; Finazzo, J.; Warner, R.L. Oxygen and carbon dioxide fluxes from barley shoots depend on nitrate assimilation. Plant Physiol. 1989, 91, 352–356. [Google Scholar] [CrossRef]
- Cen, Y.P.; Turpin, D.H.; Layzell, D.B. Whole-plant gas exchange and reductive biosynthesis in white lupin. Plant Physiol. 2001, 126, 1555–1565. [Google Scholar] [CrossRef]
- Matt, P.; Geiger, M.; Walch-Liu, P.; Engels, C. Elevated carbon dioxide increases nitrate uptake and nitrate reductase activity when tobacco is growing on nitrate, but increases ammonium uptake and inhibits nitrate reductase activity when tobacco is growing on ammonium nitrate. Plant Cell Environ. 2011, 24, 1119–1137. [Google Scholar] [CrossRef]
- Passama, L.; Gojon, A.; Robin, P.; Salsac, L. In situ nitrate reductase activity as an indicator of nitrate availability. Plant Soil 1987, 102, 145–148. [Google Scholar] [CrossRef]
- Kaiser, W.M.; Kandlbinder, A.; Stoimenova, M.; Glaab, J. Discrepancy between nitrate reduction rates in intact leaves and nitrate reductase activity in leaf extracts: What limits nitrate reduction in situ? Planta 2000, 210, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.Q.; Crawford, N.M. Identification and characterization of a chlorate-resistant mutant of Arabidopsis thaliana with mutations in both nitrate reductase structural genes NIA1 and NIA2. Mol. Gen. Genet. 1993, 239, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.L.; Kleinhofs, A. Nitrate utilization by nitrate reductase-deficient barley mutants. Plant Physiol. 1981, 67, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Eichelberger, K.D.; Lambert, R.J.; Below, F.E. Divergent phenotypic recurrent selection for nitrate reductase activity in maize. II. Efficient use of fertilizer nitrogen. Crop Sci. 1989, 29, 1398–1402. [Google Scholar] [CrossRef]
- Andrews, M.; Condron, L.M.; Kemp, P.D.; Topping, J.F. Elevated CO₂ effects on nitrogen assimilation and growth of C₃ vascular plants are similar regardless of N-form assimilated. J. Exp. Bot. 2019, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Condron, L.M.; Kemp, P.D. Will rising atmospheric CO2 concentration inhibit nitrate assimilation in shoots but enhance it in roots of C3 plants? Physiol. Plant 2020, 170, 40–45. [Google Scholar] [CrossRef]
- Obata, T.; Florian, A.; Timm, S.; Bauwe, H.; Fernie, A.R. On the metabolic interactions of (photo)respiration. J. Exp. Bot. 2016, 67, 3003–3014. [Google Scholar] [CrossRef]
- Scheibe, R. Malate valves to balance cellular energy supply. Physiol. Plant 2004, 120, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Voss, I.; Sunil, B.; Scheibe, R.; Raghavendra, A.S. Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol. 2013, 15, 713–722. [Google Scholar] [CrossRef]
- Taniguchi, M.; Miyake, H. Redox-shuttling between chloroplast and cytosol: Integration of intra-chloroplast and extra-chloroplast metabolism. Curr. Opin. Plant Biol. 2012, 15, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Backhausen, J.E.; Emmerlich, A.; Holtgrefe, S.; Horton, P.; Nast, G.; Rogers, J.J.; Müller-Röber, B.; Scheibe, R. Transgenic potato plants with altered expression levels of chloroplast NADP-malate dehydrogenase: Interactions between photosynthetic electron transport and malate metabolism in leaves and in isolated intact chloroplasts. Planta 1998, 207, 105–114. [Google Scholar] [CrossRef]
- Bloom, A.J. The increasing importance of distinguishing among plant nitrogen sources. Curr. Opin. Plant. Biol. 2015, 25, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Dutilleul, C.; Lelarge, C.; Prioul, J.-L.; De Paepe, R.; Foyer, C.H.; Noctor, G. Mitochondria-driven changes in leaf NAD status exert a crucial influence on the control of nitrate assimilation and the integration of carbon and nitrogen metabolism. Plant Physiol. 2005, 139, 64–78. [Google Scholar] [CrossRef]
- Schneidereit, J.; Häusler, R.E.; Fiene, G.; Kaiser, W.M.; Weber AP, M. Antisense repression reveals a crucial role of the plastidic 2-oxoglutarate/malate translocator DiT1 at the interface between carbon and nitrogen metabolism. Plant J. 2005, 45, 206–224. [Google Scholar] [CrossRef]
- Cossins, E.A. The fascinating world of folate and one-carbon metabolism. Can. J. Bot. 2000, 78, 691–708. [Google Scholar]
- Hanson, A.D.; Roje, S. One-carbon metabolism in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 119–137. [Google Scholar] [CrossRef]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef]
- Gorelova, V.; Ambach, L.; Rébeillé, F.; Stove, C.; Van Der Straeten, D. Folates in Plants: Research Advances and Progress in Crop Biofortification. Front. Chem. 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Abadie, C.; Tcherkez, G. Plant sulphur metabolism is stimulated by photorespiration. Commun. Biol. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Tcherkez, G.; Tea, I. 32S/34S isotope fractionation in plant sulphur metabolism. New Phytol. 2013, 200, 44–53. [Google Scholar] [CrossRef]
- Amrani, A.; Kamyshny, A.; Lev, O.; Aizenshtat, Z. Sulfur Stable Isotope Distribution of Polysulfide Anions in an (NH4)2Sn Aqueous Solution. Inorg. Chem. 2006, 45, 1427–1429. [Google Scholar] [CrossRef]
- Rausch, T.; Wachter, A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, G.L. The nature of the active methyl donor formed enzymatically from L-methionine and adenosine triphosphate1,2. J. Am. Chem. Soc. 1952, 74, 2942–2943. [Google Scholar] [CrossRef]
- Timm, S.; Bauwe, H. The variety of photorespiratory phenotypes—Employing the current status for future research directions on photorespiration. Plant Biol. 2013, 15, 737–747. [Google Scholar] [CrossRef]
- Eisenhut, M.; Ruth, W.; Haimovich, M.; Bauwe, H.; Kaplan, A.; Hagemann, M. The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc. Natl. Acad. Sci. USA 2008, 105, 17199–17204. [Google Scholar] [CrossRef]
- Eisenhut, M.; Bräutigam, A.; Timm, S.; Florian, A.; Tohge, T. Photorespiration is crucial for dynamic response of photosynthetic metabolism and stomatal movement to altered CO2 availability. Mol. Plant 2017, 10, 47–61. [Google Scholar] [CrossRef]
- Ziotti, A.; Silva, B.P.; Neto, M. Photorespiration is crucial for salinity acclimation in castor bean. Environ. Exp. Bot. 2019, 167, 103845. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).