1. Introduction
Cultural methods (i.e., agricultural practices) can play important roles in preventing or minimizing many plant diseases. Different practices can be implemented during seeding/planting, as the crop is growing or even after harvest. Cultural techniques aimed at minimizing disease include the choice of altitude at which to plant a particular crop, the means of preparing and cultivating the soil, the use of particular cultivars, the treatment of propagation material, the choice of particular planting times and depths, the exposure to air movement, the direction of plant rows, weed-management and irrigation practices, changes in plant nutrition, and general sanitation [
1,
2,
3]. In covered crops, farmers can also consider the type of cover spread over the crop, heating, ventilation, and soil mulch [
4]. Cultural methods that affect the environmental conditions inside the greenhouse and minimize the presence of water on the canopy can be effective ways of controlling diseases that are promoted by high humidity [
5].
Sweet basil (
Ocimum basilicum L.) is an economically important annual herb crop from the Labiatae family that is grown in polyethylene-covered structures (i.e., greenhouse structures or walk-in tunnels). Sweet basil greenhouses are common along the ridge above the Syrian-African Rift, south of the Sea of Galilee, and around and north of the Dead Sea. Crops are planted from September on, so winter and spring crops are common, but are challenged by humidity-promoted diseases [
6,
7,
8,
9].
Downy mildew of basil (
Peronospora sp.) was first identified in Uganda [
10]. The causal agent was identified as an oomycete pathogen and named
Peronospora belbahrii Thines [
11,
12]. This pathogen mainly infects the leaf blades of sweet basil [
12]. Infected leaves are distorted, asymmetric chlorosis develops on the leaf blades, and dark spores (on sporangiophores) form on the underside of the leaves [
13,
14]. Sweet basil downy mildew (SBDM) was found to be particularly severe when plants were kept wet for at least 6 to 12 h immediately after inoculation; the pathogen was most active at 20 °C, whereas at 12 and 27 °C, the disease was suppressed [
13]. Sporulation occurs when infected plants are incubated for at least 7.5 h in a dark, moisture-saturated atmosphere at 10 to 27 °C and is suppressed by light [
14]. The effects of temperature and relative humidity on SBDM have been confirmed under field conditions. Passive heating has been shown to increase root temperatures and induce resistance in sweet basil [
9].
Methods for controlling SBDM may involve fungicides, seed treatments, and/or breeding for resistance [
15,
16,
17]. Seeds have been found to be vehicles for pathogen transmission. It was, therefore, recommended that a seed-certification scheme be established and that the pathogen be controlled on the seeds [
18]. In terms of fungicide treatments, mefenoxam with copper hydroxide, azoxystrobin, and mandipropamid effectively suppress SBDM, but the economic value of those treatments has not been demonstrated [
19]. The development of resistance [
15] to chemical fungicides and the need to avoid any residues on the harvested shoots limit the use of fungicides in sweet basil.
Previous studies have examined other humidity-promoted diseases of sweet basil:
Botrytis cinerea-induced gray mold and
Sclerotinia sclerotiorum-induced white mold. Gray mold initially infects stem wounds after harvest and white mold initially infects the stem base. Both of those pathogens are necrotrophs that benefit from the film of water present on the plant organs. Increased plant spacing, the use of polyethylene mulch, and increased greenhouse aeration have been shown to reduce the incidence of both gray mold and white mold in sweet basil [
7,
8].
P. belbahrii is an oomycete biotroph that initially infects leaves. This makes it different from the two necrotrophic pathogens, but it is similar to those pathogens in that it depends on water present on the leaf for infection. Our hypothesis was that the conditions in sweet basil greenhouses are favorable for SBDM infection and that we could affect the severity of disease by manipulating those conditions. Thus, the aim of the present research was to test cultural means of controlling SBDM in greenhouses and walk-in tunnels under commercial-like conditions. The cultural means examined included increased air circulation, manipulation of the direction of the walk-in tunnels, decreased planting density, and the use of polyethylene mulch.
3. Discussion
Cultural measures can be used to suppress SBDM. The following factors were examined in the present work: tunnel orientation, air circulation in a greenhouse, polyethylene mulch, and plant spacing. The level of disease control did not vary with the different growing seasons and intermediate-level disease control was observed in both locations. Studies of cultural means of controlling downy mildews are scarce; therefore, it is only possible to compare the currently studied means with similar methods that have been examined in other patho-systems.
Nighttime air circulation in the greenhouse was increased in an effort to reduce the amount of water on the aerial plant organs and the amount of time that water was present. Humidity-promoted pathogens such as
P. belbahrii thrive in the film of water that primarily appears at night and depend on it for their reproduction and their ability to infect the plant [
9,
13]. Increased air circulation can reduce the presence of water at the boundary layer over the plant organs even under humid conditions [
20] and can limit humidity-promoted diseases [
4]. Indeed, the test carried out over three growing seasons revealed that increased air circulation at night can suppress SBDM in greenhouses. Similarly, Papas [
21] concluded that
B. cinerea in out-of-season tomato (
Solanum lycopersicum) plants grown in unheated glasshouses in Greece can be limited by adequate indoor air circulation. Similarly, in-bed air circulation has been shown to reduce
B. cinerea gray mold in lisianthus (
Eustoma grandiflorum) [
22].
In the sweet basil-growing regions in Israel, walk-in tunnels are traditionally oriented east-west. The tunnel orientation affects the microclimate in the tunnel, since it relates to the direction of the sun [
6,
9,
23] and prevailing winds. Surprisingly, the north-south orientation was associated with lower SBDM severity in all of our experiments. Nevertheless, the yields from north-south and east-west tunnels were similar. These results suggest that the temperature × hours gain in the two types of tunnels are similar, but the moisture duration was different. We are not aware of similar results in other patho-systems.
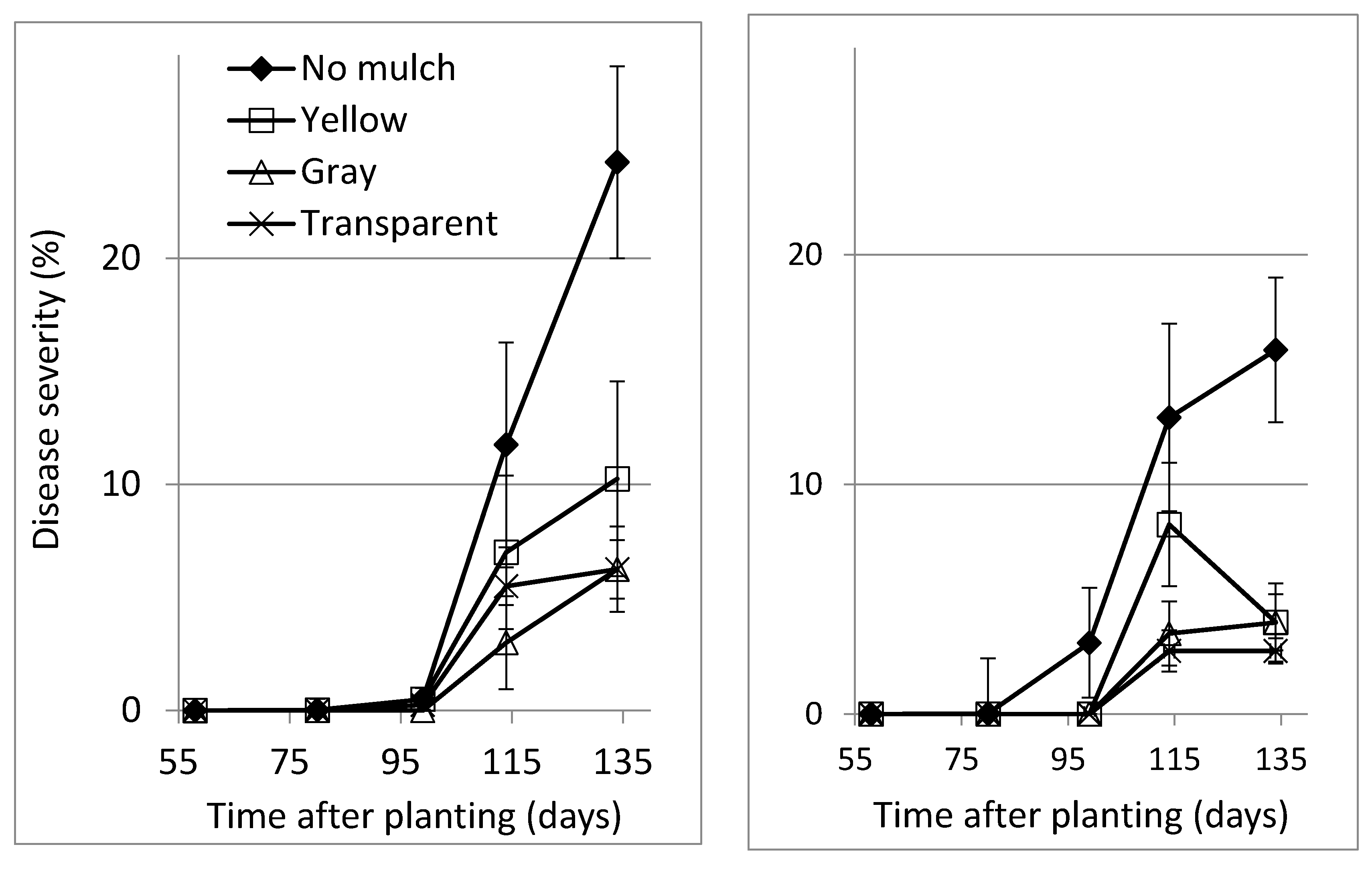
Polyethylene soil cover has been suggested for the control of gray mold induced by
B. cinerea [
24]. A plastic cover with a white upper surface reduced the incidence of
B. cinerea infection in strawberry (
Fragaria × ananassa), compared to bare soil [
25]. Similarly, in lisianthus, the use of a polyethylene barrier between the lower leaves and soil that prevented the lower leaves from coming into contact with the wet soil reduced the development of
B. cinerea along the leaves toward the stem and prevented plant mortality [
22]. The prevention of contact between the canopy and the wet soil is not relevant in the case of SBDM. However, polyethylene mulch effectively suppressed SBDM in both locations, congruent with the effects such mulch has been shown to have on
B. cinerea-induced gray mold [
8] and
S. sclerotiorum-induced white mold [
7] in sweet basil. As expected [
7,
8], polyethylene mulch increased shoot yield in two of three experiments.
In addition to decreasing evaporation from the growth medium, the polyethylene mulch also increases the bed temperature. Shtienberg et al. [
26] showed that the polyethylene mulch causes irradiation flux across the canopy and the drying of the leaves and fruits of greenhouse tomato and cucumber (
Cucumis sativus) plants. This helps to control tomato late blight (
Phytophthora infestans) and downy mildew (
Pseudoperonospora cubensis) in cucumber [
26]. Polyethylene soil cover is associated with increased yields, thanks to the accumulation of heat in the root zone and higher soil temperatures [
27]. It was suggested that passive greenhouse warming increases sweet basil’s resistance to downy mildew by warming the root zone [
9]. Recently, Gupta et al. [
28] demonstrated that warming the root zone induces systemic resistance in plants. Indeed, warming the root zone of sweet basil under field conditions resulted in shoot resistance to the necrotrophic fungi
B. cinerea and
S. sclerotiorum that continued after harvest [
7,
8]. It may be assumed that polyethylene mulch may reduce the susceptibility of harvested leaves also to SBDM.
Reducing the density of sweet basil plants reduced SBDM, as previously demonstrated for gray mold and white mold [
7,
8]. Reduced plant density has also been shown to suppress disease in other patho-systems. Vieira et al. [
29] reported decreased incidence of white mold and increased soybean yields when within-row densities were reduced. Lower plant density has also been shown to reduce stem gray mold in lisianthus [
22]. Reducing the number of blond psyllium (
Plantago ovata) seeds sown per unit area reduced the incidence of downy mildew (
Peronospora alta) in that crop [
30]. The reduction from 12 to 6 bean plants/m
2 decreased the severity of bean white mold (
S. sclerotiorum) in one of two experiments, but did not decrease yield [
31]. The severity of soybean stem canker (
Diaporthe phaseolorum var.
meridionalis) decreased proportionately to a decrease in plant densities [
32]. In downy mildew of rose (
Peronospora sparsa), reducing the density of container-grown plants had a measurable effect on the progress of downy mildew [
33].
The reduction in sweet basil planting density resulted in reduced canopy volume at the beginning of the season. But, after the second harvest, the canopy was dense. Nevertheless, SBDM levels were lower in the reduced planting despite the dense canopy. The mechanism of this control could not be studied with the biotroph
P. belbahrii, but our experience with
B. cinerea and
S. sclerotiorum pointed to reduced shoot susceptibility to pathogens [
7,
8]. We hypothesize that the reduction in planting density also affects the plants’ susceptibility to SBDM.
We calculated the correlation between disease severity values and the intensity of disease reduction across experiments. There was a negative correlation between the disease in the denser plots and disease reduction in the plots in which plants were planted at the lower density, pointing to the fact that under conditions of lower disease pressure, increased plant spacing provides more pronounced disease suppression. Surprisingly, such a negative correlation was not found for the polyethylene mulch practice. In some experiments, we also examined the possibility of combining polyethylene mulch with reduced planting density, but that combination did not provide synergistic disease control (results not shown). When applied in combination with chemical fungicide, neither reduced plant density nor the use of polyethylene provided synergistic disease control (results not shown). As described, there was also no synergistic effect between increased air circulation and transparent or gray polyethylene mulch.
4. Materials and Methods
Experiments were carried out at two experimental stations (Sites 1 and 2, described in detail below) under semi-commercial conditions during the years 2013–2015. Sweet basil cv. Peri [
34] plants were used in all of these experiments. Plugs were prepared in a commercial nursery (Hishtil, Ashkelon, Israel) and transplanted 3 to 4 weeks after seeding. Each plug contained 3 to 5 plants, but the plugs are usually referred to as plants. “Peri” is susceptible to
P. belbahrii [
9]. The experiments were carried out in greenhouses (Site 1) and in walk-in tunnels (Site 2). Downy mildew epidemics occurred naturally at the field sites, following the placement of infected basil plants next to the plots as described below.
4.1. Inoculation with P. belbahrii and Disease Evaluation
Spores of
P. belbahrii were harvested in water by washing sporulating leaves of sweet basil plants that were kept in an experimental greenhouse at the Volcani Center, Agricultural Research Organization, Israel. The suspension was then filtered through cheesecloth. The concentration of spores was determined using a hemocytometer and a light microscope, and adjusted to 1 × 10
3 cells ml
−1. Potted sweet basil plants were inoculated by spraying with a spore suspension (5 mL plant
−1), incubated at high RH (>95%) in the dark in a growth chamber at 22 ± 1 °C for 12 h and then incubated in a greenhouse chamber at 22 ± 2 °C for 1 week, and incubated at high RH (>95%) in the dark in a growth chamber at 22 ± 1 °C for 12 h and then incubated in a greenhouse chamber at 22 ± 2 °C for symptom development [
9]. The potted sweet basil plants subjected to this artificial inoculation served as a source of inoculum to ensure even inoculum loads across the greenhouses and walk-in tunnels. The plants were placed at the borders of each plot.
The evaluation of the severity of sweet basil downy mildew (SBDM) in the plots included all plants except those along the 1 m edges of each plot. The severity of SBDM was determined periodically in all plants of each plot in each experiment on a scale of 0 to 100, in which 0 = all plants visually healthy, 10 = 10% of the leaf area in the plot covered by typical downy mildew symptoms of chlorosis and/or dry necrotic lesions or
P.
belbahrii spores on the undersides of the leaves, and 100 = all leaves on all plants in the plot show typical downy mildew symptoms/signs [
9].
4.2. Shoot Weight
In selected experiments, shoots longer than 15 cm were harvested and weighed three to five times during the growing season, as detailed below. The yield was collected separately for each plot, sorted for quality, and calculated per m2 bed. The cumulative yield figures for the various harvests were calculated and those figures are presented.
4.3. Site 1—Eden Experimental Station
Experiments were conducted at the Eden Experimental Station (32°46′79 N, 35°48′88 E; 120 m below mean sea level) at the Emek Hamaayanot Research and Development Center. The regional climate is Mediterranean, semiarid with winter rains and a dry, hot summer. At this site, experiments were carried out in two 400 m2 greenhouses. The structures were covered with 150 µm-thick Sunsaver Clear IR AV polyethylene (Ginegar Plastic Products, Kibutz Ginegar, Israel). The greenhouses were aerated during the day and closed during the night (18:00 to 07:00). At night, the greenhouses were heated to 12 °C to prevent physiological damage to the leaves. There were five bays in each greenhouse and the bays were separated with 1.8 m-high transparent polyethylene.
The potting material was tuff (volcanic gravel; 3 to 6 mm particles) placed in plastic containers that were 1 m wide × 15 cm deep × 20 m long (Mapal, Mevo Hama, Israel). Plants were irrigated daily according to local extension service recommendations, allowing 30% drainage, and fertigated proportionally with 5-3-8 N-P-K fertilizer at a rate of 2 L/1000 L water. The nutrient concentrations were therefore 8.6, 1.0, and 4.0 mM N, P, and K, respectively. Fertigation was performed using a 17 mm drip-irrigation pipe with a 1 L/h dripper embedded in the pipe every 20 cm. Plots were 5 m long each, containing 108–125 plants/plot at the higher plant density (24–25 plants/m2) mentioned below.
Experiments were carried out over three consecutive growing seasons, with planting dates of 9 September (fall 2013), 24 February (spring 2014), and 19 January (winter 2015). Treatments consisted of different cultural methods, as detailed below and in
Table 9. Plots consisted of one bed (1 m wide and 4.5 to 5.0 m long) and there were 4–8 plot replicates.
Fall 2013 experiments: SBDM was first observed on 27 November 2013, 80 days after planting. There were five shoot harvests, starting 7 October 2013 (29 days after planting).
Spring 2014 experiments: SBDM was first observed on 20 March 2014, 24 days after planting. There were six shoot harvests, starting 26 March 2014 (30 days after planting).
Winter 2015 experiments: SBDM was first observed on 14 April 2015, 90 days after planting. Shoots were harvested five times, starting 1 March (45 days after planting).
4.4. Cultural Methods Applied at Site 1
Air circulation: Four fans (60 cm diam., Adirom Heating and Ventilation Engineering Ltd., Ashkelon, Israel) were installed 2 m above the beds, facing the canopy of plants that were planted at the area of one third of a greenhouse bay toward the north or south edge of the greenhouse. The fans were operated once every hour for 15 min from 19:00 until 08:00. Beds were covered with gray polyethylene mulch and the planting density was 24–25 plants/m2.
Planting density: Sweet basil plants were planted at two densities: 24–30 plants/m
2, as is customary in the area, and 14–15 plants/m
2. The higher planting density was also used in experiments in which planting density was not a tested parameter. The beds were left bare or covered with gray or transparent polyethylene (
Table 9).
Polyethylene mulch: The beds were either left uncovered (bare growing medium) or covered with sheets of polyethylene. Several types of polyethylene were examined: (1) transparent 30 µm-thick Sunsaver Clear IR polyethylene (Ginegar), (2) gray-black 30 µm-thick Mulch-More polyethylene (Ginegar) with the gray-colored side visible and the black-colored side facing the ground, and (3) yellow-brown 30 µm-thick Mulch-More polyethylene (Ginegar) with the yellow side visible and the brown side facing the ground. The plant density was 24–25 plants/m
2 (
Table 9).
4.5. Site 2—Zohar Experimental Station
This research station is located in the Sedom area south of the Dead Sea and is part of the Northern Arava Research and Development Center. It is located at 30°94′656.2 N, 35°40′341.7 E at 354 m below mean sea level. The weather at the Zohar Station is arid. In the winter, rain is rare and the mean daytime temperature is 22 °C. The summers are dry and hot, with an average daily temperature of 33 °C. The work at the Zohar Experimental Station was carried out in 10 walk-in tunnels. Each tunnel was 40 m long and 5 m wide (200 m2). The structures were covered with 100 µm-thick Sunsaver Clear IR AV polyethylene (Ginegar Plastic Products, Ginegar, Israel). The front and back openings of each tunnel were covered with 50-mesh netting. Five round aeration openings (50 cm diam.) were cut along the length of the tunnels and covered with 50-mesh netting. One-meter-wide sandy soil beds were planted with 30 plants/m2, unless otherwise noted. Plants were irrigated with local brackish water (4 decisiemens per meter), according to the local extension service recommendations, and fertigated with 1.0 L/1000 L 8-2-4 N-P-K fertilizer. Nutrient concentrations were therefore 6.9, 0.33, and 1.0 mM N, P, and K, respectively. Fertigation was performed using a 17 mm drip-irrigation pipe with a 1.2 L/h dripper embedded in the pipe every 20 cm. Each plot consisted of two beds that were each 9 m long, unless otherwise mentioned.
Experiments were carried out over two consecutive growing seasons, with planting dates of 19 February (spring 2014 season) and 11 November 2014 (winter 2015 season). Treatments consisted of different cultural methods, as detailed below and in
Table 10. Plots consisted of two beds (1 m wide and 9 m long) containing 270 plants/plot of 30 plants/m
2 and there were 5‒10 plot replicates.
Spring 2014 experiments: SBDM was first observed on 20 March 2014, 31 days after planting. There were six shoot harvests, beginning 15 March 2014 (36 days after planting).
Winter 2015 experiments: SBDM was first observed on 21 January 2015, 71 days after planting. Shoots were harvested four times starting 8 December 2014 (27 days after planting).
4.6. Cultural Methods Applied at Site 2
Tunnel direction: Walk-in tunnels were oriented north-south or east-west with either bare soil or with transparent polyethylene mulch. The planting density in the plots was 30 plants/m
2 (
Table 10).
Planting density: Sweet basil plants were planted at two densities: 30 plants/m
2, as is the common local practice, or 15 plants/m
2. The higher planting density was also used in experiments in which the planting density was not a tested parameter. The soil was covered with transparent polyethylene (
Table 10).
Polyethylene soil mulch: The beds were either left uncovered (bare growing medium) or covered with transparent polyethylene (30 µm-thick Sunsaver Clear IR polyethylene; Ginegar). The beds were planted with 30 plants/m
2 in tunnels oriented either north-south or east-west (
Table 10).
4.7. Experimental Design and Statistical Analysis
Treatments in each year and each field experiment were replicated 4–10 times. Replicates of each treatment were arranged randomly. Disease severity was evaluated in each plot (replicate). Area under the disease severity progress curve (AUDPC) values were also calculated. Data in percentages were arcsine-transformed before further analysis. Disease severity (%) and AUDPC (% × days) data were analyzed using ANOVA and Tukey’s HSD test. Standard errors (SE) of the means were calculated and disease levels were statistically separated following a one-way analysis of variance. Treatments in experiments with combined two-treatment factors were statistically separated following a two-way analysis of variance. Statistical analyses were performed using JMP 5.0 software (SAS Institute, Cary, NC, USA).
Disease reduction was calculated as follows:
The combined effect of the control measures used was estimated using the Abbott formula [
35,
36]. The expected disease reduction (control efficacy) and the combined suppressive activity were calculated as:
where a = disease reduction due to one measure when applied alone, b = disease reduction due to the other measure when applied alone, CE
exp = expected control efficacy of the combined treatment if the two measures act additively, CE
obs = observed disease reduction for the combined treatment, and SF = the synergy factor achieved by the combined treatment. When SF = 1, the interaction between the control measures is additive. When SF < 1, the interaction is antagonistic, and when SF > 1, the interaction is synergistic [
26,
35,
36]. The same formula was used to calculate SF in the context of yield.