Effects of Monochromatic Illumination with LEDs Lights on the Growth and Photosynthetic Performance of Auxenochlorella protothecoides in Photo- and Mixotrophic Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Trophic Conditions and Monochromatic Illumination on the Growth Rates of A. protothecoides
2.1.1. Photoautotrophic Conditions
2.1.2. Mixotrophic Conditions
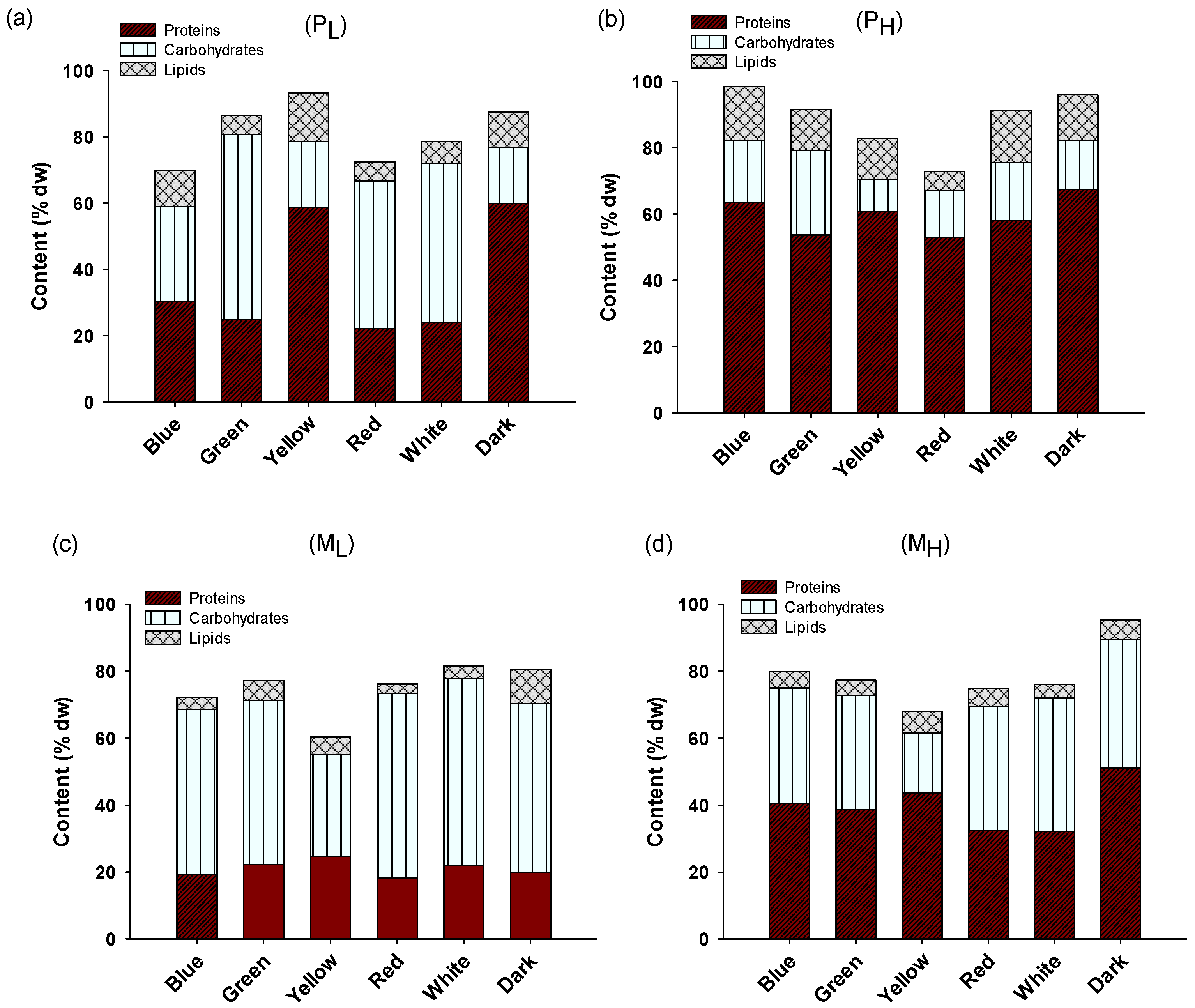
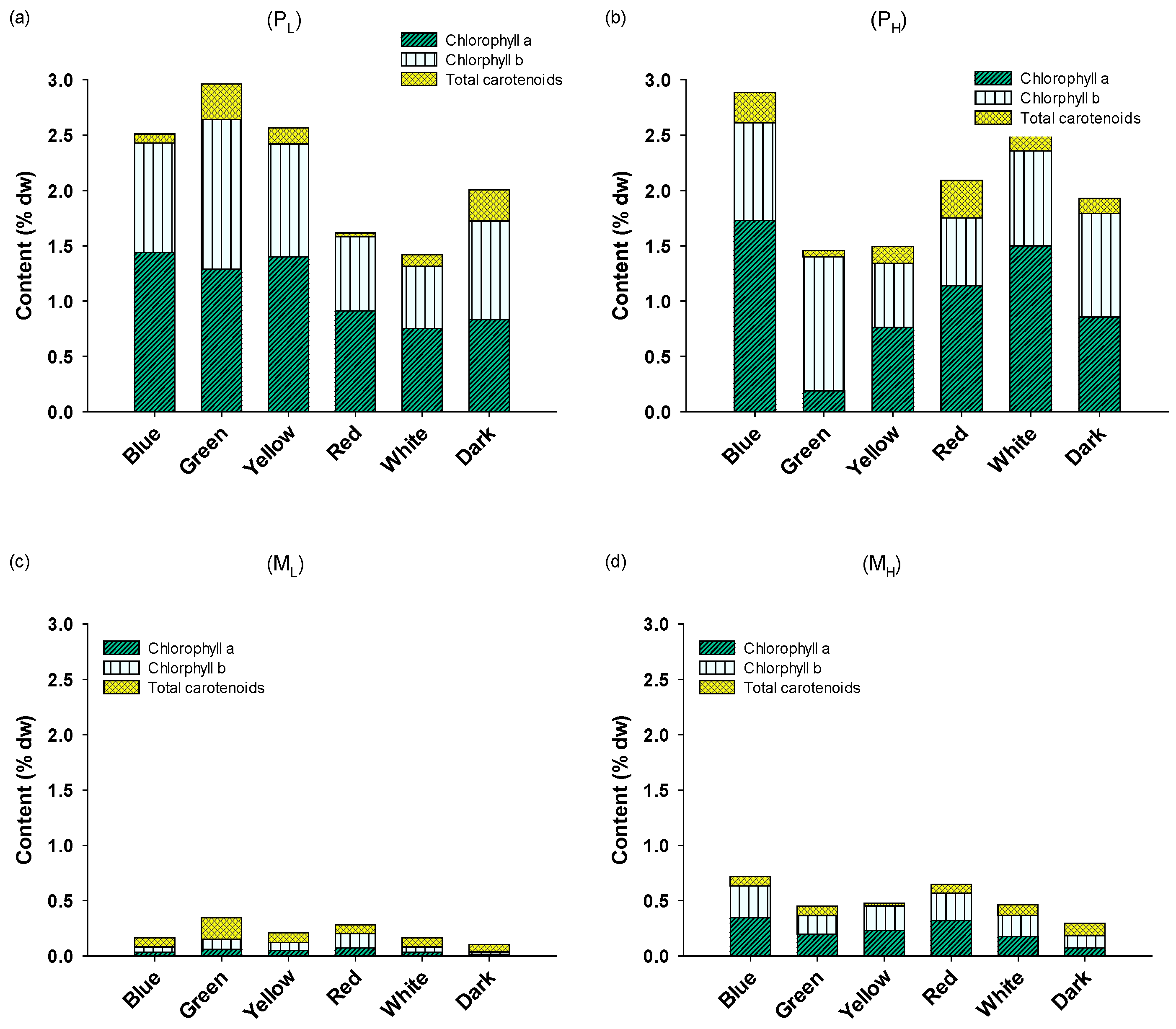
2.2. Biochemical Composition and Pigment Content of A. protothecoides
2.3. Chlorophyll Fluorescence Studies
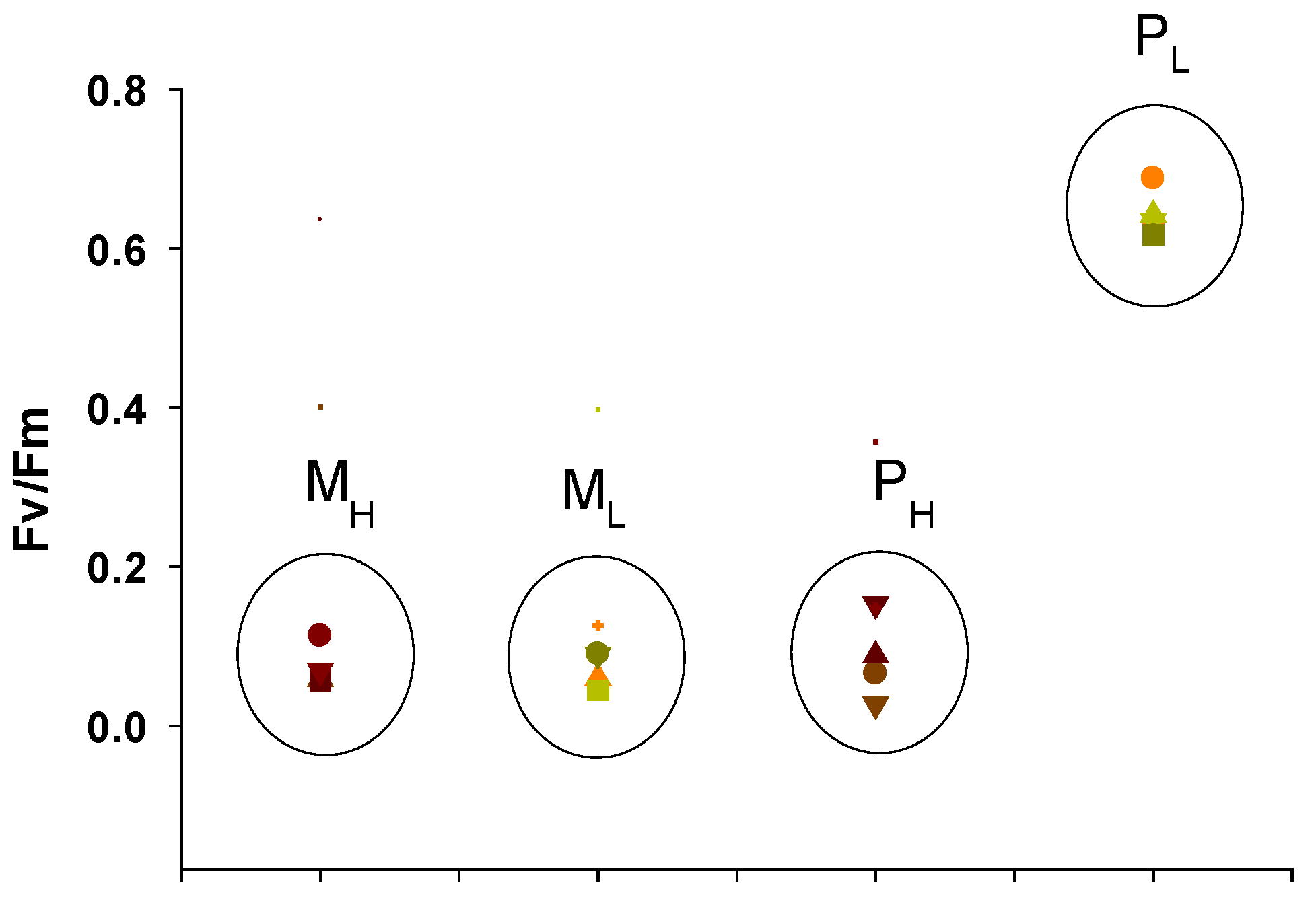
2.3.1. Maximum Quantum Yields of Primary Photochemistry and Non-Photochemical Quenching (NPQ)
2.3.2. QA− Re-Oxidation Kinetics on Cultures with White Light
2.3.3. Inactive PSII RCs and QB Non-Reducing RCs on Cultures with White Light
3. Materials and Methods
3.1. Microorganism and Cultivation Conditions
3.2. Experimental Design
3.3. Analytical Methods
3.3.1. Biomass Analysis
3.3.2. Chlorophyll Fluorescence Measurements and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Pang, N.; Gu, X.; Chen, S.; Kirchhoff, H.; Lei, H.; Roje, S. Exploiting mixotrophy for improving productivities of biomass and co-products of microalgae. Renew. Sustain. Energy Rev. 2019, 112, 450–460. [Google Scholar] [CrossRef]
- D’Imporzano, G.; Silvia, S.; Davide, V.; Barbara, S.; Fabrizio, A. Microalgae Mixotrophic Growth: Opportunity for Stream Depuration and Carbon Recovery. In Prospects and Challenges in Algal Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 141–177. [Google Scholar]
- Paranjape, K.; Leite, G.B.; Hallenbeck, P.C. Strain variation in microalgal lipid production during mixotrophic growth with glycerol. Bioresour. Technol. 2016, 204, 80–88. [Google Scholar] [CrossRef][Green Version]
- Cerón-García, M.C.; Macías-Sánchez, M.D.; Sánchez-Mirón, A.; García-Camacho, F.; Molina-Grima, E. A process for biodiesel production involving the heterotrophic fermentation of Chlorella protothecoides with glycerol as the carbon source. Appl. Energy 2013, 103, 341–349. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Walker, T.H. Biomass and lipid production of heterotrophic microalgae Chlorella protothecoides by using biodiesel-derived crude glycerol. Biotechnol. Lett. 2011, 33, 1973. [Google Scholar] [CrossRef]
- O’Grady, J.; Morgan, J.A. Heterotrophic growth and lipid production of Chlorella protothecoides on glycerol. Bioprocess Biosyst. Eng. 2011, 34, 121–125. [Google Scholar] [CrossRef]
- Xiao, Y.; Lu, Y.; Dai, J.; Wu, Q. Industrial fermentation of auxenochlorella protothecoides for production of biodiesel and Its application in vehicle diesel engines. Front. Bioeng. Biotechnol. 2015, 3, 164. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, K. Heterotrophic Culture of Microalgae Using Biodiesel Derived Glycerol. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2014. [Google Scholar]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Masojídek, J.; Vonshak, A.; Torzillo, G. Chlorophyll fluorescence applications in microalgal mass cultures. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 277–292. [Google Scholar]
- Keren, N.; Krieger-Liszkay, A. Photoinhibition: Molecular mechanisms and physiological significance. Physiol. Plant 2011, 142, 1–5. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Carvalho, A.; Silva, S.; Baptista, J.; Malcata, F. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Lababpour, A.; Hada, K.; Shimahara, K.; Katsuda, T.; Katoh, S. Effects of nutrient supply methods and illumination with blue light emitting diodes (LEDs) on astaxanthin production by Haematococcus pluvialis. J. Biosci. Bioeng. 2004, 98, 452–456. [Google Scholar] [CrossRef]
- Markou, G. Effect of Various Colors of Light-Emitting Diodes (LEDs) on the Biomass Composition of Arthrospira platensis Cultivated in Semi-continuous Mode. Appl. Biochem. Biotechnol. 2014, 172, 2758–2768. [Google Scholar] [CrossRef]
- Schulze, P.S.; Barreira, L.A.; Pereira, H.G.; Perales, J.A.; Varela, J.C. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef]
- Ra, C.-H.; Kang, C.-H.; Jung, J.-H.; Jeong, G.-T.; Kim, S.-K. Effects of light-emitting diodes (LEDs) on the accumulation of lipid content using a two-phase culture process with three microalgae. Bioresour. Technol. 2016, 212, 254–261. [Google Scholar] [CrossRef]
- Pattison, P.; Tsao, J.; Brainard, G.; Bugbee, B. LEDs for photons, physiology and food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Kirchhoff, H.; Gargouri, M.; Feng, J.; Cousins, A.B.; Pienkos, P.T.; Gang, D.R.; Chen, S. Assessment of photosynthesis regulation in mixotrophically cultured microalga Chlorella sorokiniana. Algal Res. 2016, 19, 30–38. [Google Scholar] [CrossRef]
- Liu, X.; Duan, S.; Li, A.; Xu, N.; Cai, Z.; Hu, Z. Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum. J. Appl. Phycol. 2009, 21, 239–246. [Google Scholar] [CrossRef]
- Jacobi, A.; Steinweg, C.; Sastre, R.R.; Posten, C. Advanced photobioreactor LED illumination system: Scale-down approach to study microalgal growth kinetics. Eng. Life Sci. 2012, 12, 621–630. [Google Scholar] [CrossRef]
- Ma, R.; Thomas-Hall, S.R.; Chua, E.T.; Eltanahy, E.; Netzel, M.E.; Netzel, G.; Lu, Y.; Schenk, P.M. LED power efficiency of biomass, fatty acid, and carotenoid production in Nannochloropsis microalgae. Bioresour. Technol. 2018, 252, 118–126. [Google Scholar] [CrossRef]
- Jung, J.-H.; Sirisuk, P.; Ra, C.H.; Kim, J.-M.; Jeong, G.-T.; Kim, S.-K. Effects of green LED light and three stresses on biomass and lipid accumulation with two-phase culture of microalgae. Process Biochem. 2019, 77, 93–99. [Google Scholar] [CrossRef]
- Hultberg, M.; Jönsson, H.L.; Bergstrand, K.-J.; Carlsson, A.S. Impact of light quality on biomass production and fatty acid content in the microalga Chlorella vulgaris. Bioresour. Technol. 2014, 159, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Ra, C.H.; Kang, C.-H.; Jung, J.-H.; Jeong, G.-T.; Kim, S.-K. Enhanced biomass production and lipid accumulation of Picochlorum atomus using light-emitting diodes (LEDs). Bioresour. Technol. 2016, 218, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- de Mooij, T.; de Vries, G.; Latsos, C.; Wijffels, R.H.; Janssen, M. Impact of light color on photobioreactor productivity. Algal Res. 2016, 15, 32–42. [Google Scholar] [CrossRef]
- Chen, Z.; Su, B. Influence of Medium Frequency Light/Dark Cycles on the Cultivation of Auxenochlorella pyrenoidosa. Appl. Sci. 2020, 10, 5093. [Google Scholar] [CrossRef]
- Das, P.; Lei, W.; Aziz, S.S.; Obbard, J.P. Enhanced algae growth in both phototrophic and mixotrophic culture under blue light. Bioresour. Technol. 2011, 102, 3883–3887. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-J.; Kwon, H.-K.; Jeon, J.-Y.; Yang, H.-S. Effect of monochromatic light emitting diode on the growth of four microalgae species (Chlorella vulgaris, Nitzschia sp., Phaeodactylum tricornutum, Skeletonema sp.). J. Korean Soc. Mar. Environ. Saf. 2015, 21, 1–8. [Google Scholar] [CrossRef]
- Wang, S.K.; Stiles, A.R.; Guo, C.; Liu, C.Z. Microalgae cultivation in photobioreactors: An overview of light characteristics. Eng. Life Sci. 2014, 14, 550–559. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, J.; Zhu, H.; Muhammad, A.; Deng, H.; Hu, Z.; Wu, Q. Inhibition of glucose assimilation in Auxenochlorella protothecoides by light. Biotechnol. Biofuels 2020, 13, 146. [Google Scholar] [CrossRef]
- Zhan, J.; Rong, J.; Wang, Q. Mixotrophic cultivation, a preferable microalgae cultivation mode for biomass/bioenergy production, and bioremediation, advances and prospect. Int. J. Hydrogen Energy 2017, 42, 8505–8517. [Google Scholar] [CrossRef]
- Heredia-Arroyo, T.; Wei, W.; Hu, B. Oil Accumulation via Heterotrophic/Mixotrophic Chlorella protothecoides. Appl. Biochem. Biotechnol. 2010, 162, 1978–1995. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Hampel, K.H.; Lane, C.D.; Kessler, B.A.; White, N.M.; Moats, K.M.; Allnutt, F.T. High-productivity lipid production using mixed trophic state cultivation of Auxenochlorella (Chlorella) protothecoides. Bioprocess Biosyst. Eng. 2015, 38, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Huang, W.; Xu, J.; Wang, Z.; Xu, J.; Yuan, Z. Metabolic changes of starch and lipid triggered by nitrogen starvation in the microalga Chlorella zofingiensis. Bioresour. Technol. 2014, 152, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Yang, S.; Wang, H.; Huo, H.; Guo, B.; Liu, N.; Zhang, A.; Niu, S. Regulation of biomass, pigments, and lipid production by Chlorella vulgaris 31 through controlling trophic modes and carbon sources. J. Appl. Phycol. 2020, 32, 1569–1579. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Li, X.; Li, W.; Zhai, J.; Wei, H.; Wang, Q. Effect of ammonium nitrogen on microalgal growth, biochemical composition and photosynthetic performance in mixotrophic cultivation. Bioresour. Technol. 2019, 273, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Antal, T.; Mattila, H.; Hakala-Yatkin, M.; Tyystjarvi, T.; Tyystjarvi, E. Acclimation of photosynthesis to nitrogen deficiency in Phaseolus vulgaris. Planta 2010, 232, 887–898. [Google Scholar] [CrossRef]
- Mamedov, F.; Rintamaki, E.; Aro, E.M.; Andersson, B.; Styring, S. Influence of protein phosphorylation on the electron-transport properties of Photosystem II. Photosynth. Res. 2002, 74, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Vidotti, A.D.S.; Riaño-Pachón, D.M.; Mattiello, L.; Giraldi, L.A.; Winck, F.V.; Franco, T.T. Analysis of autotrophic, mixotrophic and heterotrophic phenotypes in the microalgae Chlorella vulgaris using time-resolved proteomics and transcriptomics approaches. Algal Res. 2020, 51, 102060. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T.L. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef]
- Kochert, G. Carbohydrate determination by phenol-sulfuric acid method. In Handbook of Phycological Methods. Physiological and Biochemical Methods; Hellebust, J.A., Craige, J.S., Eds.; Cambridge University Press: London, UK, 1978; pp. 95–97. [Google Scholar]
- Izard, J.; Limberger, R.J. Rapid screening method for quantitation of bacterial cell lipids from whole cells. J. Microbiol. Methods 2003, 55, 411–418. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- PSI. Operation Manual. Available online: http://www.psi.cz/download/document/manuals/aquapen/AquaPen_Manual.pdf (accessed on 1 February 2016).
- Campbell, D.; Hurry, V.; Clarke, A.K.; Gustafsson, P.; Öquist, G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol. Mol. Biol. Rev. 1998, 62, 667–683. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dabrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Belatik, A.; Hotchandani, S.; Carpentier, R. Inhibition of the water oxidizing complex of photosystem II and the reoxidation of the quinone acceptor QA− by Pb 2+. PLoS ONE 2013, 8, e68142. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.P.; Ames, W.M.; Nilsson, H.; Lohmiller, T.; Pantazis, D.A.; Rapatskiy, L.; Nowaczyk, M.M.; Neese, F.; Boussac, A.; Messinger, J. Ammonia binding to the oxygen-evolving complex of photosystem II identifies the solvent-exchangeable oxygen bridge (μ-oxo) of the manganese tetramer. Proc. Natl. Acad. Sci. USA 2013, 110, 15561–15566. [Google Scholar] [CrossRef]
- Oyala, P.H.; Stich, T.A.; Debus, R.J.; Britt, R.D. Ammonia Binds to the Dangler Manganese of the Photosystem II Oxygen-Evolving Complex. J. Am. Chem. Soc. 2015, 137, 8829–8837. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Deng, C.; Zhang, D.; Wang, J.; Mu, G.; Chen, Y. Toxic effects of amoxicillin on the photosystem II of Synechocystis sp. characterized by a variety of in vivo chlorophyll fluorescence tests. Aquat. Toxicol. 2008, 89, 207–213. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Culture |  | |||
| MH | ML | PH | PL | ||
| ABS/RC | 54.37 ± 4.59 | 26.56 ± 3.7 | 167.23 ± 22.81 | 1.28 ± 0.11 | |
| DIo/RC | 50.42 ± 3.46 | 24.60 ± 4.72 | 160.94 ± 33.47 | 0.40 ± 0.02 | |
| φDo | 0.93 ± 0.09 | 0.91 ± 0.07 | 0.96 ± 0.08 | 0.31 ± 0.03 | |
| PIABS | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 | 4.50 ± 0.48 | |
| NPQ | 0.04 ± 0.002 | 0.417 ± 0.031 | 0.097 ± 0.009 | 0.517 ± 0.027 | |
| qp | 0.01 ± 0.001 | 0.107 ± 0.005 | 0.01 ± 0.001 | 0.783 ± 0.081 | |
| Kinetic Phases | MH | ML | PH | PL | 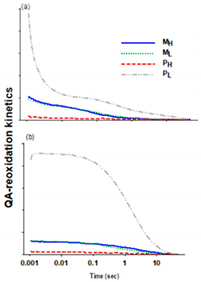 |
| Without DCMU | |||||
| Fast Phase (t1/2, ms) | 116 ± 15 | 207 ± 56 | 237 ± 16 | 10.3 ± 0.3 | |
| Medium Phase (t1/2, s) | 11.3 ± 0.47 | 20.1 ± 2.7 | 46.5 ± 12.4 | 10.1 ± 0.8 | |
| Slow Phase (t1/2, s) | 12,172 ± 1192 | Not computable | 18,783 ± 1691 | 1685 ± 190 | |
| With 50 μM DCMU | |||||
| Fast Phase (t1/2, ms) | 849 ± 61 | 4801 ± 3269 | 763 ± 56 | 22,847 ± 1438 | |
| Medium Phase (t1/2, s) | 43 ± 4.5 | 34.5 ± 0.5 | 46.4 ± 4.3 | 8.2 ± 0.9 | |
| Slow Phase (t1/2, s) | 2001 ± 304 | 3698 ± 557 | 9677 ± 1056 | 328 ± 25 | |
| Culture | Inactive PSII RCs | QB Non-Reducing RCs |
|---|---|---|
| MH | Negative value | 21.36% ± 3.35% |
| ML | Negative value | 22.61% ± 0.93% |
| PH | Negative value | 23.24% ± 5.3% |
| PL | 8.5% ± 1.3% | 11.10% ± 1.34% |
| Culture Name | Glycerol (g/L) | KHCO3 (g/L) | Peptone (g/L) |
|---|---|---|---|
| MH | 10 | - | 4 |
| ML | 10 | - | 0.5 |
| PH | 0 | 2.5 | 4 |
| PL | 0 | 2.5 | 0.5 |
| Parameters | Formulae | Terms |
|---|---|---|
| VJ | (F2ms − F0)/(Fm − F0) | Variable fluorescence at the J step |
| VI | (F60ms − F0)/(Fm − F0) | Variable fluorescence at the I step |
| M0 | 4*(F300μs − F0)/(Fm − F0) | Approximated initial slope of the fluorescence transients |
| φPo | TRo/ABS = 1 − (F0/Fm) = Fv/Fm | Maximum quantum yield for primary photochemistry (at t = 0) |
| φDo | = 1 − ΦPo-(F0/Fm) | Quantum yield of energy dissipation (at t = 0) |
| PIABS | (RC/ABS) [φPo/(1 φPo)] . [Ψo/(1 − Ψo)] | Performance index |
| ABS/RC | M0*(1/VJ)*(1/φPo) | Absorption flux per RC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markou, G.; Diamantis, A.; Korozi, E.; Tsagou, V.; Kefalogianni, I.; Chatzipavlidis, I. Effects of Monochromatic Illumination with LEDs Lights on the Growth and Photosynthetic Performance of Auxenochlorella protothecoides in Photo- and Mixotrophic Conditions. Plants 2021, 10, 799. https://doi.org/10.3390/plants10040799
Markou G, Diamantis A, Korozi E, Tsagou V, Kefalogianni I, Chatzipavlidis I. Effects of Monochromatic Illumination with LEDs Lights on the Growth and Photosynthetic Performance of Auxenochlorella protothecoides in Photo- and Mixotrophic Conditions. Plants. 2021; 10(4):799. https://doi.org/10.3390/plants10040799
Chicago/Turabian StyleMarkou, Giorgos, Alexandros Diamantis, Evagelina Korozi, Vasiliki Tsagou, Io Kefalogianni, and Iordanis Chatzipavlidis. 2021. "Effects of Monochromatic Illumination with LEDs Lights on the Growth and Photosynthetic Performance of Auxenochlorella protothecoides in Photo- and Mixotrophic Conditions" Plants 10, no. 4: 799. https://doi.org/10.3390/plants10040799
APA StyleMarkou, G., Diamantis, A., Korozi, E., Tsagou, V., Kefalogianni, I., & Chatzipavlidis, I. (2021). Effects of Monochromatic Illumination with LEDs Lights on the Growth and Photosynthetic Performance of Auxenochlorella protothecoides in Photo- and Mixotrophic Conditions. Plants, 10(4), 799. https://doi.org/10.3390/plants10040799







