Magnesium Foliar Supplementation Increases Grain Yield of Soybean and Maize by Improving Photosynthetic Carbon Metabolism and Antioxidant Metabolism
Abstract
1. Introduction
2. Results
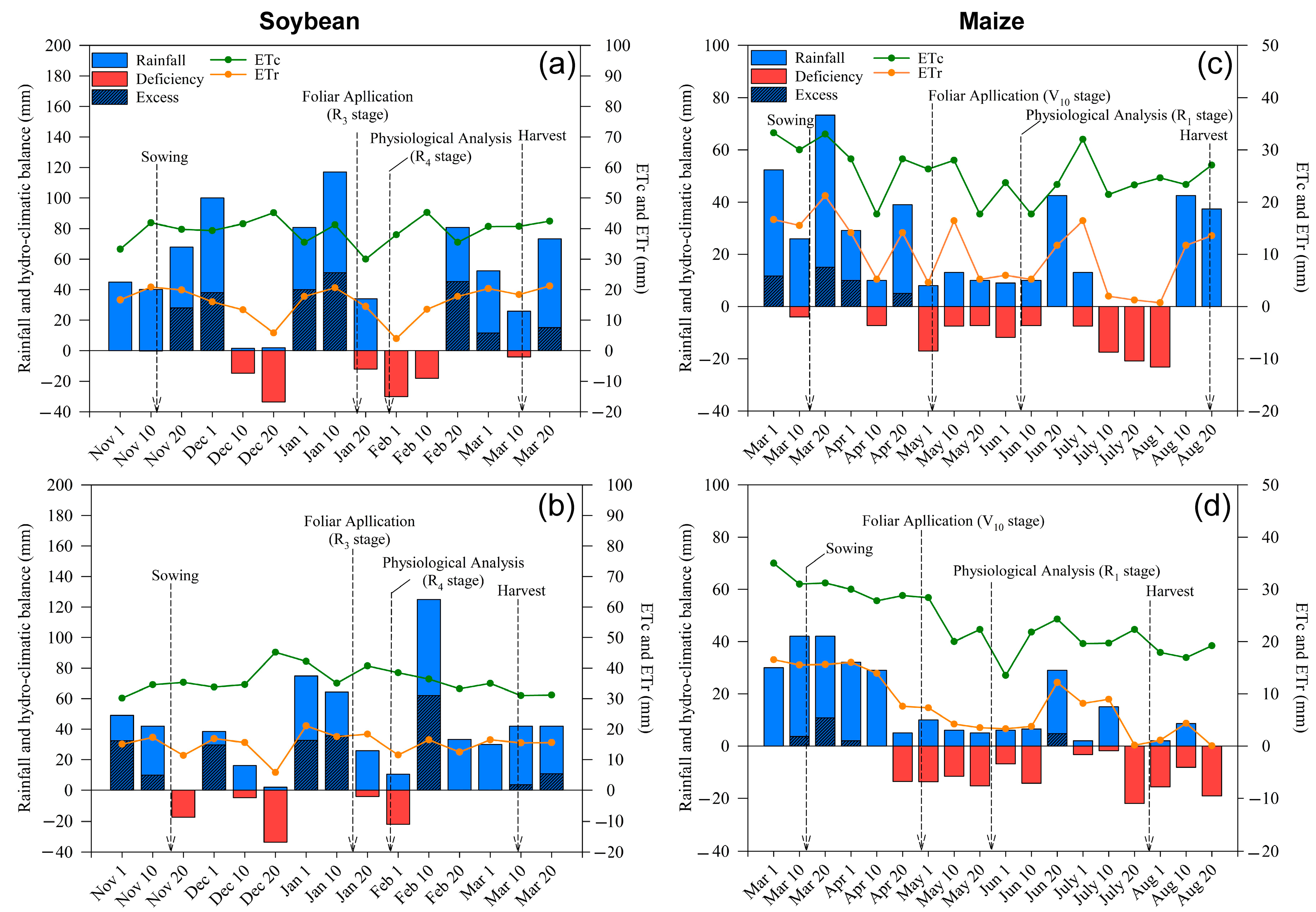
2.1. Weather Conditions
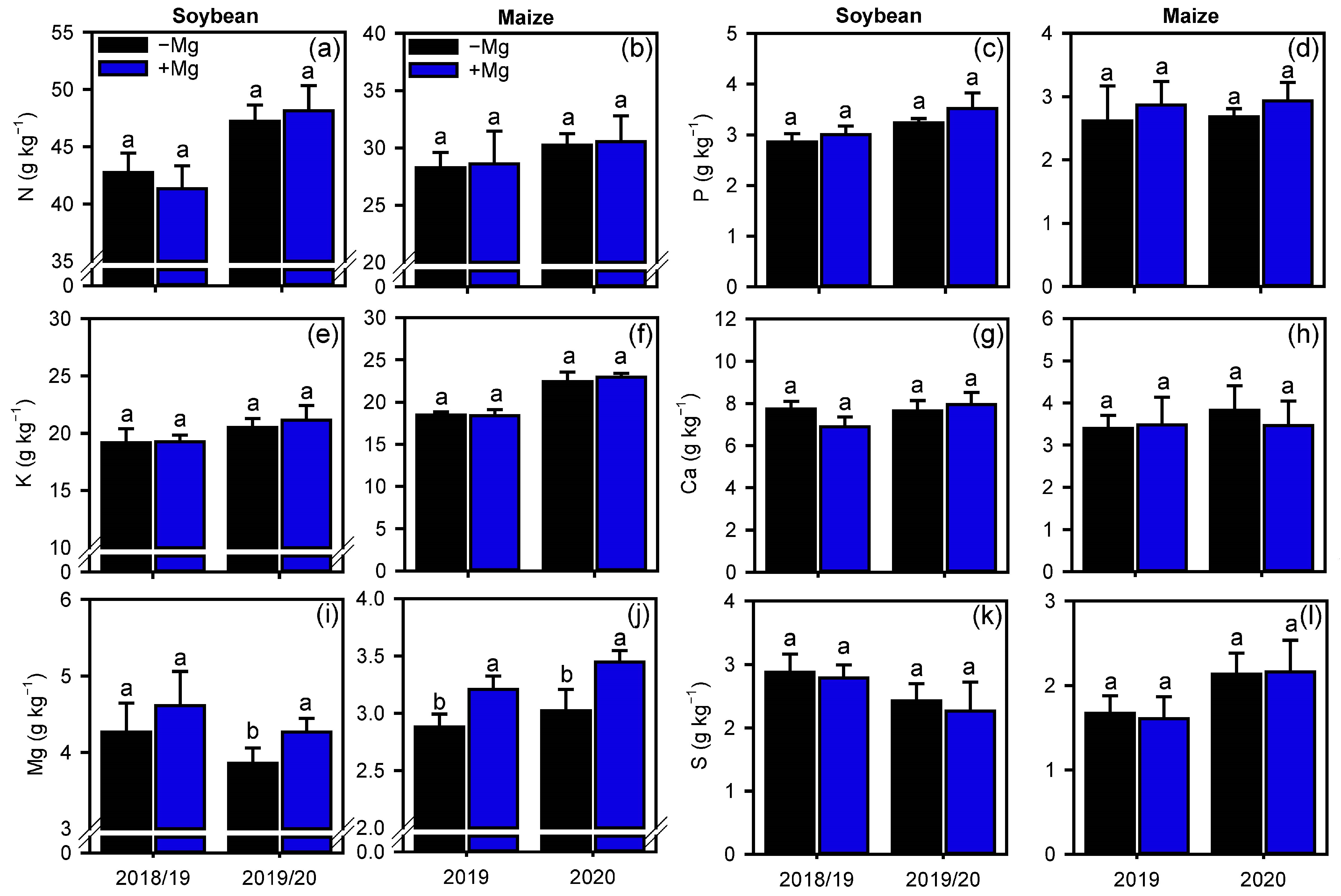
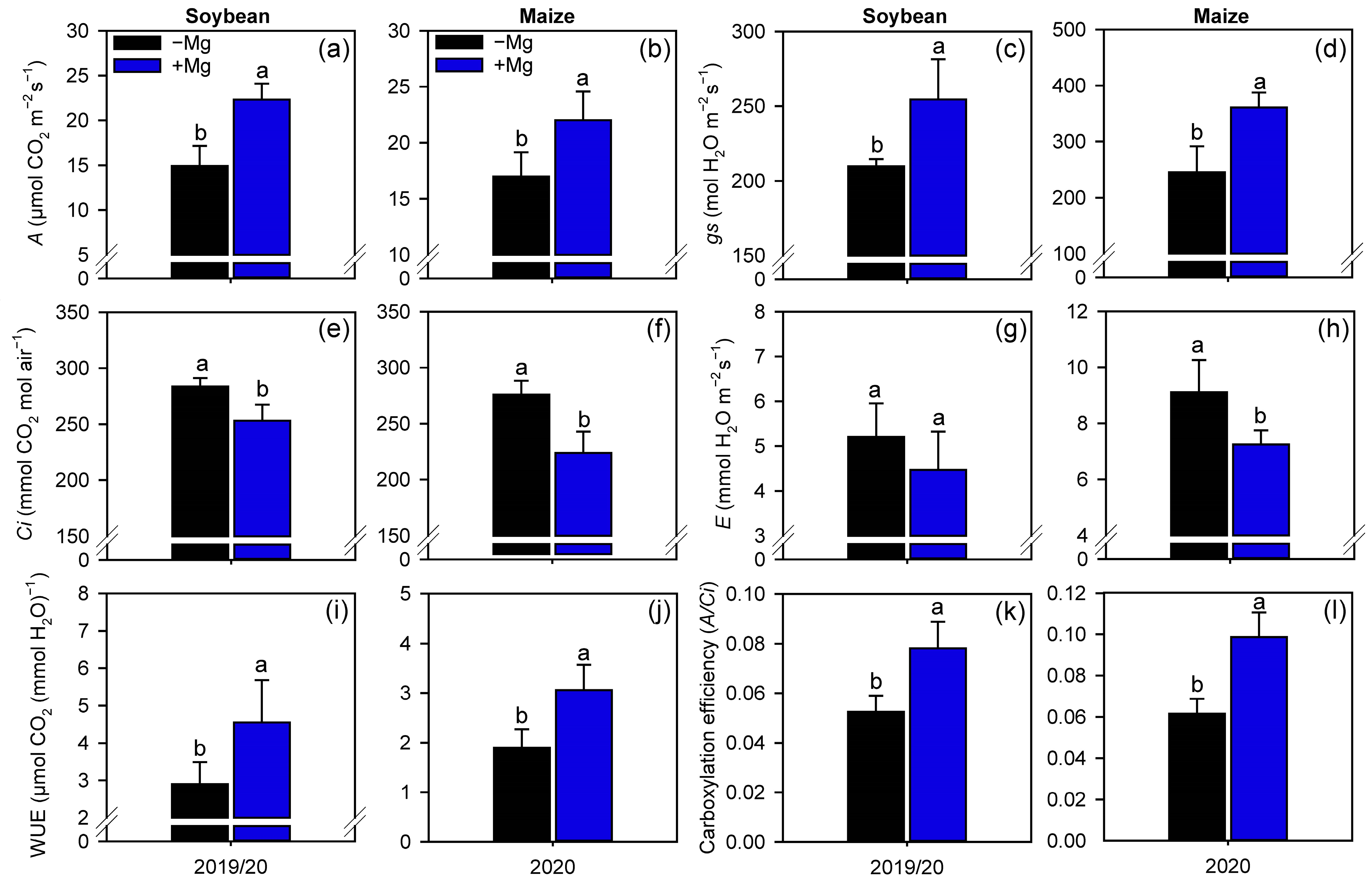
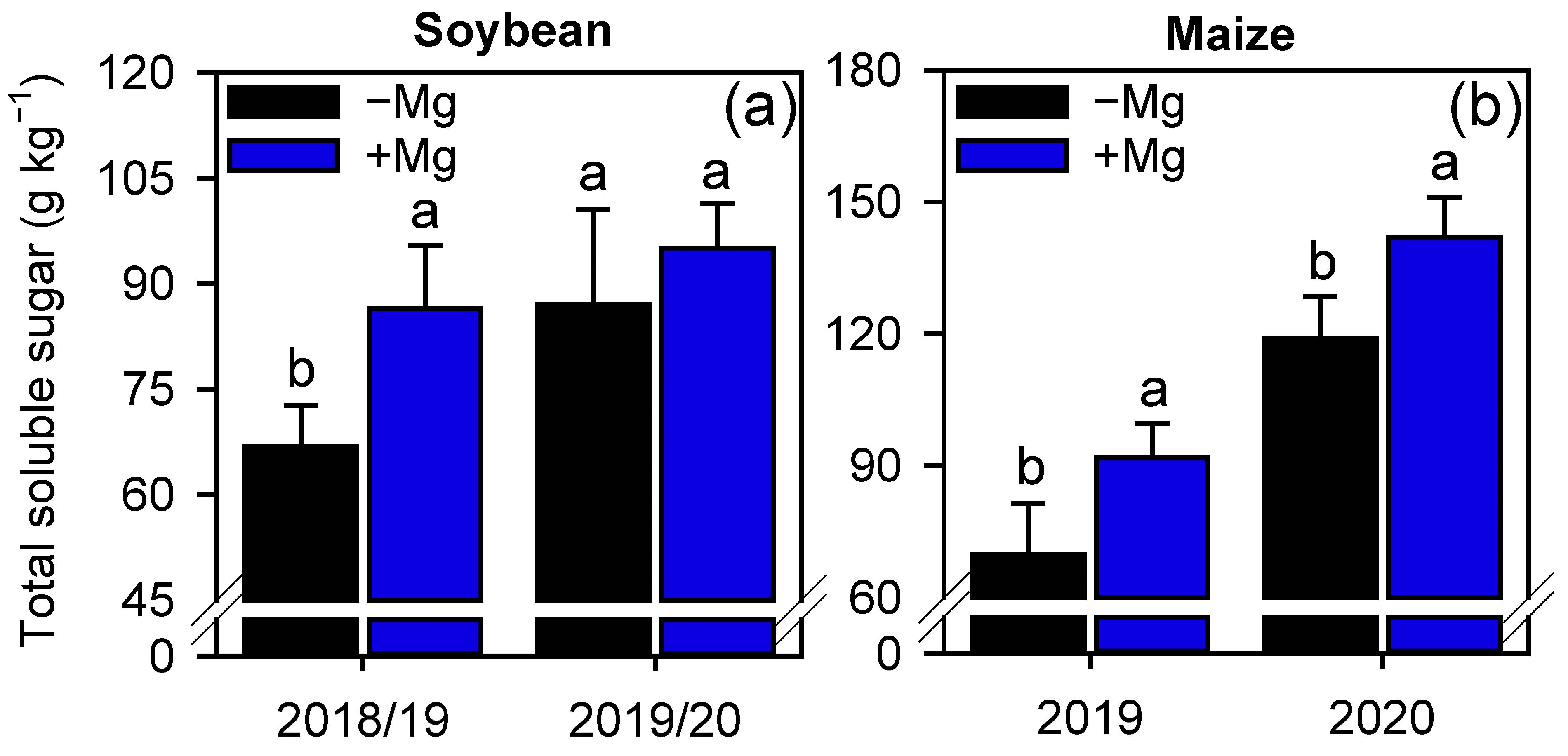
2.2. Crop Nutrition, Photosynthetic Parameters, and Carbon Assimilation
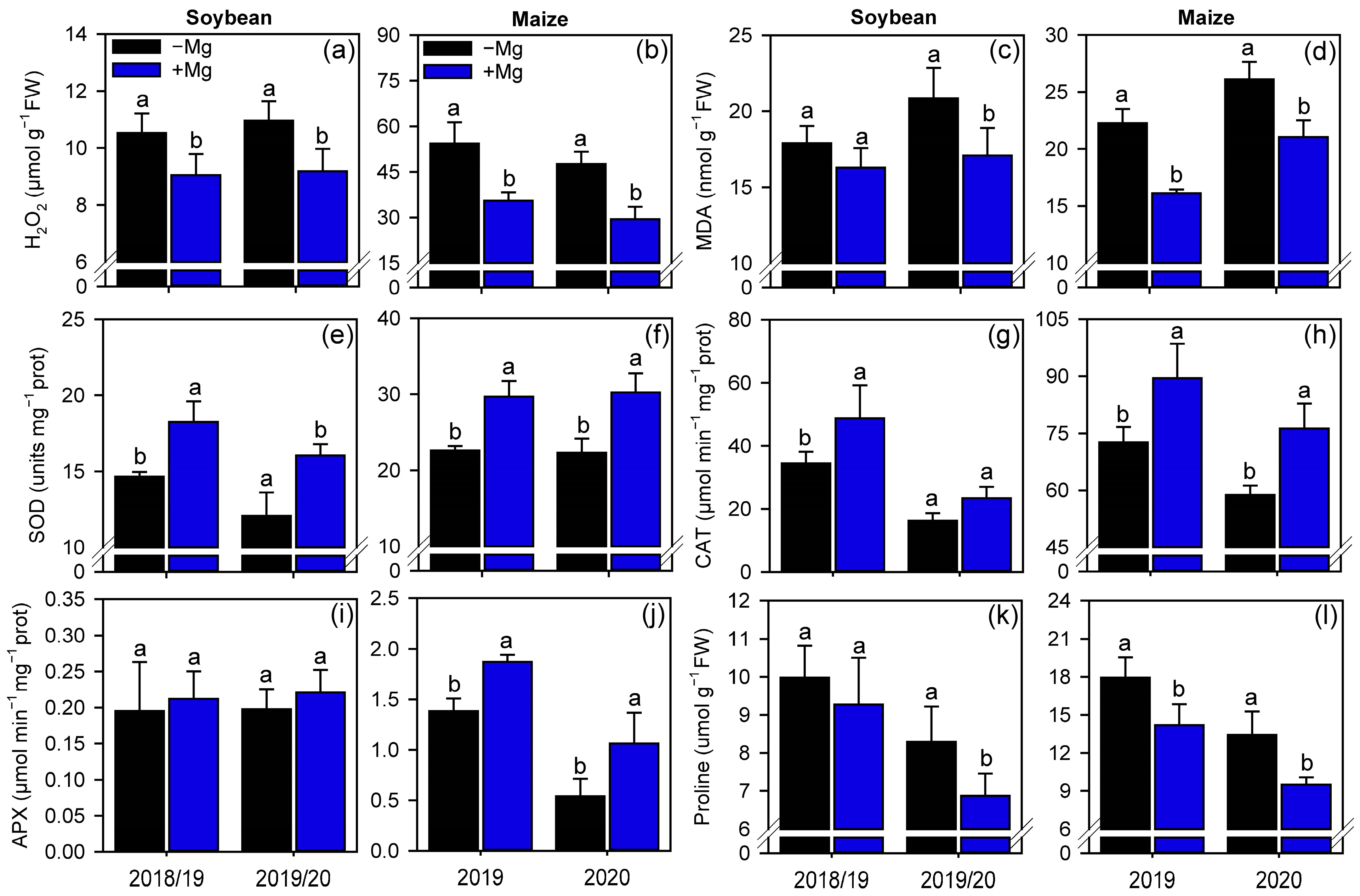
2.3. Oxidative Stress and Antioxidant Enzymes
2.4. Grain Yield
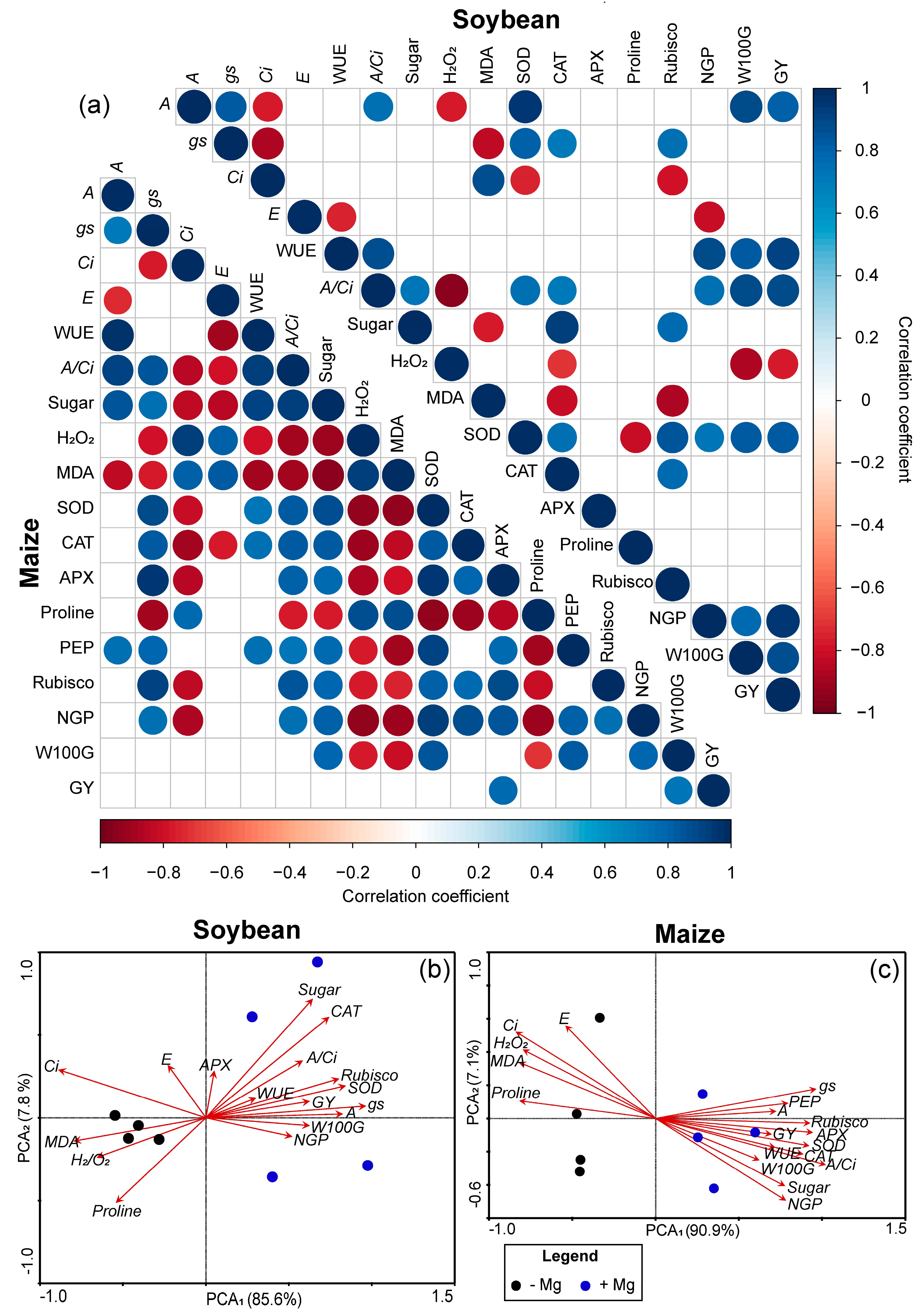
2.5. Pearson’s Correlation and PCA among Soybean and Maize Parameters
3. Discussion
4. Materials and Methods
4.1. Field Description
4.2. Experimental Design and Treatment Description
4.3. Field Management
4.3.1. Soybean Crop
4.3.2. Maize Crop
4.4. Plant Sampling and Laboratory Analyzes
4.4.1. Crop Nutrition
4.4.2. Gas Exchange Parameters
4.4.3. Photosynthetic Enzymes
4.4.4. Total Soluble Sugar Concentration
4.4.5. Oxidative Stress and Antioxidant Enzymes
4.4.6. Agronomic Parameters and Grain Yield
4.5. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Kameritsch, P. Relative association of Rubisco with manganese and magnesium as a regulatory mechanism in plants. Physiol. Plant. 2017, 161, 545–559. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, Q.; Zhou, M.; Li, C.; Gong, X.; Liu, C.; Qu, C.; Wang, L.; Si, W.; Hong, F. Magnesium deficiency results in damage of nitrogen and carbon cross-talk of maize and improvement by cerium addition. Biol. Trace Elem. Res. 2012, 148, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Rosolem, C.A. Recomendação e aplicação de nutrientes via foliar. Lavras: Ufla/Faepe 2002, pp. 63–98.
- Igamberdiev, A.U.; Kleczkowski, L.A. Membrane potential, adenylate levels and Mg2+ are interconnected via adenylate kinase equilibrium in plant cells. Biochim. Biophys. Acta (Bba)-Bioenerg. 2003, 1607, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, Y.; Asukagawa, N.; Ishikawa, Y.; Ohta, E.; Sakata, M. Estimation of cytoplasmic free Mg2+ levels and phosphorylation potentials in mung bean root tips by in vivo 31P NMR spectroscopy. Plant. Cell Physiol. 1988, 29, 919–924. [Google Scholar]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Graham, I.A.; Martin, T. Control of photosynthesis, allocation and partitioning by sugar regulated gene expression. In Photosynthesis. Advances in Photosynthesis and Respiration; Leegood, R.C., Sharkey, T.D., von Caemmerer, S., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 233–248. [Google Scholar]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; FAO/OECD Publishing: Rome, Italy; Paris, France, 2020. [Google Scholar] [CrossRef]
- Moretti, L.G.; Lazarini, E.; Bossolani, J.W.; Parente, T.L.; Caioni, S.; Araujo, R.S.; Hungria, M. Can additional inoculations increase soybean nodulation and grain yield? Agron. J. 2018, 110, 715–721. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Kuramae, E.E.; Bossolani, J.W.; Moreira, A.; Costa, N.R.; Alves, C.J.; Pascoaloto, I.M.; Rondina, A.B.L.; Hungria, M. Effects of growth-promoting bacteria on soybean root activity, plant development, and yield. Agron. J. 2020, 112, 418–428. [Google Scholar] [CrossRef]
- Bossolani, J.W.; Moretti, L.G.; Portugal, J.R.; Rossi, R.; Crusciol, C.A.C. Thermomagnesium: A By-Product of Ni Ore Mining as a Clean Fertilizer Source for Maize. Agronomy 2021, 11, 525. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Bossolani, J.W.; Garcia, A.; Rossi, R.; Moreira, A. Thermomagnesium as a fertilizer for soybean: Carbohydrate metabolism, silicon–magnesium fertilizer, and grain yield. J. Plant. Nutr. 2021, 1–15. [Google Scholar] [CrossRef]
- Buchmann, N.; Ehleringer, J.R. CO2 concentration profiles, and carbon and oxygen isotopes in C3 and C4 crop canopies. Agric. For. Meteorol. 1998, 89, 45–58. [Google Scholar] [CrossRef]
- Wrobel, K.; Karasiński, J.; Tupys, A.; Negrete, M.A.A.; Halicz, L.; Wrobel, K.; Bulska, E. Magnesium–isotope fractionation in chlorophyll-a extracted from two plants with different pathways of carbon fixation (C3, C4). Molecules 2020, 25, 1644. [Google Scholar] [CrossRef] [PubMed]
- Gou, W.; Zheng, P.; Tian, L.; Gao, M.; Zhang, L.; Akram, N.A.; Ashraf, M. Exogenous application of urea and a urease inhibitor improves drought stress tolerance in maize (Zea mays L.). J. Plant. Res. 2017, 130, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Xu, S.; Li, J.L.; Cui, L.J.; Chen, Y.N.; Wang, J.Z. Effects of foliar application of nitrogen on the photosynthetic performance and growth of two fescue cultivars under heat stress. Biol. Plant. 2008, 52, 113–116. [Google Scholar] [CrossRef]
- da Silva, D.M.; de Souza, K.R.D.; Vilas Boas, L.V.; Alves, Y.S.; Alves, J.D. The effect of magnesium nutrition on the antioxidant response of coffee seedlings under heat stress. Sci. Hortic. (Amst.) 2017, 224, 115–125. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Bossolani, J.W.; Rossi, R.; Moreira, A. Agricultural repurposing of nickel slag residue. J. Plant Nutr. 2020, 44, 1141–1150. [Google Scholar] [CrossRef]
- Tränkner, M.; Jaghdani, S.J. Minimum magnesium concentrations for photosynthetic efficiency in wheat and sunflower seedlings. Plant Physiol. Biochem. 2019, 144, 234–243. [Google Scholar] [CrossRef]
- Jaghdani, S.J.; Jahns, P.; Tränkner, M. Mg deficiency induces photo-oxidative stress primarily by limiting CO2 assimilation and not by limiting photosynthetic light utilization. Plant Sci. 2021, 302, 110751. [Google Scholar] [CrossRef] [PubMed]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Hauer-Jákli, M.; Tränkner, M. Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense: A systematic review and meta-analysis from 70 years of research. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Altarugio, L.M.; Loman, M.H.; Nirschl, M.G.; Silvano, R.G.; Zavaschi, E.; de Mello e Silva Carneiro, L.; Vitti, G.C.; de Cerqueira Luz, P.H.; Otto, R. Yield performance of soybean and corn subjected to magnesium foliar spray. Pesqui. Agropecu. Bras. 2017, 52, 1185–1191. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.A.; Al-Khaishany, M.Y.Y.; Al-Qutami, M.A.; Ali, H.M.; Al-Whaibi, M.H.; Al-Wahibi, M.S.; Alharby, H.F. Mitigation of adverse effects of heat stress on Vicia faba by exogenous application of magnesium. Saudi J. Biol. Sci. 2018, 25, 1393–1401. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.C.; Schroth, G.; Becker, F.J.; Mandarino, J.M.G. Soybean yield and nutritional status response to nitrogen sources and rates of foliar fertilization. Agron. J. 2017, 109, 629–635. [Google Scholar] [CrossRef]
- Ahmad, Z.; Waraich, E.A.; Akhtar, S.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, O.B.A.; Tapera, T.; Labuschagne, M.; Rizwan, M. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol. Plant. 2018, 40, 1–13. [Google Scholar] [CrossRef]
- Boaretto, R.M.; Hippler, F.W.R.; Ferreira, G.A.; Azevedo, R.A.; Quaggio, J.A.; Mattos, D. The possible role of extra magnesium and nitrogen supply to alleviate stress caused by high irradiation and temperature in lemon trees. Plant Soil 2020, 457, 57–70. [Google Scholar] [CrossRef]
- Hoober, J.K.; Eggink, L.L.; Chen, M. Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts. Photosynth. Res. 2007, 94, 387–400. [Google Scholar] [CrossRef]
- Fiedor, L.; Kania, A.; Myśliwa-Kurdziel, B.; Orzeł, Ł.; Stochel, G. Understanding chlorophylls: Central magnesium ion and phytyl as structural determinants. Biochim. Biophys. Acta (Bba)-Bioenerg. 2008, 1777, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plantarum 2008, 133, 692–704. [Google Scholar] [CrossRef]
- Sage, R.F.; Sharkey, T.D.; Seemann, J.R. The in-vivo response of the ribulose-1,5-bisphosphate carboxylase activation state and the pool sizes of photosynthetic metabolites to elevated CO2 in Phaseolus vulgaris L. Planta 1988, 174, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F. The evolution of C4 photosynthesis. New Phytol. 2004, 161, 341–370. [Google Scholar] [CrossRef]
- Lawson, T.; Flexas, J. Fuelling life: Recent advances in photosynthesis research. Plant. J. 2020, 101, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F.; Sage, T.L.; Kocacinar, F. Photorespiration and the evolution of C4 photosynthesis. Annu. Rev. Plant. Biol. 2012, 63, 19–47. [Google Scholar] [CrossRef] [PubMed]
- Ehleringer, J.R.; Monson, R.K. Evolutionary and ecological aspects of photosynthetic pathway variation. Annu. Rev. Ecol. Syst. 1993, 24, 411–439. [Google Scholar] [CrossRef]
- Douce, R.; Heldt, H.-W. Photorespiration. In Photosynthesis. Advances in Photosynthesis and Respiration; Leegood, R.C., Sharkey, T.D., von Caemmerer, S., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 115–136. [Google Scholar]
- Kubien, D.S.; Whitney, S.M.; Moore, P.V.; Jesson, L.K. The biochemistry of Rubisco in Flaveria. J. Exp. Bot. 2008, 59, 1767–1777. [Google Scholar] [CrossRef]
- Samborska, I.A.; Kalaji, H.M.; Sieczko, L.; Goltsev, V.; Borucki, W.; Jajoo, A. Structural and functional disorder in the photosynthetic apparatus of radish plants under magnesium deficiency. Funct. Plant. Biol. 2018, 45, 668–679. [Google Scholar] [CrossRef]
- Canizella, B.T.; Moreira, A.; Moraes, L.A.C.; Fageria, N.K. Efficiency of Magnesium Use by Common Bean Varieties Regarding Yield, Physiological Components, and Nutritional Status of Plants. Commun. Soil Sci. Plant. Anal. 2015, 46, 1376–1390. [Google Scholar] [CrossRef]
- Peng, H.Y.; Qi, Y.P.; Lee, J.; Yang, L.T.; Guo, P.; Jiang, H.X.; Chen, L.S. Proteomic analysis of Citrus sinensis roots and leaves in response to long-term magnesium-deficiency. BMC Genom. 2015, 16, 1–24. [Google Scholar] [CrossRef]
- Tang, N.; Li, Y.; Chen, L.S. Magnesium deficiency-induced impairment of photosynthesis in leaves of fruiting Citrus reticulata trees accompanied by up-regulation of antioxidant metabolism to avoid photo-oxidative damage. J. Plant. Nutr. Soil Sci. 2012, 175, 784–793. [Google Scholar] [CrossRef]
- Andersson, I. Catalysis and regulation in Rubisco. J. Exp. Bot. 2008, 59, 1555–1568. [Google Scholar] [CrossRef]
- Hazra, S.; Henderson, J.N.; Liles, K.; Hilton, M.T.; Wachter, R.M. Regulation of Ribulose-1, 5-bisphosphate Carboxylase/Oxygenase (Rubisco) Activase product inhibition, cooperativity, and magnesium activation. J. Biol. Chem. 2015, 290, 24222–24236. [Google Scholar] [CrossRef]
- White, P.J. Ion uptake mechanisms of individual cells and roots: Short-distance transport. In Marschner’s Mineral Nutrition of HigherPlants; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 7–47. [Google Scholar]
- Ceylan, Y.; Kutman, U.B.; Mengutay, M.; Cakmak, I. Magnesium applications to growth medium and foliage affect the starch distribution, increase the grain size and improve the seed germination in wheat. Plant Soil 2016, 406, 145–156. [Google Scholar] [CrossRef]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Ba, Q.; Zhang, L.; Chen, S.; Li, G.; Wang, W. Effects of foliar application of magnesium sulfate on photosynthetic characteristics, dry matter accumulation and its translocation, and carbohydrate metabolism in grain during wheat grain filling. Cereal Res. Commun. 2020, 48, 157–163. [Google Scholar] [CrossRef]
- Cunningham, C. Characterization of dry spells in southeastern Brazil during the monsoon season. Int. J. Climatol. 2020, 40, 4609–4621. [Google Scholar] [CrossRef]
- Correia, P.M.P.; da Silva, A.B.; Roitsch, T.; Carmo-Silva, E.; Marques da Silva, J. Photoprotection and optimization of sucrose usage contribute to faster recovery of photosynthesis after water deficit at high temperatures in wheat. Physiol. Plant. 2020. [Google Scholar] [CrossRef]
- de Nioa, S.N., Jr.; Sentelhas, P.C. Soybean-maize off-season double crop system in Brazil as affected by El Niño Southern Oscillation phases. Agric. Syst. 2019, 173, 254–267. [Google Scholar] [CrossRef]
- Kibria, M.G.; Barton, L.; Rengel, Z. Foliar application of magnesium mitigates soil acidity stress in wheat. J. Agron. Crop. Sci. 2020, 207, 378–389. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant. Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; Shanker, A., Shanker, C., Eds.; IntechOpen: London, UK, 2016; pp. 463–480. [Google Scholar]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer: Berlin, Germany, 2018; ISBN 3319750887. [Google Scholar]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M.; Khan, M.M.A. Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol. Plant. 2010, 32, 121–132. [Google Scholar] [CrossRef]
- Trotel, P.; Bouchereau, A.; Niogret, M.F.; Larher, F. The fate of osmo-accumulated proline in leaf discs of rape (Brassica napus L.) incubated in a medium of low osmolarity. Plant Sci. 1996, 118, 31–45. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxygen stress and superoxide dismutases. Plant Physiol. 1993, 101, 7–12. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Jahn, R.; Blume, H.P.; Asio, V.B.; Spaargaren, O.; Schad, P. Guidelines for Soil Description, 4th ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- USDA. Keys to Soil Taxonomy, 12th ed.; USDA—Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.D.D.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Unicamp Center of Meteorological and Climatic Research Applied to Agriculture. Botucatu. Municipalities Climate of São Paulo State. Available online: www.cpa.unicamp.br/outras-informacoes/clima_muni_086.html (accessed on 20 June 2020).
- Cassel, D.K.; Nielsen, D.R. Field Capacity and Available Water Capacity. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy, Soil Sciense Society of America: Madison, WI, USA, 1986; pp. 901–926. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotraspiration guidelines for computing crop water requirements. In FAO Irrigation & Drainage Paper 56; FAO, Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; p. 50. [Google Scholar]
- de Souza Rolim, G.; Sentelhas, P.C.; Barbieri, V. Planilhas no ambiente Excel TM para os cálculos de balanços hídricos: Normal, sequencial, de cultura e de produtividade real e potencial. Rev. Bras. Agrometeorol. 1998, 6, 133–137. [Google Scholar]
- Thornthwaite, C.W.; Mather, J.R. The Water Balance Climatology; Laboratory of Climatology: New Jersey, NJ, USA, 1995. [Google Scholar]
- Donagema, G.K.; Viana, J.H.M.; Almeida, B.G.; Ruiz, H.A.; Klein, V.A.; Dechen, S.C.F.; Fernandes, R.B.A. Granulometric analysis. In Soil Analysis Methods Manual; Teixeira, P.C., Donagema, G.K., Fontana, A., Teixeira, W.G., Eds.; Embrapa Solos: Brasilia, Brazil, 2017; pp. 95–116. [Google Scholar]
- van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001; ISBN 9788585564056. [Google Scholar]
- Quaggio, J.A.; van Raij, B. Correção da acidez do solo. In Recomendações de Adubação e Calagem Para o Estado de São Paulo; van Raij, B., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico: Campinas, Brazil, 1997; pp. 14–19. ISBN 0100-3100. [Google Scholar]
- Fehr, W.R.; Caviness, C.E. Iowa State University Cooperative Extension Service, Special Report 80; Iowa State University of Science and Technology: Ames, IA, USA, 1977. [Google Scholar]
- Moretti, L.G.; Crusciol, C.A.C.; Bossolani, J.W.; Momesso, L.; Garcia, A.; Kuramae, E.E.; Hungria, M. Bacterial consortium and microbial metabolites increase grain quality and soybean yield. J. Soil Sci. Plant. Nutr. 2020, 20, 1923–1934. [Google Scholar] [CrossRef]
- Embrapa Tecnologias de produção de soja, Sistemas de Produção 17, 1st ed.Seixas, C.D.S.; Neumaier, N.; Balbinot-Junior, A.A.; Krzyzanowski, F.C.; Campos-Leite, R.M.V.B. (Eds.) Embrapa Soja: Londrina, Brazil, 2020; pp. 180–221. [Google Scholar]
- Ritchie, S.W.; Hanway, J.J.; Benson, G.O. How a Corn Plant Develops; Iowa State University Cooperative Extension Service: Ames, IA, USA, 1993. [Google Scholar]
- Ambrosano, E.J.; Tanaka, R.T.; Mascarenhas, H.A.A.; van Raij, B.; Quaggio, J.A.; Cantarella, H. Leguminosas e oleaginosas. In Recomendações de adubação e calagem para o Estado de São Paulo; van Raij, B., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico: Campinas, Brazil, 1997; pp. 187–203. [Google Scholar]
- Cantarella, H.; van Raij, B.; Camargo, C.E.O. Adubação de Cereais. In Recomendações de Adubação e Calagem Para o Estado de São Paulo, 2nd ed.; van Raij, B., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico: Campinas, Brazil, 1997; pp. 43–50. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Evaluation of Nutritional Status of Plants: Principles and Applications, 2nd ed.; POTAFOS: Piracicaba, Brazil, 1997. [Google Scholar]
- Degl’Innocenti, E.; Guidi, L.; Soldatini, G.F. Effect of chronic O 3 fumigation on the activity of some Calvin cycle enzymes in two poplar clones. Photosynthetica 2002, 40, 121–126. [Google Scholar] [CrossRef]
- Reid, C.D.; Tissue, D.T.; Fiscus, E.L.; Strain, B.R. Comparison of spectrophotometric and radioisotopic methods for the assay of Rubisco in ozone-treated plants. Physiol. Plant. 1997, 101, 398–404. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.A.; Alas, R.M.; Smith, R.J.; Lea, P.J. Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol. Plant. 1998, 104, 280–292. [Google Scholar] [CrossRef]
- Gratão, P.L.; Monteiro, C.C.; Antunes, A.M.; Peres, L.E.P.; Azevedo, R.A. Acquired tolerance of tomato (Lycopersicon esculentum cv. Micro-Tom) plants to cadmium-induced stress. Ann. Appl. Biol. 2008, 153, 321–333. [Google Scholar] [CrossRef]
- Torello, W.A.; Rice, L.A. Effects of NaCl stress on proline and cation accumulation in salt sensitive and tolerant turfgrasses. Plant Soil 1986, 93, 241–247. [Google Scholar] [CrossRef]
- Mauad, M.; Crusciol, C.A.C.; Nascente, A.S.; Filho, H.G.; Lima, G.P.P. Effects of silicon and drought stress on biochemical characteristics of leaves of upland rice cultivars1. Rev. Cienc. Agron. 2016, 47, 532–539. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (Complete Samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics: Essays in Honor of Ingram Olkin; Olkin, I., Ghurye, S.G., Hoeffding, W., Madow, W.G., Mann, H.B., Eds.; Stanford University Press: Palo Alto, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Bartlett, M.S. Properties of sufficiency and statistical tests. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1937, 160, 268–282. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, V.A.; Crusciol, C.A.C.; Bossolani, J.W.; Moretti, L.G.; Portugal, J.R.; Mundt, T.T.; de Oliveira, S.L.; Garcia, A.; Calonego, J.C.; Lollato, R.P. Magnesium Foliar Supplementation Increases Grain Yield of Soybean and Maize by Improving Photosynthetic Carbon Metabolism and Antioxidant Metabolism. Plants 2021, 10, 797. https://doi.org/10.3390/plants10040797
Rodrigues VA, Crusciol CAC, Bossolani JW, Moretti LG, Portugal JR, Mundt TT, de Oliveira SL, Garcia A, Calonego JC, Lollato RP. Magnesium Foliar Supplementation Increases Grain Yield of Soybean and Maize by Improving Photosynthetic Carbon Metabolism and Antioxidant Metabolism. Plants. 2021; 10(4):797. https://doi.org/10.3390/plants10040797
Chicago/Turabian StyleRodrigues, Vitor Alves, Carlos Alexandre Costa Crusciol, João William Bossolani, Luiz Gustavo Moretti, José Roberto Portugal, Tamara Thaís Mundt, Sirlene Lopes de Oliveira, Ariani Garcia, Juliano Carlos Calonego, and Romulo Pisa Lollato. 2021. "Magnesium Foliar Supplementation Increases Grain Yield of Soybean and Maize by Improving Photosynthetic Carbon Metabolism and Antioxidant Metabolism" Plants 10, no. 4: 797. https://doi.org/10.3390/plants10040797
APA StyleRodrigues, V. A., Crusciol, C. A. C., Bossolani, J. W., Moretti, L. G., Portugal, J. R., Mundt, T. T., de Oliveira, S. L., Garcia, A., Calonego, J. C., & Lollato, R. P. (2021). Magnesium Foliar Supplementation Increases Grain Yield of Soybean and Maize by Improving Photosynthetic Carbon Metabolism and Antioxidant Metabolism. Plants, 10(4), 797. https://doi.org/10.3390/plants10040797






