Transcriptome Analysis of Needle and Root of Pinus Massoniana in Response to Continuous Drought Stress
Abstract
1. Introduction
2. Results
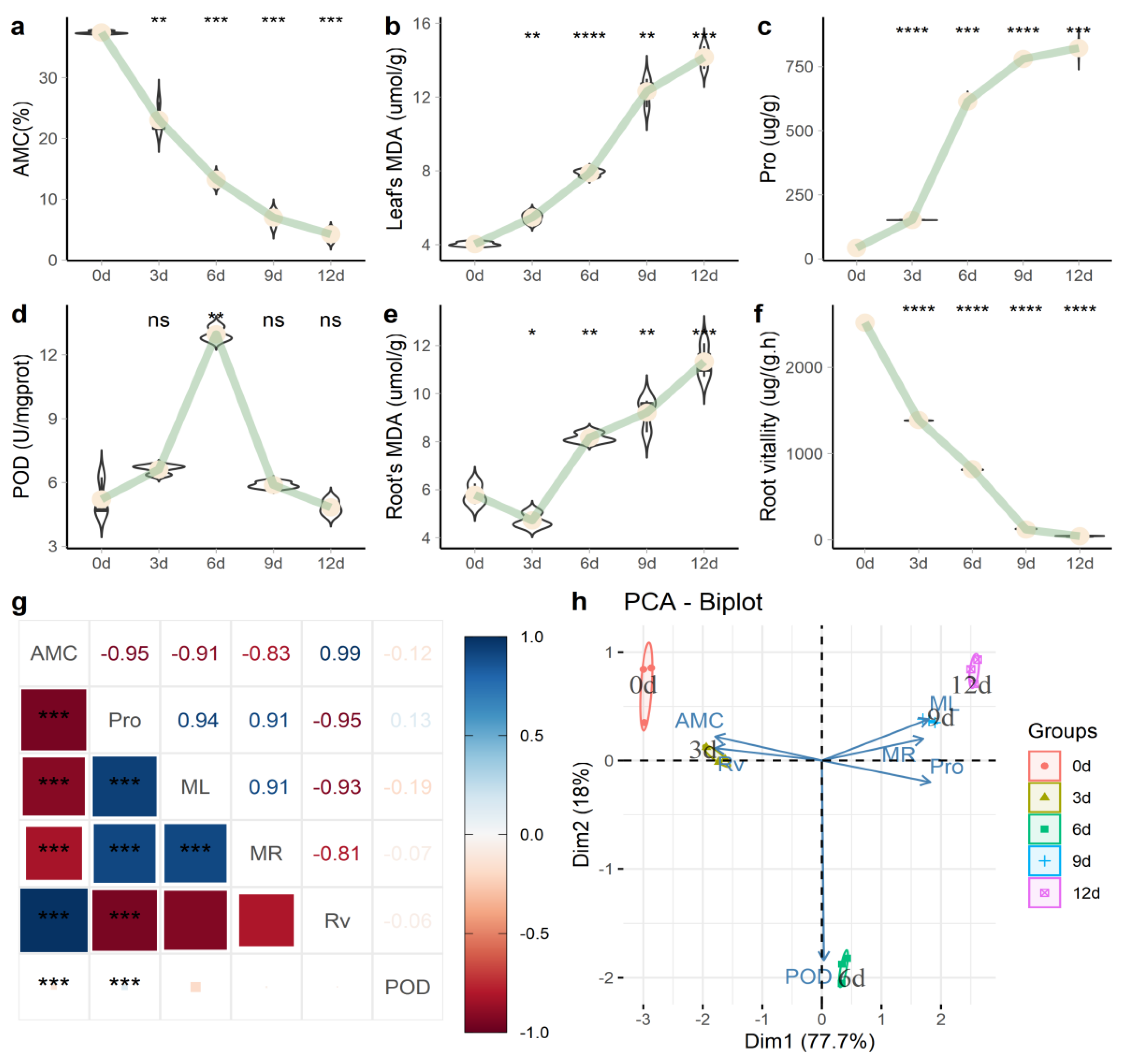
2.1. Physiological and Biochemical Analyses
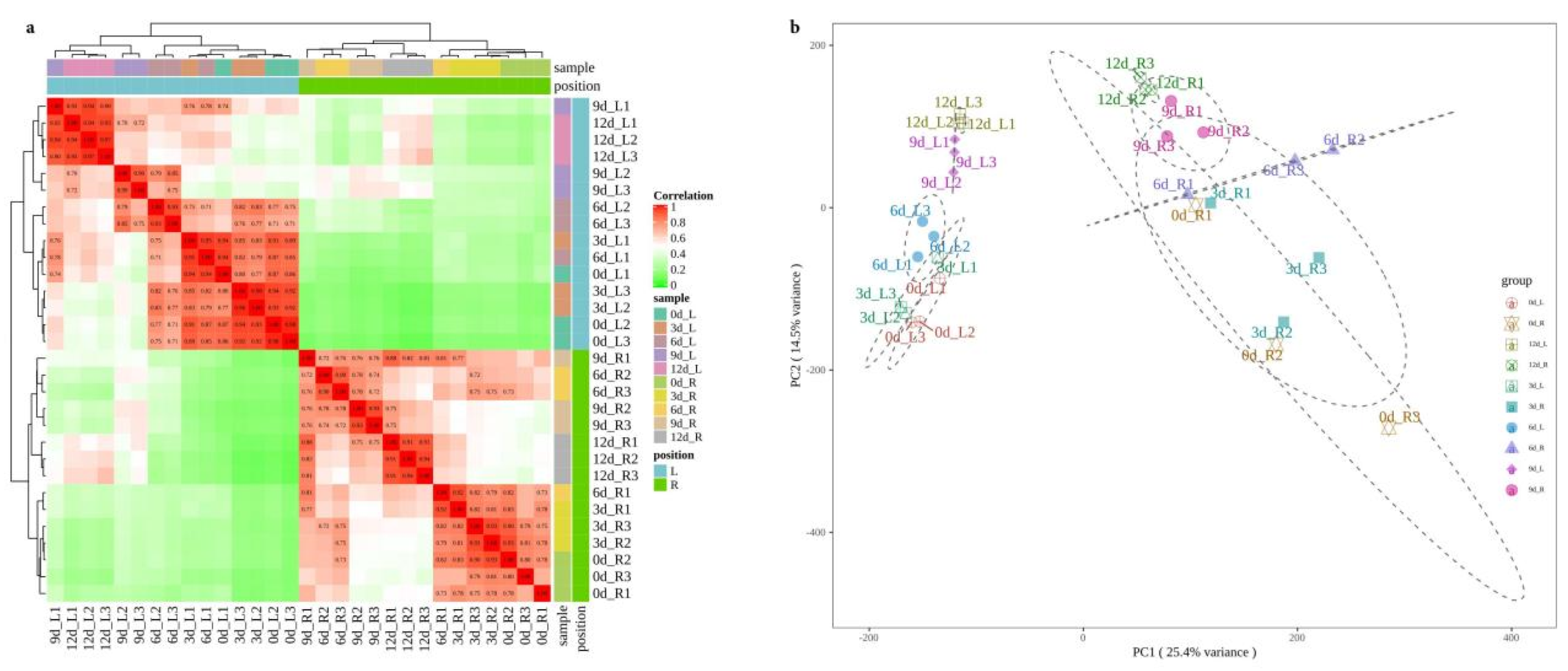
2.2. Quality Control, Annotation, and Correlation Analysis
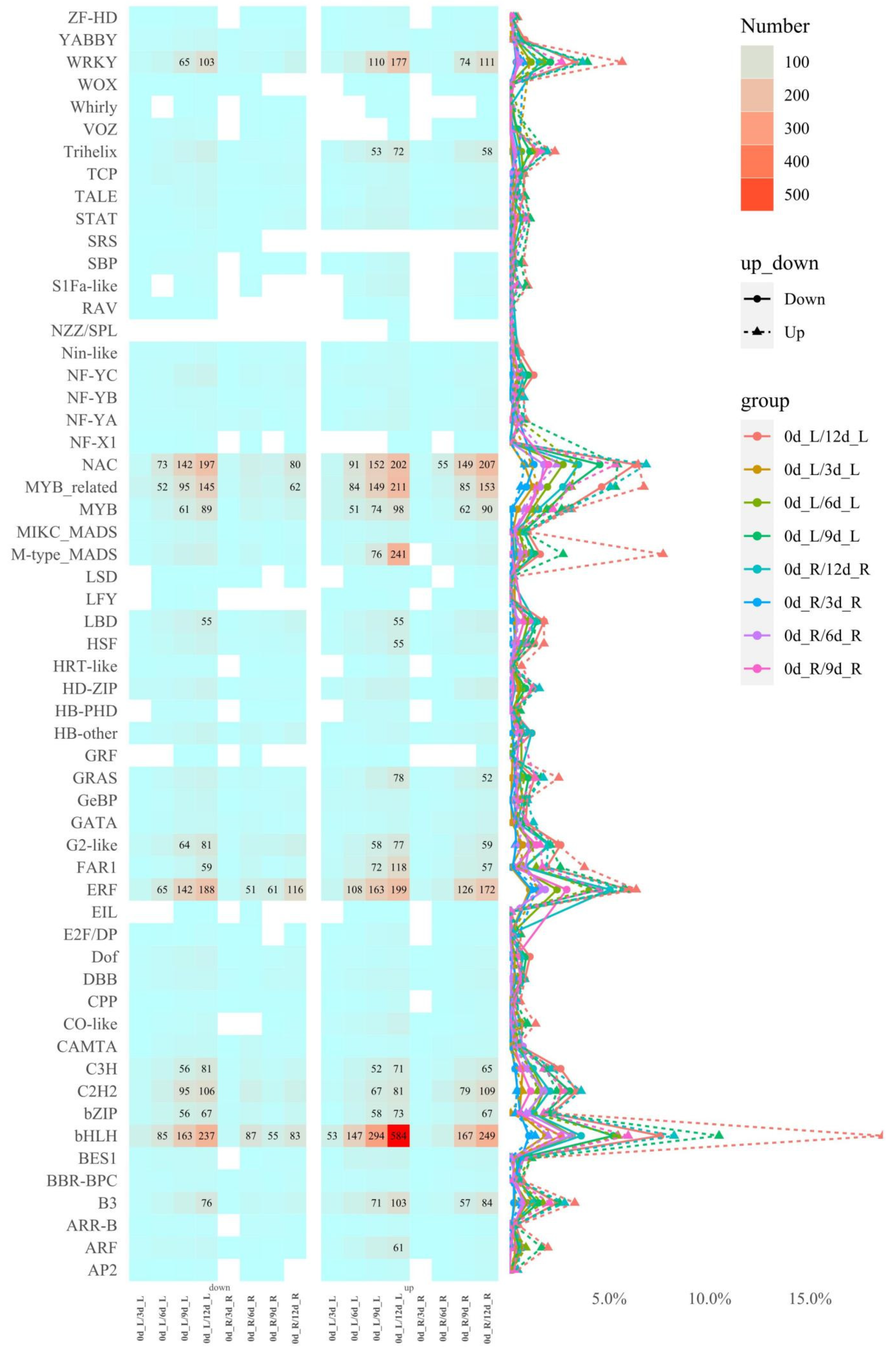
2.3. Short Time-Series Expression Miner Analysis
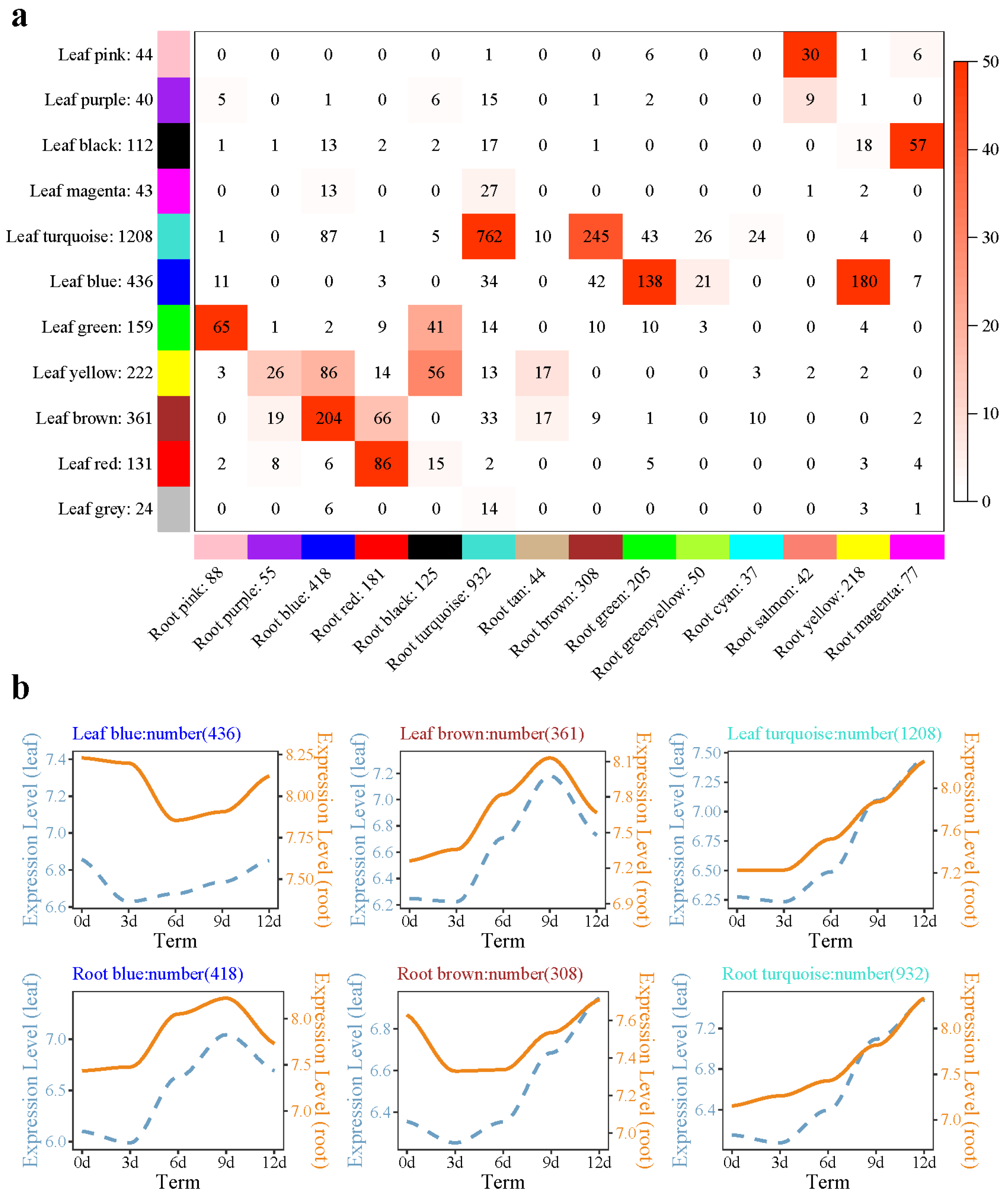
2.4. Weighted Gene Coexpression Network Analysis (WGCNA) Analysis
3. Discussion
4. Materials and Methods
4.1. Acquisition of Test Materials
4.2. Determination of Physiological Indicators
4.3. Establishment of Transcriptome Library
4.4. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, L.; Xie, Y.; Fan, S.; Wang, Z.; Wang, F.; Zhang, B.; Li, H.; Song, J.; Kong, L. Comparative analysis of root transcriptome profiles between drought-tolerant and susceptible wheat genotypes in response to water stress. Plant Sci. 2018, 272, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, Z.; Chen, D.; Zhao, S.; Zhou, F.; Cao, X.; Fang, K. Growth decline of Pinus Massoniana in response to warming induced drought and increasing intrinsic water use efficiency in humid subtropical China. Dendrochronologia 2019, 57, 125609. [Google Scholar] [CrossRef]
- Stocker, T. (Ed.) Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Sun, M.; Huang, D.; Zhang, A.; Khan, I.; Yan, H.; Wang, X.; Zhang, X.; Zhang, J.; Huang, L. Transcriptome analysis of heat stress and drought stress in pearl millet based on Pacbio full-length transcriptome sequencing. BMC Plant Biol. 2020, 20, 323. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.; Doron-Faigenboim, A.; Kelly, G.; Bourstein, R.; Attia, Z.; Zhou, J.; Moshe, Y.; Moshelion, M.; David-Schwartz, R. Transcriptome analysis of Pinus halepensis under drought stress and during recovery. Tree Physiol. 2018, 38, 423–441. [Google Scholar] [CrossRef]
- Tan, J.; Tang, S.S.; Chen, H. Advances of Drought Resistance in Pinus massoniana. Guangxi For. Sci. 2017, 46, 1–7. [Google Scholar] [CrossRef]
- Deng, X.; Xiao, W.; Shi, Z.; Zeng, L.; Lei, L. Combined Effects of Drought and Shading on Growth and Non-Structural Carbohydrates in Pinus massoniana Lamb. Seedlings. Forests 2020, 11, 18. [Google Scholar] [CrossRef]
- Quan, W.; Ding, G. Root tip structure and volatile organic compound responses to drought stress in Masson pine (Pinus massoniana Lamb.). Acta Physiol. Plant. 2017, 39, 258. [Google Scholar] [CrossRef]
- Quan, W.; Ding, G. Dynamic of volatiles and endogenous hormones in pinus massoniana needles under drought stress. Sci. Silvae Sin. 2017, 53, 49–55. [Google Scholar]
- Du, M.; Ding, G.; Zhao, X. Responses to continuous drought stress and drought resistance of different masson pine families. Sci. Silvae Sin. 2017, 53, 21–29. [Google Scholar]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Du, M. The Molecular Mechanism of Masson Pine Drought-Resistant Germplasm in Response to Drought Stress. Guizhou University, 2018. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CDFD&dbname=CDFDLAST2019&filename=1019819015.nh&v=CVf9cfJqo7Cnn1DdPs3YUIEplNbmZqz6z99igqCDzt%25mmd2BXNEwfR9z9%25mmd2F%25mmd2F70XLLVjp0l (accessed on 1 March 2021). (In Chinese).
- Du, M.; Ding, G.; Cai, Q. The Transcriptomic Responses of Pinus massoniana to Drought Stress. Forests 2018, 9, 326. [Google Scholar] [CrossRef]
- Garg, R.; Singh, V.K.; Rajkumar, M.S.; Kumar, V.; Jain, M. Global transcriptome and coexpression network analyses reveal cultivar-specific molecular signatures associated with seed development and seed size/weight determination in chickpea. Plant J. 2017, 91, 1088–1107. [Google Scholar] [CrossRef]
- Taïbi, K.; Del Campo, A.D.; Mulet, J.M.; Flors, J.; Aguado, A.; Del Campo, A. Testing Aleppo pine seed sources response to climate change by using trial sites reflecting future conditions. New For. 2014, 45, 603–624. [Google Scholar] [CrossRef][Green Version]
- Taïbi, K.; Del Campo, A.D.; Aguado, A.; Mulet, J.M. The effect of genotype by environment interaction, phenotypic plasticity and adaptation on Pinus halepensis reforestation establishment under expected climate drifts. Ecol. Eng. 2015, 84, 218–228. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. ASCORBATE AND GLUTATHIONE: Keeping Active Oxygen Under Control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Lv, D.; Wang, P.; Wang, X.-C.; Chen, J.; Song, C.-P. An Arabidopsis Glutathione Peroxidase Functions as Both a Redox Transducer and a Scavenger in Abscisic Acid and Drought Stress Responses. Plant Cell 2006, 18, 2749–2766. [Google Scholar] [CrossRef]
- Aguadé, D.; Poyatos, R.; Gómez, M.; Oliva, J.; Martinez-Vilalta, J. The role of defoliation and root rot pathogen infection in driving the mode of drought-related physiological decline in Scots pine (Pinus sylvestris L.). Tree Physiol. 2015, 35, 229–242. [Google Scholar] [CrossRef]
- Wang, D.; Huang, G.; Duan, H.; Lei, X.; Liu, W.; Wu, J.; Fan, H. Effects of drought and nitrogen addition on growth and leaf physiology of pinus massoniana seedlings. Pak. J. Bot. 2019, 51, 1575–1585. [Google Scholar] [CrossRef]
- Zhang, X. Photosynthetic Physiology for Pinus massoniana in Jin Yun Mountain; Beijing Forestry University: Beijing, China, 2013; p. 48. Available online: https://kns.cnki.net/KNS8/Detail?sfield=fn&QueryID=29&CurRec=1&recid=&FileName=1013213698.nh&DbName=CMFD201302&DbCode=CMFD&yx=&pr=&URLID= (accessed on 1 March 2021). (In Chinese)
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) Function as Transcriptional Activators in Abscisic Acid Signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.I.; Yang, Z.; Gong, Q.; Chen, E.; Wang, X.; Zhao, G.; Ge, X.; Zhang, X.; Li, F. GaMYB85, an R2R3 MYB gene, in transgenic Arabidopsis plays an important role in drought tolerance. BMC Plant Biol. 2017, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, W.; Yao, Y.; Wei, Y.; Chan, Z. Alcohol dehydrogenase 1 (ADH1) confers both abiotic and biotic stress resistance in Arabidopsis. Plant Sci. 2017, 262, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Myint, T.; Ismawanto, S.; Namasivayam, P.; Napis, S.; Abdulla, M.P. Expression analysis of the ADH genes in Arabidopsis plants exposed to PEG-induced water stress. World J. Agric. Res. 2015, 3, 57–65. [Google Scholar]
- Luan, S. Protein phosphatases and signaling cascades in higher plants. Trends Plant Sci. 1998, 3, 271–275. [Google Scholar] [CrossRef]
- Manohar, M.; Wang, D.; Manosalva, P.M.; Choi, H.W.; Kombrink, E.; Klessig, D.F. Members of the abscisic acid co-receptor PP2C protein family mediate salicylic acid-abscisic acid crosstalk. Plant Direct 2017, 1, e00020. [Google Scholar] [CrossRef] [PubMed]
- Schaller, F.; Biesgen, C.; Mufcssig, C.; Altmann, T.; Weiler, E.W. 12-Oxophytodienoate reductase 3 (OPR3) is the isoenzyme involved in jasmonate biosynthesis. Planta 2000, 210, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; Ellis, C.; Devoto, A. The Jasmonate Signal Pathway. Plant Cell 2002, 14 (Suppl. 1), S153–S164. [Google Scholar] [CrossRef] [PubMed]
- Strassner, J.; Schaller, F.; Frick, U.B.; Howe, G.A.; Weiler, E.W.; Amrhein, N.; Schaller, A. Characterization and cDNA-microarray expression analysis of 12-oxophytodienoate reductases reveals differential roles for octadecanoid biosynthesis in the local versus the systemic wound response. Plant J. 2002, 32, 585–601. [Google Scholar] [CrossRef]
- Tani, T.; Sobajima, H.; Okada, K.; Chujo, T.; Arimura, S.-I.; Tsutsumi, N.; Nishimura, M.; Seto, H.; Nojiri, H.; Yamane, H. Identification of the OsOPR7 gene encoding 12-oxophytodienoate reductase involved in the biosynthesis of jasmonic acid in rice. Planta 2008, 227, 517–526. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; Palma, J.M.; Rodriguez-Ruiz, M. Plant peroxisomes: A nitro-oxidative cocktail. Redox Biol. 2017, 11, 535–542. [Google Scholar] [CrossRef]
- Islinger, M.; Grille, S.; Fahimi, H.D.; Schrader, M. The peroxisome: An update on mysteries. Histochem. Cell Biol. 2012, 137, 547–574. [Google Scholar] [CrossRef]
- Bolte, K.; Rensing, S.A.; Maier, U.G. The evolution of eukaryotic cells from the perspective of peroxisomes: Phylogenetic analyses of peroxisomal beta-oxidation enzymes support mitochondria-first models of eukaryotic cell evolution. BioEssays 2015, 37, 195–203. [Google Scholar] [CrossRef]
- Pan, R.; Liu, J.; Hu, J. Peroxisomes in plant reproduction and seed-related development. J. Integr. Plant Biol. 2018, 61, 784–802. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, L.; Sanad, M.N.; Jarvis, D.E.; Steel, P.; Murphy, K.; Smertenko, A. Impact of heat and drought stress on peroxisome proliferation in quinoa. Plant J. 2019, 99, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Sanad, M.N.M.E.; Smertenko, A.; Garland-Campbell, K.A. Differential Dynamic Changes of Reduced Trait Model for Analyzing the Plastic Response to Drought Phases: A Case Study in Spring Wheat. Front. Plant Sci. 2019, 10, 504. [Google Scholar] [CrossRef]
- Saravitz, D.M.; Pharr, D.M.; Carter, T.E. Galactinol Synthase Activity and Soluble Sugars in Developing Seeds of Four Soybean Genotypes. Plant Physiol. 1987, 83, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, M.G.; Ishizaki, T.; Valencia, M.; Ogawa, S.; Dedicova, B.; Ogata, T.; Yoshiwara, K.; Maruyama, K.; Kusano, M.; Saito, K.; et al. Overexpression of an Arabidopsis thaliana galactinol synthase gene improves drought tolerance in transgenic rice and increased grain yield in the field. Plant Biotechnol. J. 2017, 15, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Fan, J.; Xu, C. Peroxisomal fatty acid β-oxidation negatively impacts plant survival under salt stress. Plant Signal. Behav. 2019, 14, 1–3. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef]
- Nakagawa, A.C.S.; Itoyama, H.; Ariyoshi, Y.; Ario, N.; Tomita, Y.; Kondo, Y.; Iwaya-Inoue, M.; Ishibashi, Y. Drought stress during soybean seed filling affects storage compounds through regulation of lipid and protein metabolism. Acta Physiol. Plant. 2018, 40, 111. [Google Scholar] [CrossRef]
- De Miguel, M.; Guevara, M.Á.; Sánchez-Gómez, D.; de María, N.; Díaz, L.M.; Mancha, J.A.; de Simón, B.F.; Cadahía, E.; Desai, N.; Aranda, I.; et al. Organ-specific metabolic responses to drought in Pinus pinaster Ait. Plant Physiol. Biochem. 2016, 102, 17–26. [Google Scholar] [CrossRef]
- Taïbi, K.; Del Campo, A.D.; Vilagrosa, A.; Bellés, J.M.; López-Gresa, M.P.; Pla, D.; Calvete, J.J.; López-Nicolás, J.M.; Mulet, J.M. Drought Tolerance in Pinus halepensis Seed Sources As Identified by Distinctive Physiological and Molecular Markers. Front. Plant Sci. 2017, 8, 1202. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Ding, G.J.; Wen, X.P. Cloning of Glutathione Peroxidase Pm GPX6 Gene from Pinus massoniana and the Study on Drought Tolerance of Transgenic Arabidopsis thaliana. For. Res. 2016, 29, 839–846. [Google Scholar]
- Baozhang, B.; Jinzhi, J.; Liping, H.; Song, B. Improvement of TTC method determining root activity in corn. Maize Sci. 1994, 2, 44–47. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Pan, L.; Yang, Z.; Wang, J.; Wang, P.; Ma, X.; Zhou, M.; Li, J.; Gang, N.; Feng, G.; Zhao, J.; et al. Comparative proteomic analyses reveal the proteome response to short-term drought in Italian ryegrass (Lolium multiflorum). PLoS ONE 2017, 12, e0184289. [Google Scholar] [CrossRef]
- Xuekui, W. The Principle and Technology of Plant Physiology and Biochemistry Experiment; Higher Education Press: Beijjing, China, 2006. (In Chinese) [Google Scholar]
- Liu, J.J.; Wei, Z.; Li, J.H. Effects of copper on leaf membrane structure and root activity of maize seedling. Bot. Stud. 2014, 55, 1–6. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Finn, R.D.; Eddy, S.R.; Bateman, A.; Punta, M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013, 41, e121. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A. Ambiguous Fragment Assignment for High-Throughput Sequencing Experiments; University of California: Berkeley, CA, USA, 2013. [Google Scholar]
- Anders, S.; Huber, W. Differential Expression of RNA-Seq Data at the Gene Level—The DESeq Package; European Molecular Biology Laboratory (EMBL): Heidelberg, Germany, 2012. [Google Scholar]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, F.; Zhao, Y.; Wang, X.-R.; Liu, Q.; Ran, J. Transcriptome Analysis of Needle and Root of Pinus Massoniana in Response to Continuous Drought Stress. Plants 2021, 10, 769. https://doi.org/10.3390/plants10040769
Xiao F, Zhao Y, Wang X-R, Liu Q, Ran J. Transcriptome Analysis of Needle and Root of Pinus Massoniana in Response to Continuous Drought Stress. Plants. 2021; 10(4):769. https://doi.org/10.3390/plants10040769
Chicago/Turabian StyleXiao, Feng, Yang Zhao, Xiu-Rong Wang, Qiao Liu, and Jie Ran. 2021. "Transcriptome Analysis of Needle and Root of Pinus Massoniana in Response to Continuous Drought Stress" Plants 10, no. 4: 769. https://doi.org/10.3390/plants10040769
APA StyleXiao, F., Zhao, Y., Wang, X.-R., Liu, Q., & Ran, J. (2021). Transcriptome Analysis of Needle and Root of Pinus Massoniana in Response to Continuous Drought Stress. Plants, 10(4), 769. https://doi.org/10.3390/plants10040769





