Bitter Melon (Momordica charantia L.) Fruit Bioactives Charantin and Vicine Potential for Diabetes Prophylaxis and Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement and Handling of Raw Material
2.2. Reagents and Standards
2.3. Quantification of Charantin
2.4. Quantification of Vicine
2.5. Efficacy Study
2.6. Diet and Dosage
2.7. Testing
2.8. Statistical Analysis
3. Results
3.1. Quantification of Charantin and Vicine
3.2. Feed and Water Intake
3.3. Body Weight Gain
3.4. Estimation of Urine and Reducing Sugar Excretion
3.5. Estimation of Kidney Weight
3.6. Estimation of Glomerular Filtration Rate
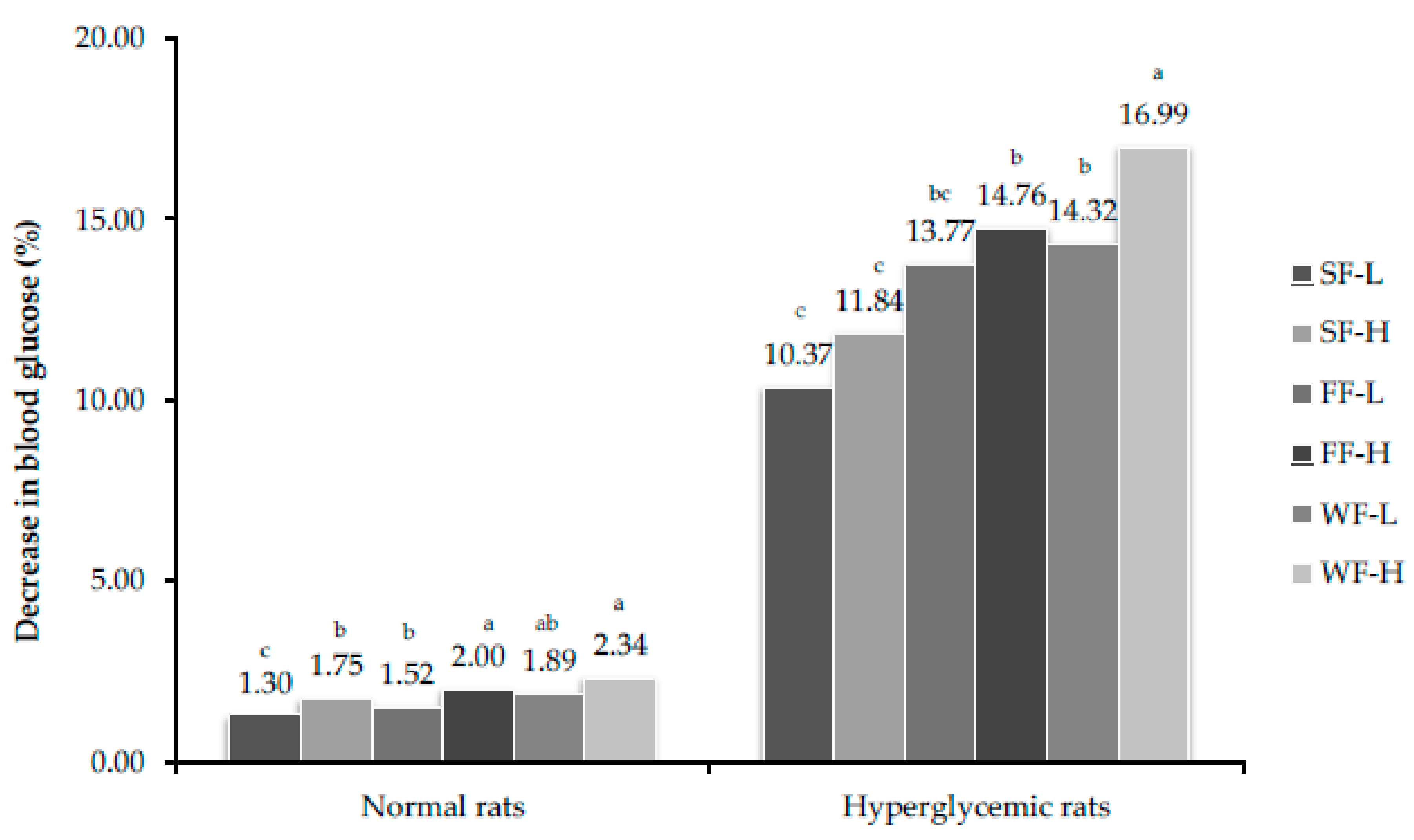
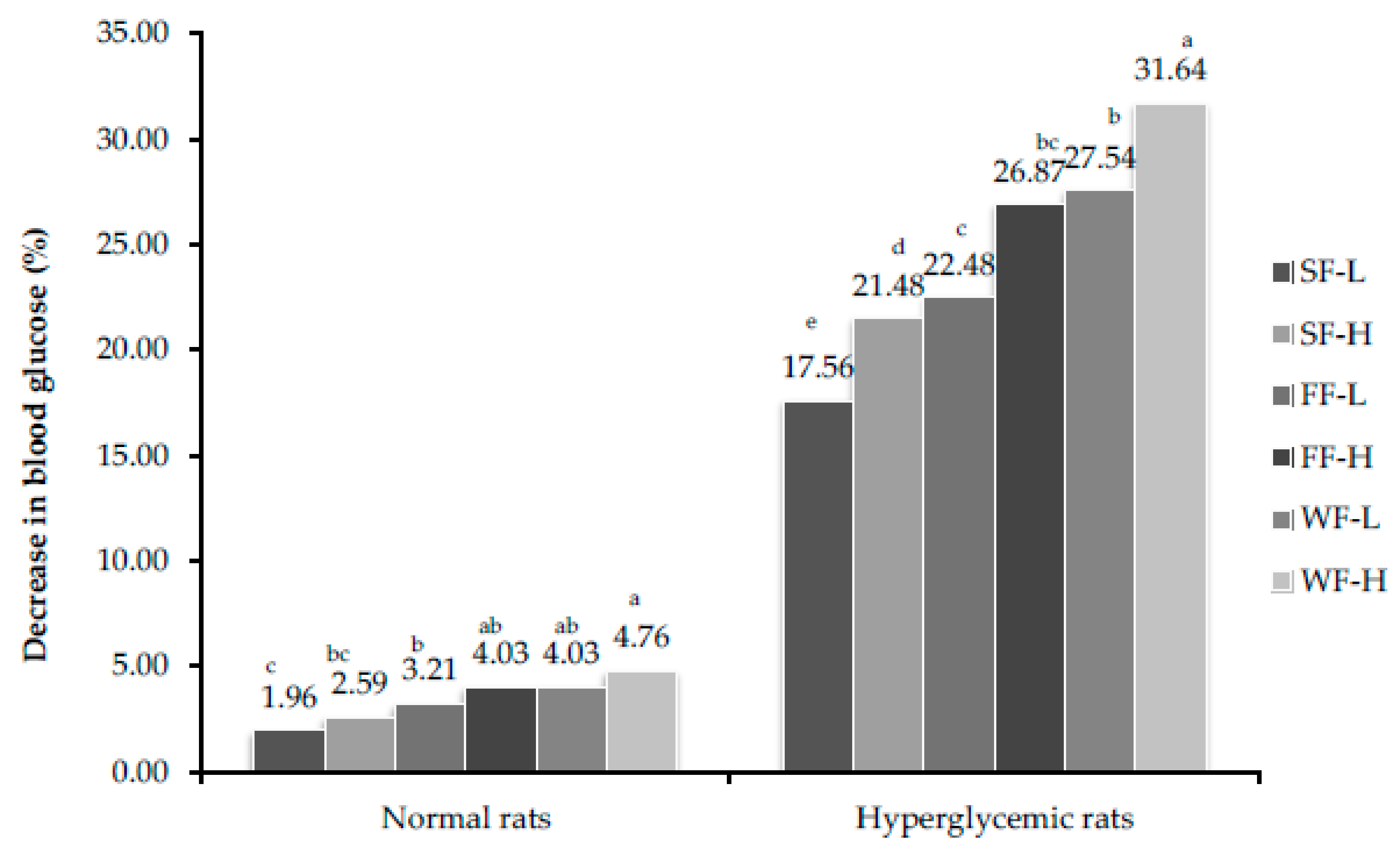
3.7. Estimation of Blood Glucose
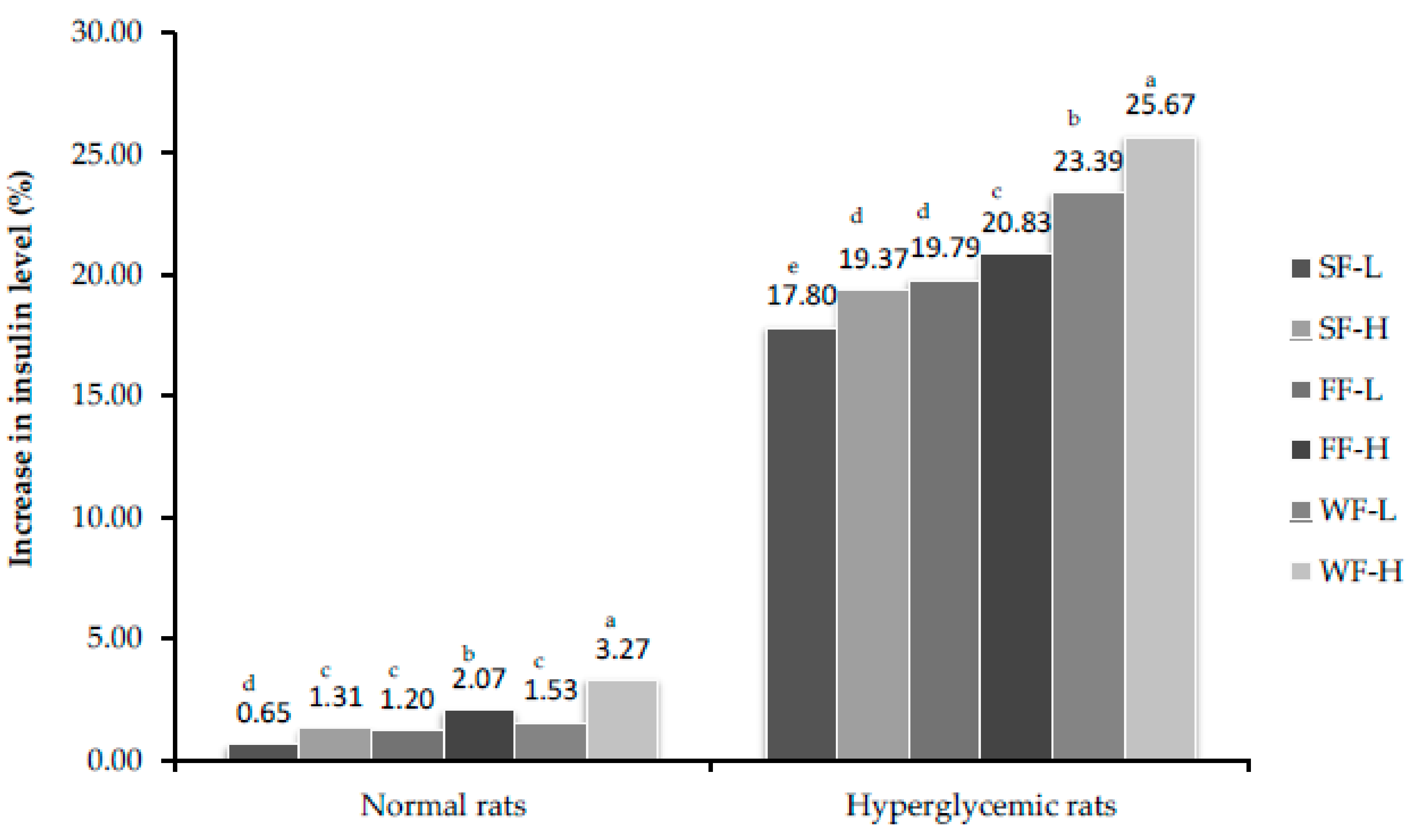
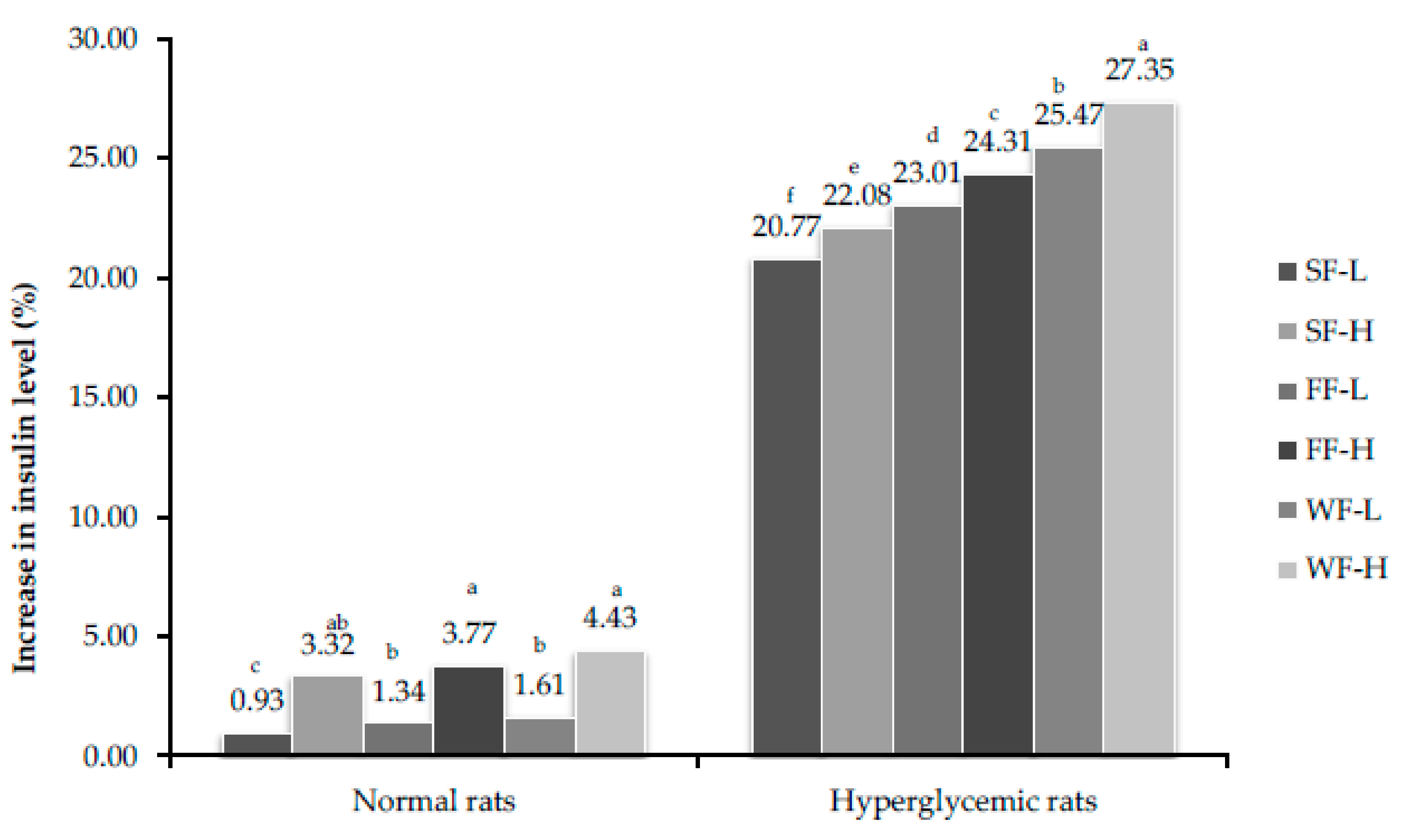
3.8. Estimation of Insulin Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Gupta, M.; Sharma, S.; Gautam, A.; Bhadauria, R. Momordica charantia Linn. (Karela): Nature’s silent healer. Int. J. Pharm. Sci. Rev. Res. 2011, 11, 32–37. [Google Scholar]
- Virdi, J.; Sivakami, S.; Shahani, S.; Suthar, A.C.; Banavalikar, M.M.; Biyani, M.K. Antihyperglycemic effects of three extracts from Momordica charantia. J. Ethnopharmacol. 2003, 88, 107–111. [Google Scholar] [CrossRef]
- Kulkarni, P.; Lohidasan, S.; Mahadik, K. Isolation, characterisation and investigation of in vitro antidiabetic and antioxidant activity of phytoconstituents from fruit of Momordica charantia Linn. Nat. Prod. Res. 2019, 1–3. [Google Scholar] [CrossRef]
- Kim, S.K.; Jung, J.; Jung, J.H.; Yoon, N.; Kang, S.S.; Roh, G.S.; Hahm, J.R. Hypoglycemic efficacy and safety of Momordica charantia (bitter melon) in patients with type 2 diabetes mellitus. Complement. Ther. Med. 2020, 52, 102524. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Navarrete, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; González-Ortiz, M.; Méndez-Del Villar, M. Momordica charantia Administration Improves Insulin Secretion in Type 2 Diabetes Mellitus. J. Med. Food 2018, 21, 672–677. [Google Scholar] [CrossRef]
- Thent, Z.C.; Das, S.; Zaidun, N.H. Emerging Trends On Drug Delivery Strategy of Momordica charantia against Diabetes and its Complications. Curr. Drug Deliv. 2018, 15, 453–460. [Google Scholar] [CrossRef]
- Xu, X.; Shan, B.; Liao, C.H.; Xie, J.H.; Wen, P.W.; Shi, J.Y. Anti-diabetic properties of Momordica charantia L. polysaccharide in alloxan-induced diabetic mice. Int. J. Biol. Macromol. 2015, 81, 538–543. [Google Scholar] [CrossRef]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef]
- Tan, S.P.; Kha, T.C.; Parks, S.E.; Roach, P.D. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: A review. Food. Rev. Int. 2016, 32, 181–202. [Google Scholar] [CrossRef]
- Ooi, C.P.; Yassin, Z.; Hamid, T.A. Momordica charantia for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Kasbia, G.S.; Arnason, J.T.; Imbeault, P. No effect of acute, single dose oral administration of Momordica charantia Linn., on glycemia, energy expenditure and appetite: A pilot study in non-diabetic overweight men. J. Ethnopharmacol. 2009, 126, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.V.; Lee, N.C.; Hirpara, H.; Phung, O.J. The effect of bitter melon (Mormordica charantia) in patients with diabetes mellitus: A systematic review and meta-analysis. Nutr. Diabetes 2014, 4, e145. [Google Scholar] [CrossRef]
- Peter, E.L.; Kasali, F.M.; Deyno, S.; Mtewa, A.; Nagendrappa, P.B.; Tolo, C.U.; Ogwang, P.E.; Sesaazi, D. Momordica charantia L. lowers elevated glycaemia in type 2 diabetes mellitus patients: Systematic review and meta-analysis. J. Ethnopharmacol. 2019, 231, 311–324. [Google Scholar] [CrossRef]
- Pitipanapong, J.; Chitprasert, S.; Goto, M.; Jiratchariyakul, W.; Sasaki, M.; Shotipruk, A. New approach for extraction of charantin from Momordica charantia with pressurized liquid extraction. Sep. Purif. Technol. 2007, 52, 416–422. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zhang, X.; Liu, M.; Hu, Z. Analysis of vicine in bitter melon with high performance liquid chromatography. Anal. Lett. 2003, 36, 1597–1605. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Owen, J.A.; Iggo, B.; Scandrett, F.J.; Stewart, C.P. The determination of creatinine in plasma or serum, and in urine; a critical examination. Biochem. J. 1954, 58, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Chung, H.Y.; He, L.Q.; Oura, H. Effectiveness of green tea tannin on rats with chronic renal failure. Biosci. Biotechnol. Biochem. 1996, 60, 1000–1005. [Google Scholar] [CrossRef][Green Version]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Ahn, J.; Choi, W.; Kim, S.; Ha, T. Anti-diabetic effect of watermelon (Citrullus vulgaris Schrad) on Streptozotocin-induced diabetic mice. Food Sci. Biotechnol. 2011, 20, 251–254. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Han, C.; Wang, J. Optimization of conditions for charantin extraction in PEG/Salt aqueous two-phase systems using response surface methodology. Open Complemen. Med. J. 2009, 1, 46–50. [Google Scholar] [CrossRef]
- Desai, S.; Tatke, P.; Mane, T.; Gabhe, S. Isolation, characterization and quantitative HPLC-DAD analysis of components of charantin from fruits of Momordica charantia. Food Chem. 2020, 345, 128717. [Google Scholar] [CrossRef]
- Parmar, K.; Patel, S.; Patel, J.; Patel, B.; Patel, M. Effects of bittergourd (Momordica charantia) fruit juice on glucose tolerance and lipid profile in type-II diabetic rats. Int. J. Drug Dev. Res. 2011, 3, 139–146. [Google Scholar]
- Chen, Q.; Chan, L.L.; Li, E.T. Bitter melon (Momordica charantia) reduces adiposity, lowers serum insulin and normalizes glucose tolerance in rats fed a high fat diet. J. Nutr. 2003, 133, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Reyes, B.A.; Bautista, N.D.; Tanquilut, N.C.; Anunciado, R.V.; Leung, A.B.; Sanchez, G.C.; Magtoto, R.L.; Castronuevo, P.; Tsukamura, H.; Maeda, K.I. Anti-diabetic potentials of Momordica charantia and Andrographis paniculata and their effects on estrous cyclicity of alloxan-induced diabetic rats. J. Ethnopharmacol. 2006, 105, 196–200. [Google Scholar] [CrossRef]
- Huang, H.L.; Hong, Y.W.; Wong, Y.H.; Chen, Y.N.; Chyuan, J.H.; Huang, C.J.; Chao, P.M. Bitter melon (Momordica charantia L.) inhibits adipocyte hypertrophy and down regulates lipogenic gene expression in adipose tissue of diet-induced obese rats. Br. J. Nutr. 2008, 99, 230–239. [Google Scholar] [CrossRef]
- Klomann, S.D.; Mueller, A.S.; Pallauf, J.; Krawinkel, M.B. Antidiabetic effects of bitter gourd extracts in insulin-resistant db/db mice. Br. J. Nutr. 2010, 104, 1613–1620. [Google Scholar] [CrossRef]
- Jafri, S.; Ismail, M.; Zaman, G. Effect of Momordica charantia (karela) in alloxan induced diabetic rats. Pak. J. Sci. 2009, 61, 220. [Google Scholar]
- Nandini, C.D.; Sambaiah, K.; Salimath, P.V. Dietary fibres ameliorate decreased synthesis of heparan sulphate in streptozotocin induced diabetic rats. J. Nutr. Biochem. 2003, 14, 203–210. [Google Scholar] [CrossRef]
- Christiansen, J.S.; Gammelgaard, J.; Frandsen, M.; Parving, H.H. Increased kidney size, glomerular filtration rate and renal plasma flow in short-term insulin-dependent diabetics. Diabetologia 1981, 20, 451–456. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, B.; Hegedüs, L.; Mathiesen, E.; Deckert, T. Kidney volume in type 1 (insulin-dependent) diabetic patients with normal or increased urinary albumin excretion: Effect of long-term improved metabolic control. Scand. J. Clin. Lab. Investig. 1991, 51, 31–36. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, M. Regeneration of beta cells in islets of Langerhans of pancreas of alloxan diabetic rats by acetone extract of Momordica charantia (Linn.) (bitter gourd) fruits. Ind. J. Exp. Biol. 2007, 45, 1055–1062. [Google Scholar] [PubMed]
- Bhushan, M.S.; Rao, C.; Ojha, S.; Vijayakumar, M.; Verma, A. An analytical review of plants for anti diabetic activity with their phytoconstituent and mechanism of action. Int. J. Pharm. Sci. Res. 2010, 1, 29–46. [Google Scholar] [CrossRef]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef]
- Akhtar, N.; Khan, B.A.; Majid, A.; Khan, H.M.; Mahmood, T.; Gulfishan; Saeed, T. Pharmaceutical and biopharmaceutical evaluation of extracts from different plant parts of indigenous origin for their hypoglycemic responses in rabbits. Acta Pol. Pharm. 2011, 68, 919–925. [Google Scholar] [PubMed]
- Uebanso, T.; Arai, H.; Taketani, Y.; Fukaya, M.; Yamamoto, H.; Mizuno, A.; Uryu, K.; Hada, T.; Takeda, E. Extracts of Momordica charantia suppress postprandial hyperglycemia in rats. J. Nutr. Sci. Vitaminol. 2007, 53, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-H.; Lee, S.-H.; Hue, J.-J.; Lee, K.-N.; Nam, S.Y.; Yun, Y.W.; Jeong, S.-w.; Lee, Y.H.; Lee, B.J. Effect of bitter melon (Momordica charantia) on anti-diabetic activity in C57BLI/6J db/db mice. Korean J. Vet. Res. 2008, 48, 327–336. [Google Scholar]
- Abdollah, M.; Zuki, A.; Goh, Y.; Rezaeizadeh, A.; Noordin, M. The effects of Momordica charantia on the liver in streptozotocin-induced diabetes in neonatal rats. Afr. J. Biotechnol. 2010, 9, 5004–5012. [Google Scholar]
- Shibib, B.A.; Khan, L.A.; Rahman, R. Hypoglycaemic activity of Coccinia indica and Momordica charantia in diabetic rats: Depression of the hepatic gluconeogenic enzymes glucose-6-phosphatase and fructose-1,6-bisphosphatase and elevation of both liver and red-cell shunt enzyme glucose-6-phosphate dehydrogenase. Biochem. J. 1993, 292, 267–270. [Google Scholar] [CrossRef]
- Gadang, V.; Gilbert, W.; Hettiararchchy, N.; Horax, R.; Katwa, L.; Devareddy, L. Dietary bitter melon seed increases peroxisome proliferator-activated receptor-γ gene expression in adipose tissue, down-regulates the nuclear factor-κB expression, and alleviates the symptoms associated with metabolic syndrome. J. Med. Food 2011, 14, 86–93. [Google Scholar] [CrossRef]
- Joseph, B.; Jini, D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013, 3, 93–102. [Google Scholar] [CrossRef]
- Fernandes, N.P.; Lagishetty, C.V.; Panda, V.S.; Naik, S.R. An experimental evaluation of the antidiabetic and antilipidemic properties of a standardized Momordica charantia fruit extract. BMC Complement. Altern. Med. 2007, 7, 29. [Google Scholar] [CrossRef]
- Mohammady, I.; Elattar, S.; Mohammed, S.; Ewais, M. An evaluation of anti-diabetic and anti-lipidemic properties of Momordica charantia (bitter melon) fruit extract in experimentally induced diabetes. Life Sci. J. 2012, 9, 363–374. [Google Scholar]
- Xiang, L.; Huang, X.; Chen, L.; Rao, P.; Ke, L. The reparative effects of Momordica charantia Linn. extract on HIT-T15 pancreatic beta-cells. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. 1), 249–252. [Google Scholar] [PubMed]

| Groups | Water Intake (mL/24 h) | |||
|---|---|---|---|---|
| 28th day | 56th day | |||
| Normal Rats | Hyperglycemic Rats | Normal Rats | Hyperglycemic Rats | |
| Control | 23.7 ± 1.15 d | 29.35 ± 2.15 a | 27.9 ± 2.12 d | 36.65 ± 2.05 a |
| SF-L | 24.1 ± 1.34 c | 25.94 ± 1.49 b | 28.1 ± 1.77 c,d | 31.02 ± 2.34 b |
| SF-H | 24.3 ± 1.40 c | 24.85 ± 1.46 c | 28.4 ± 2.15 c | 29.01 ± 2.17 d |
| FF-L | 24.4 ± 1.88 b,c | 25.03 ± 2.11 b | 28.3 ± 1.63 c | 30.85 ± 1.69 c |
| FF-H | 24.7 ± 1.35 b | 24.07 ± 1.75 d | 28.6 ± 2.19 b | 28.79 ± 1.56 e |
| WF-L | 24.8 ± 2.01 a,b | 24.91 ± 1.68 b | 28.7 ± 2.03 b | 30.71 ± 2.62 c |
| WF-H | 25.2 ± 1.48 a | 23.86 ± 1.33 d | 29.00 ± 1.59 a | 28.63 ± 2.22 e |
| Groups | Feed Intake (g) | |||
|---|---|---|---|---|
| 28th day | 56th day | |||
| Normal Rats | Hyperglycemic Rats | Normal Rats | Hyperglycemic Rats | |
| Control | 15.1 ± 1.12 a | 17.03 ± 1.78 a | 21.2 ± 2.49 a | 23.22 ± 1.33 a |
| SF-L | 14.6 ± 0.76 b | 14.96 ± 1.39 b | 20.5 ± 2.03 b | 20.94 ± 1.11 b |
| SF-H | 14.3 ± 1.38 b | 13.80 ± 1.01 c | 20.1 ± 1.98 b | 19.78 ± 1.04 c |
| FF-L | 14.3 ± 1.22 b | 14.94 ± 0.78 b | 20.4 ± 1.62 b | 20.87 ± 0.92 b |
| FF-H | 14.1 ± 0.94 b,c | 13.76 ± 1.39 c | 19.8 ± 0.99 b,c | 19.45 ± 1.17 c |
| WF-L | 13.9 ± 0.45 c | 14.82 ± 1.54 b | 19.9 ± 1.07 b,c | 20.73 ± 1.43 b |
| WF-H | 13.7 ± 0.88 c | 13.64 ± 1.44 c | 19.6 ± 0.69 c | 19.36 ± 1.36 c |
| Groups | Body Weight (G) | |||
|---|---|---|---|---|
| 28th Day | 56th Day | |||
| Normal Rats | Hyperglycemic Rats | Normal Rats | Hyperglycemic Rats | |
| Control | 158.66 ± 3.01 a | 145.55 ± 2.75 c | 215.90 ± 4.22 a | 181.34 ± 2.61 c |
| SF-L | 155.91 ± 2.77 b | 151.18 ± 2.04 b | 208.67 ± 4.06 b | 203.66 ± 3.59 b |
| SF-H | 154.75 ± 3.52 c | 151.66 ± 1.89 a,b | 208.40 ± 2.88 b | 205.33 ± 3.11 a |
| FF-L | 154.62 ± 1.95 c | 151.43 ± 2.09 b | 208.33 ± 2.54 b | 204.50 ± 3.49 b |
| FF-H | 154.34 ± 2.67 c | 152.57 ± 2.67 a | 208.13 ± 1.97 b | 206.56 ± 3.34 a |
| WF-L | 154.32 ± 3.00 c | 151.71 ± 0.95 b | 208.26 ± 2.42 b | 204.66 ± 2.68 b |
| WF-H | 153.68 ± 2.18 d | 152.83 ± 1.90 a | 208.01 ± 2.97 b | 206.60 ± 2.36 a |
| Groups | Urine Volume (mL/24 h) | |||
|---|---|---|---|---|
| 28th Day | 56th Day | |||
| Normal Rats | Hyperglycemic Rats | Normal Rats | Hyperglycemic Rats | |
| Control | 14.3 ± 2.23 a | 20.1 ± 1.01 a | 18.3 ± 1.13 a | 26.8 ± 2.85 a |
| SF-L | 13.7 ± 1.72 b | 16.5 ± 2.19 b | 17.6 ± 1.31 b | 21.3 ± 3.03 b |
| SF-H | 13.6 ± 1.63 b | 15.8 ± 1.43 b | 17.5 ± 0.99 b | 20.65 ± 1.26 b |
| FF-L | 13.3 ± 0.76 b,c | 15.7 ± 1.29 b | 17.3 ± 1.84 b | 20.4 ± 1.64 b |
| FF-H | 13.3 ± 1.33 b,c | 14.9 ± 2.07 b | 16.9 ± 1.22 b,c | 20.2 ± 2.13 b,c |
| WF-L | 13.2 ± 0.89 c | 15.2 ± 0.96 c | 16.8 ± 1.71 c | 19.9 ± 0.78 c |
| WF-H | 13.0 ± 2.13 c | 14.7 ± 1.56 d | 16.7 ± 2.17 c | 19.7 ± 1.16 c |
| Groups | Reducing Sugar in Urine (G) | |||
|---|---|---|---|---|
| 28th Day | 56th Day | |||
| Normal Rats | Hyperglycemic Rats | Normal Rats | Hyperglycemic Rats | |
| Control | 0.02 ± 0.00 a | 2.91 ± 0.32 a | 0.02 ± 0.01 a | 4.32 ± 0.75 a |
| SF-L | 0.03 ± 0.02 a | 2.16 ± 0.11 b | 0.03 ± 0.02 a | 2.18 ± 0.42 b |
| SF-H | 0.02 ± 0.01 a | 1.86 ± 0.21 c | 0.02 ± 0.01 a | 1.96 ± 0.38 b |
| FF-L | 0.05 ± 0.02 a | 1.99 ± 0.36 c | 0.04 ± 0.02 a | 2.02 ± 0.33 b |
| FF-H | 0.02 ± 0.00 a | 1.58 ± 0.58 c | 0.02 ± 0.01 a | 1.74 ± 0.27 c |
| WF-L | 0.02 ± 0.01 a | 1.87 ± 0.13 c | 0.03 ± 0.00 a | 1.89 ± 0.14 b |
| WF-H | 0.03 ± 0.01 a | 1.43 ± 0.19 d | 0.02 ± 0.00 a | 1.63 ± 0.36 c |
| Groups | Kidney Weight (G) in Normal Rats | Kidney Weight (G) in Hyperglycemic Rats | ||
|---|---|---|---|---|
| Right | Left | Right | Left | |
| Control | 0.69 ± 0.04 a | 0.68 ± 0.01 a | 0.89 ± 0.06 a | 0.87 ± 0.03 a |
| SF-L | 0.64 ± 0.03 b | 0.63 ± 0.02 b | 0.78 ± 0.03 b | 0.78 ± 0.02 b |
| SF-H | 0.63 ± 0.02 b | 0.62 ± 0.01 b | 0.76 ± 0.04 c | 0.77 ± 0.02 b |
| FF-L | 0.64 ± 0.02 b | 0.63 ± 0.03 b | 0.76 ± 0.03 c | 0.75 ± 0.04 c |
| FF-H | 0.62 ± 0.01 c | 0.62 ± 0.04 b | 0.74 ± 0.03 d | 0.72 ± 0.03 d |
| WF-L | 0.64 ± 0.03 b | 0.62 ± 0.02 b | 0.69 ± 0.02 e | 0.70 ± 0.01 d,e |
| WF-H | 0.62 ± 0.02 c | 0.62 ± 0.02 b | 0.68 ± 0.02 e | 0.68 ± 0.02 e |
| Groups | Glomerular Filtration Rate (mL/min) | |
|---|---|---|
| Normal Rats | Hyperglycemic Rats | |
| Control | 1.13 ± 0.13 a | 3.32 ± 0.33 a |
| SF-L | 1.06 ± 0.09 b | 2.76 ± 0.27 b |
| SF-H | 1.04 ± 0.11 b | 2.70 ± 0.28 b |
| FF-L | 1.04 ± 0.16 b | 2.75 ± 0.22 b |
| FF-H | 1.03 ± 0.07 b,c | 2.63 ± 0.31 b |
| WF-L | 1.04 ± 0.09 b | 2.45 ± 0.21 c |
| WF-H | 1.01 ± 0.14 c | 2.33 ± 0.14 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahwish; Saeed, F.; Sultan, M.T.; Riaz, A.; Ahmed, S.; Bigiu, N.; Amarowicz, R.; Manea, R. Bitter Melon (Momordica charantia L.) Fruit Bioactives Charantin and Vicine Potential for Diabetes Prophylaxis and Treatment. Plants 2021, 10, 730. https://doi.org/10.3390/plants10040730
Mahwish, Saeed F, Sultan MT, Riaz A, Ahmed S, Bigiu N, Amarowicz R, Manea R. Bitter Melon (Momordica charantia L.) Fruit Bioactives Charantin and Vicine Potential for Diabetes Prophylaxis and Treatment. Plants. 2021; 10(4):730. https://doi.org/10.3390/plants10040730
Chicago/Turabian StyleMahwish, Farhan Saeed, M. Tauseef Sultan, Ayesha Riaz, Sagheer Ahmed, Nicusor Bigiu, Ryszard Amarowicz, and Rosana Manea. 2021. "Bitter Melon (Momordica charantia L.) Fruit Bioactives Charantin and Vicine Potential for Diabetes Prophylaxis and Treatment" Plants 10, no. 4: 730. https://doi.org/10.3390/plants10040730
APA StyleMahwish, Saeed, F., Sultan, M. T., Riaz, A., Ahmed, S., Bigiu, N., Amarowicz, R., & Manea, R. (2021). Bitter Melon (Momordica charantia L.) Fruit Bioactives Charantin and Vicine Potential for Diabetes Prophylaxis and Treatment. Plants, 10(4), 730. https://doi.org/10.3390/plants10040730







