Non-Thermal Plasma Treatment Influences Shoot Biomass, Flower Production and Nutrition of Gerbera Plants Depending on Substrate Composition and Fertigation Level
Abstract
1. Introduction
2. Results
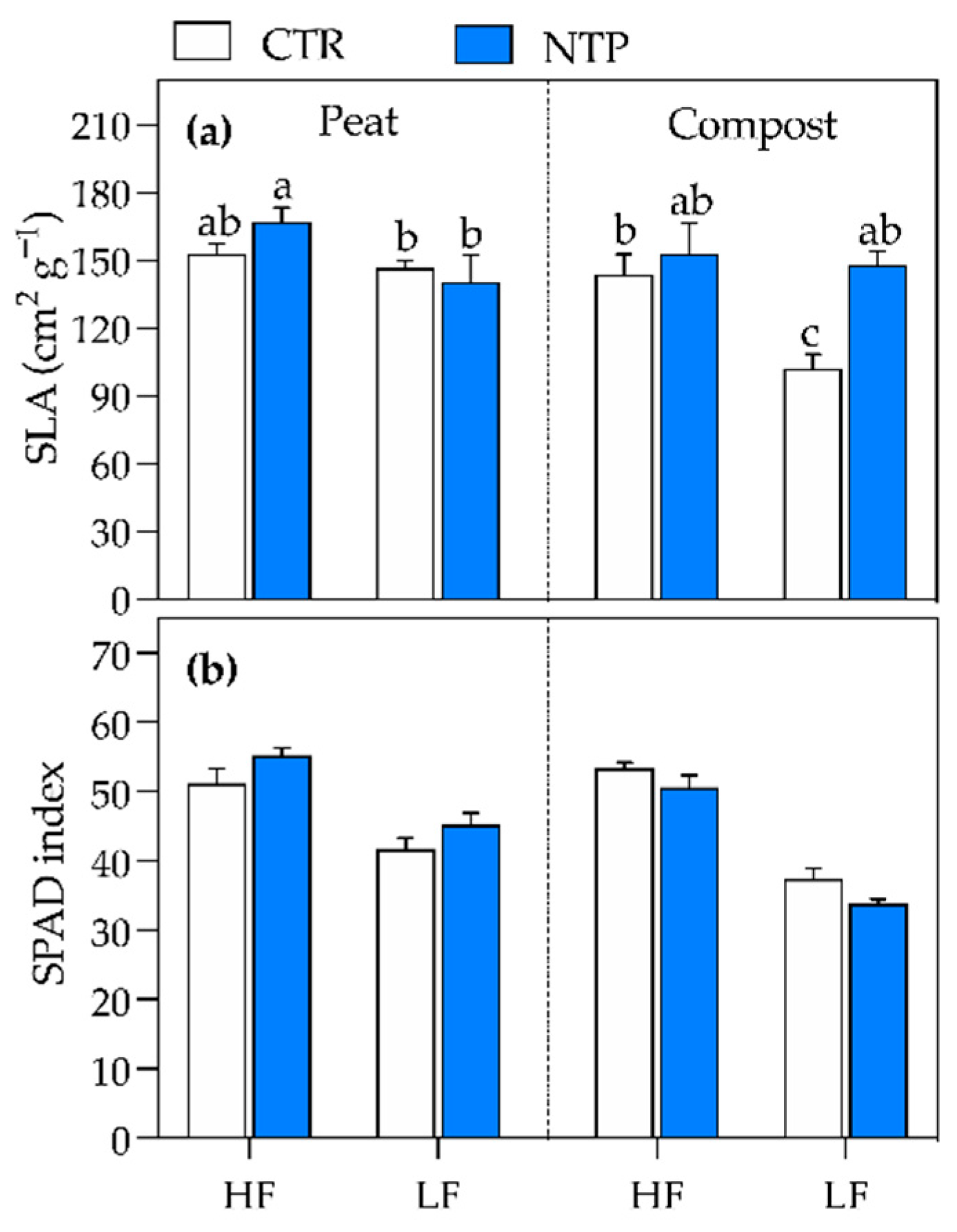
2.1. Plant Biomass Analyses
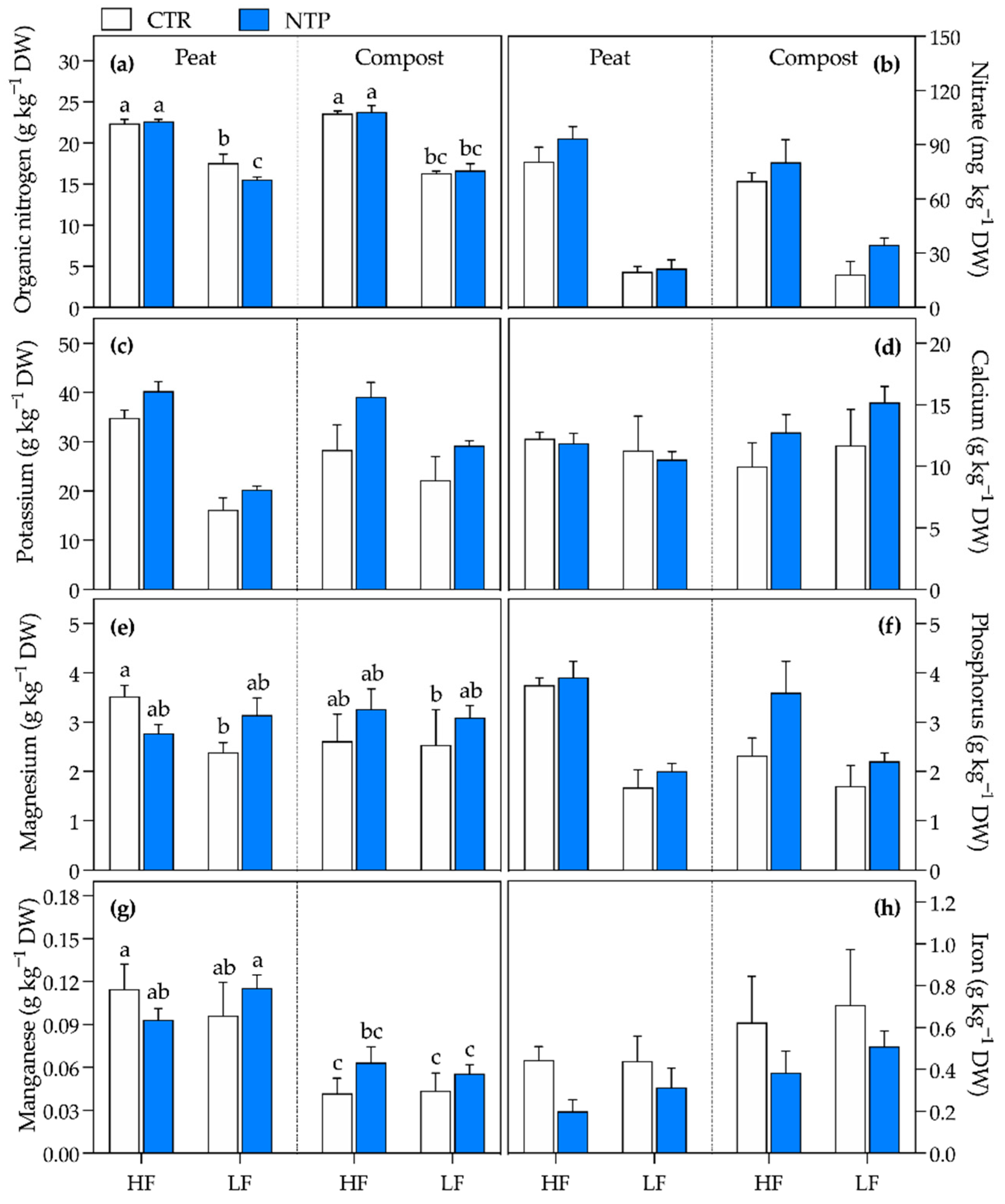
2.2. Leaf Elemental Concentrations
2.3. Fungi and Bacteria within the Substrate
3. Discussion
4. Materials and Methods
4.1. Plant Material, Treatments and Growing Conditions
4.2. Plant Sampling and Mineral Element Quantification
4.3. Quantification of Fungi and Bacteria
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradu, C.; Kutasi, K.; Magureanu, M.; Puač, N.; Živković, S. Reactive nitrogen species in plasma-activated water: Generation, chemistry and application in agriculture. J. Phys. Appl. Phys. 2020, 53, 223001. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.D.; Von Woedtke, T. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Ito, M.; Oh, J.S.; Ohta, T.; Shiratani, M.; Hori, M. Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Process. Polym. 2018, 15, 1700073. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, Ľ.; Hensel, K. Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Process. Polym. 2019, 16, 1800131. [Google Scholar] [CrossRef]
- Carlile, B.; Coules, A. Towards sustainability in growing media. Acta Hortic. 2013, 1013, 341–349. [Google Scholar] [CrossRef]
- Gruda, N. Sustainable peat alternative growing media. In XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010), International Symposium on Greenhouse 2010 and Soilless Cultivation. Acta Hortic. 2010, 927, 973–979. [Google Scholar]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Raviv, M. The future of composts as ingredients of growing media. Acta Hortic. 2011, 891, 19–32. [Google Scholar] [CrossRef]
- Massa, D.; Malorgio, F.; Lazzereschi, S.; Carmassi, G.; Prisa, D.; Burchi, G. Evaluation of two green composts for peat substitution in geranium (Pelargonium zonale L.) cultivation: Effect on plant growth, quality, nutrition, and photosynthesis. Sci. Hort. 2018, 228, 213–221. [Google Scholar] [CrossRef]
- Restrepo, A.P.; Medina, E.; Pérez-Espinosa, A.; Agulló, E.; Bustamante, M.A.; Mininni, C.; Bernal, M.P.; Moral, R. Substitution of peat in horticultural seedlings: Suitability of digestate-derived compost from cattle manure and maize silage codigestion. Commun. Soil Sci. Plan. 2013, 44, 668–677. [Google Scholar] [CrossRef]
- Larcher, F.; Scariot, V. Assessment of partial peat substitutes for the production of Camellia japonica. HortScience 2009, 44, 312–316. [Google Scholar] [CrossRef]
- Savvas, D.; Karagianni, V.; Kotsiras, A.; Demopoulos, V.; Karkamisi, I.; Pakou, P. Interactions between ammonium and pH of the nutrient solution supplied to gerbera (Gerbera jamesonii) grown in pumice. Plant Soil 2003, 254, 393–402. [Google Scholar] [CrossRef]
- Barreto, M.S.; Jagtap, K.B. Assessment of substrates for economical production of gerbera (Gerbera jamesonii Bolus ex Hooker F.) flowers under protected cultivation. J. Ornam. Hortic. 2006, 9, 136–138. [Google Scholar]
- Caballero, R.; Pajuelo, P.; Ordovás, J.; Carmona, E.; Delgado, A. Evaluation and correction of nutrient availability to Gerbera jamesonii H. Bolus in various compost-based growing media. Sci. Hort. 2009, 122, 244–250. [Google Scholar] [CrossRef]
- Khalaj, M.A.; Amiri, M.; Sindhu, S.S. Study on the effect of different growing media on the growth and yield of gerbera (Gerbera jamesonii L.). J. Ornam. Plants 2011, 1, 185–189. [Google Scholar]
- Khalaj, M.A.; Suresh Kumar, P.; Roosta, H.R. Evaluation of nutrient uptake and flowering of Gerbera in response of various growing media. World J. Environ. Biosci. 2019, 8, 12–18. [Google Scholar]
- Ahmad, I.; Ahmad, T.; Gulfam, A.; Saleem, M. Growth and flowering of gerbera as influenced by various horticultural substrates. Pak. J. Bot. 2012, 44, 291–299. [Google Scholar]
- Sonneveld, C.; Voogt, W. Nutrient management in substrate systems. In Plant Nutrition of Greenhouse Crops; Sonneveld, C., Voogt, W., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 277–312. [Google Scholar]
- Zheng, Y.; Graham, T.; Richard, S.; Dixon, M. Potted gerbera production in a subirrigation system using low-concentration nutrient solutions. HortScience 2004, 39, 1283–1286. [Google Scholar] [CrossRef]
- Shrikant, M.; Jawaharlal, M. Effect of fertigation level and biostimulants on quality parameters of gerbera (Gerbera jamesonii Bolus ex Hooker F.) var. Debora under poly house conditions. Trends Biosci. 2014, 7, 1134–1137. [Google Scholar]
- Massa, D.; Lenzi, A.; Montoneri, E.; Ginepro, M.; Prisa, D.; Burchi, G. Plant response to biowaste soluble hydrolysates in hibiscus grown under limiting nutrient availability. J. Plant Nutr. 2018, 41, 396–409. [Google Scholar] [CrossRef]
- Massa, D.; Bonetti, A.; Cacini, S.; Faraloni, C.; Prisa, D.; Tuccio, L.; Petruccelli, R. Soilless tomato grown under nutritional stress increases green biomass but not yield or quality in presence of biochar as growing medium. Hortic. Environ. Biotechnol. 2019, 60, 871–881. [Google Scholar] [CrossRef]
- Arunesh, A.; Muraleedharan, A.; Sha, K.; Kumar, S.; Joshi, J.L.; Kumar, P.S.; Rajan, E.B. Studies on the effect of different growing media on the growth and flowering of Gerbera cv. Goliath. Plant Arch. 2020, 20, 653–657. [Google Scholar]
- Sonneveld, C.; Voogt, W. Effects of pH value and Mn application on yield and nutrient absorption with rockwool grown gerbera. Acta Hortic. 1997, 450, 139–148. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Mota, P.R.D.A.; Fernandes, D.M.; Ludwig, F. Development and mineral nutrition of gerbera plants as a function of electrical conductivity. Ornam. Hortic. 2016, 22, 37–42. [Google Scholar] [CrossRef][Green Version]
- Mercurio, G. Gerbera Cultivation in Greenhouse; Schreurs: De Kwakel, The Netherlands, 2002; p. 206. [Google Scholar]
- Alejandro, S.; Cailliatte, R.; Alcon, C.; Dirick, L.; Domergue, F.; Correia, D.; Castaings, L.; Briat, J.F.; Mari, S.; Curie, C. Intracellular distribution of manganese by the trans-Golgi network transporter NRAMP2 is critical for photosynthesis and cellular redox homeostasis. Plant Cell 2017, 29, 3068–3084. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma agriculture: Review from the perspective of the plant and its ecosystem. Plasma Processes Polym. 2020, e2000162. [Google Scholar] [CrossRef]
- Holubová, Ľ.; Kyzek, S.; Ďurovcová, I.; Fabová, J.; Horváthová, E.; Ševčovičová, A.; Gálová, E. Non-Thermal Plasma—A new green priming agent for plants? Int. J. Mol. Sci. 2020, 21, 9466. [Google Scholar] [CrossRef]
- Cannazzaro, S.; Di Lonardo, S.; Cacini, S.; Traversari, S.; Burchi, G.; Pane, C.; Gambineri, F.; Cursi, L.; Massa, D. Opportunities and challenges of using non-thermal plasma treatments in soilless cultures: Experience from greenhouse experiments. In Proceedings of the III International Symposium on Soilless Culture and Hydroponics: Innovation and Advanced Technology for Circular Horticulture, Cyprus, Greece, 19–22 March 2021. [Google Scholar]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phyt. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Medeiros, D.B.; Barros, J.A.; Fernie, A.R.; Araújo, W.L. Eating away at ROS to regulate stomatal opening. Trends Plant Sci. 2020, 25, 220–223. [Google Scholar] [CrossRef]
- Postiglione, A.E.; Muday, G.K. The role of ROS homeostasis in ABA-induced guard cell signaling. Front. Plant Sci. 2020, 11, 968. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Jensen, P.E.; Husted, S. Manganese deficiency in plants: The impact on photosystem II. Trends Plant Sci. 2016, 21, 622–632. [Google Scholar] [CrossRef]
- Rafi, Z.N.; Kazemi, F.; Tehranifar, A. Effects of various irrigation regimes on water use efficiency and visual quality of some ornamental herbaceous plants in the field. Agr. Water Manage. 2019, 212, 78–87. [Google Scholar] [CrossRef]
- Roosta, H.R.; Manzari Tavakkoli, M.; Hamidpour, M. Comparison of different soilless media for growing gerbera under alkalinity stress condition. J. Plant Nutr. 2016, 39, 1063–1073. [Google Scholar] [CrossRef]
- Massa, D.; Magán, J.J.; Montesano, F.F.; Tzortzakis, N. Minimizing water and nutrient losses from soilless cropping in southern Europe. Agr. Water Manag. 2020, 241, 106395. [Google Scholar] [CrossRef]
- Pane, C.; Spaccini, R.; Piccolo, A.; Celano, G.; Zaccardelli, M. Disease suppressiveness of agricultural greenwaste composts as related to chemical and bio-based properties shaped by different on-farm composting methods. Bio. Control 2019, 137, 104026. [Google Scholar] [CrossRef]
- Burchi, G.; Chessa, S.; Gambineri, F.; Kocian, A.; Massa, D.; Milazzo, P.; Rimediotti, L.; Ruggeri, A. Information technology controlled greenhouse: A system architecture. In Proceedings of the IoT Vertical and Topical Summit on Agriculture-Tuscany (IOT Tuscany), Siena, Italy, 8–9 May 2018; pp. 1–6. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plan. 1975, 6, 71–80. [Google Scholar] [CrossRef]

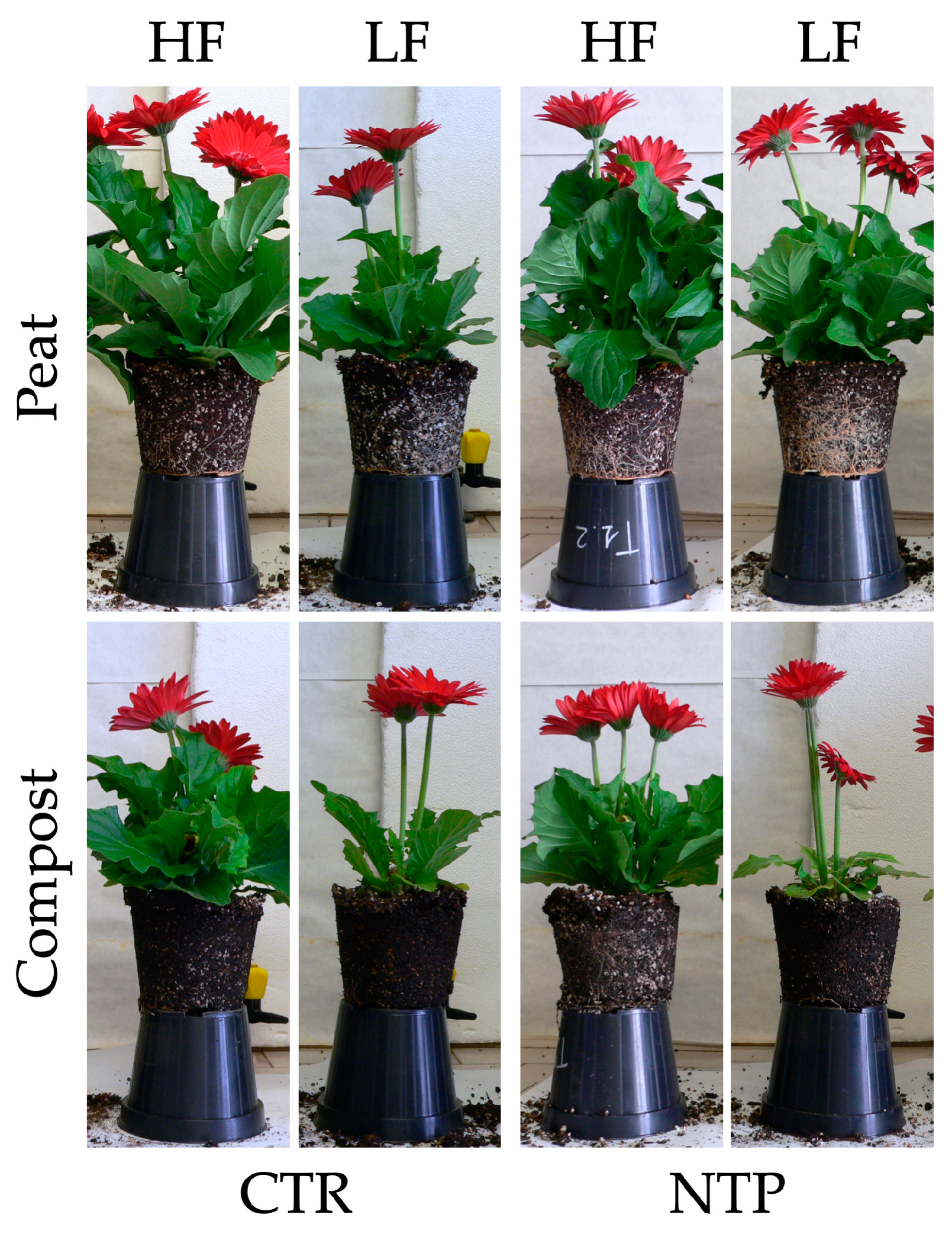
| Parameter | NTP | F | S | NTP × F | NTP × S | F × S | NTP × F × S |
|---|---|---|---|---|---|---|---|
| Leaf FW | *** | *** | *** | ns | *** | *** | * |
| Leaf area | *** | *** | *** | ns | *** | *** | ns |
| Flower FW | * | *** | *** | ns | *** | ns | ns |
| Flower number | ns | *** | *** | ns | * | ** | ns |
| Plant DW | ns | *** | *** | * | *** | ns | *** |
| Plant % DW | *** | *** | *** | ns | *** | ns | *** |
| SLA | *** | *** | *** | ns | *** | ns | *** |
| SPAD index | ns | *** | *** | ns | *** | *** | ns |
| Organic nitrogen | ns | *** | * | * | * | ** | * |
| Nitrate | *** | *** | ns | ns | ns | *** | ns |
| Potassium | *** | *** | ns | ns | ns | *** | ns |
| Calcium | ns | ns | ns | ns | ** | * | ns |
| Magnesium | * | ns | ns | * | * | ns | ** |
| Phosphorus | *** | *** | ** | ns | * | *** | ns |
| Manganese | ns | ns | *** | ns | ns | ns | * |
| Iron | *** | ns | *** | ns | ns | ns | ns |
| Peat | Compost | ||||||
|---|---|---|---|---|---|---|---|
| HF | LF | HF | LF | ||||
| CTR | NTP | CTR | NTP | CTR | NTP | CTR | NTP |
| Fungi (CFU × 103 g−1) | |||||||
| 0.52 ± 0.032 | 0.40 ± 0.128 | 0.57 ± 0.131 | 0.48 ± 0.057 | 0.10 ± 0.046 | 0.06 ± 0.009 | 0.05 ± 0.029 | 0.14 ± 0.104 |
| Bacteria (CFU × 103 g−1) | |||||||
| 7.6 ± 2.70 | 17.6 ± 4.51 | 18.9 ± 11.4 | 27.0 ± 13.4 | 32.8 ± 4.86 | 42.4 ± 13.60 | 50.8 ± 6.30 | 29.9 ± 2.70 |
| ANOVA | NTP | F | S | NTP × F | NTP × S | F × S | NTP × F × S |
| Fungi | ns | ns | *** | ns | ns | ns | ns |
| Bacteria | ns | ns | *** | * | ns | ns | ns |
| S | F | NTP | EC (µS cm−2) | pH | P-PO4 (mmol L−1) | N-NO3 (mmol L−1) |
|---|---|---|---|---|---|---|
| Peat | HF | CTR | 1114 ± 78.2 | 5.6 ± 0.14 | 0.61 ± 0.016 | 3.8 ± 0.50 |
| NTP | 1305 ± 101.8 | 5.6 ± 0.14 | 059 ± 0.010 | 4.2 ± 0.30 | ||
| LF | CTR | 582 ± 37.5 | 6.1 ± 0.17 | 0.26 ± 0.008 | 1.4 ± 0.29 | |
| NTP | 629 ± 47.6 | 5.6 ± 0.25 | 0.26 ± 0.016 | 1.2 ± 0.33 | ||
| Compost | HF | CTR | 1429 ± 25.4 | 6.6 ± 0.21 | 0.61 ± 0.062 | 4.3 ± 0.54 |
| NTP | 1630 ± 21.3 | 6.9 ± 0.06 | 0.58 ± 0.017 | 4.5 ± 0.64 | ||
| LF | CTR | 833 ± 57.8 | 7.4 ± 0.05 | 0.25 ± 0.018 | 0.8 ± 0.11 | |
| NTP | 752 ± 68.6 | 7.0 ± 0.44 | 0.25 ± 0.012 | 0.8 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannazzaro, S.; Traversari, S.; Cacini, S.; Di Lonardo, S.; Pane, C.; Burchi, G.; Massa, D. Non-Thermal Plasma Treatment Influences Shoot Biomass, Flower Production and Nutrition of Gerbera Plants Depending on Substrate Composition and Fertigation Level. Plants 2021, 10, 689. https://doi.org/10.3390/plants10040689
Cannazzaro S, Traversari S, Cacini S, Di Lonardo S, Pane C, Burchi G, Massa D. Non-Thermal Plasma Treatment Influences Shoot Biomass, Flower Production and Nutrition of Gerbera Plants Depending on Substrate Composition and Fertigation Level. Plants. 2021; 10(4):689. https://doi.org/10.3390/plants10040689
Chicago/Turabian StyleCannazzaro, Samantha, Silvia Traversari, Sonia Cacini, Sara Di Lonardo, Catello Pane, Gianluca Burchi, and Daniele Massa. 2021. "Non-Thermal Plasma Treatment Influences Shoot Biomass, Flower Production and Nutrition of Gerbera Plants Depending on Substrate Composition and Fertigation Level" Plants 10, no. 4: 689. https://doi.org/10.3390/plants10040689
APA StyleCannazzaro, S., Traversari, S., Cacini, S., Di Lonardo, S., Pane, C., Burchi, G., & Massa, D. (2021). Non-Thermal Plasma Treatment Influences Shoot Biomass, Flower Production and Nutrition of Gerbera Plants Depending on Substrate Composition and Fertigation Level. Plants, 10(4), 689. https://doi.org/10.3390/plants10040689












