Development of a Cell Suspension Culture System for Promoting Alkaloid and Vinca Alkaloid Biosynthesis Using Endophytic Fungi Isolated from Local Catharanthus roseus
Abstract
1. Introduction
2. Results
2.1. Callus Initiation and Proliferation from Local C. roseus
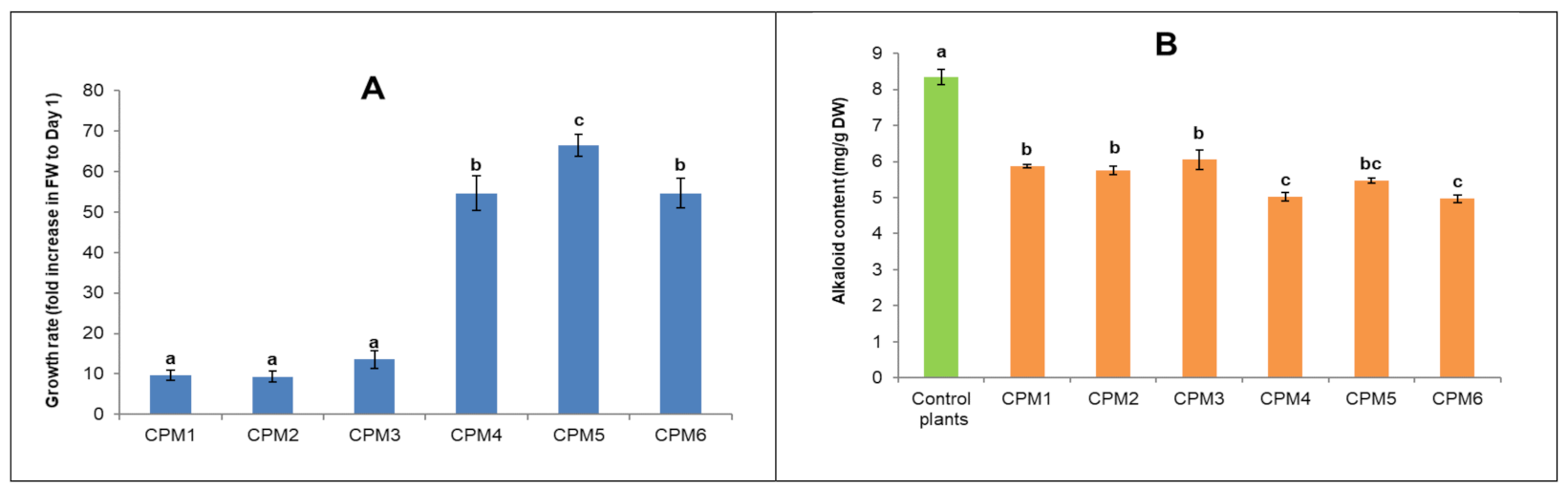
2.2. Cell Suspension Establishment
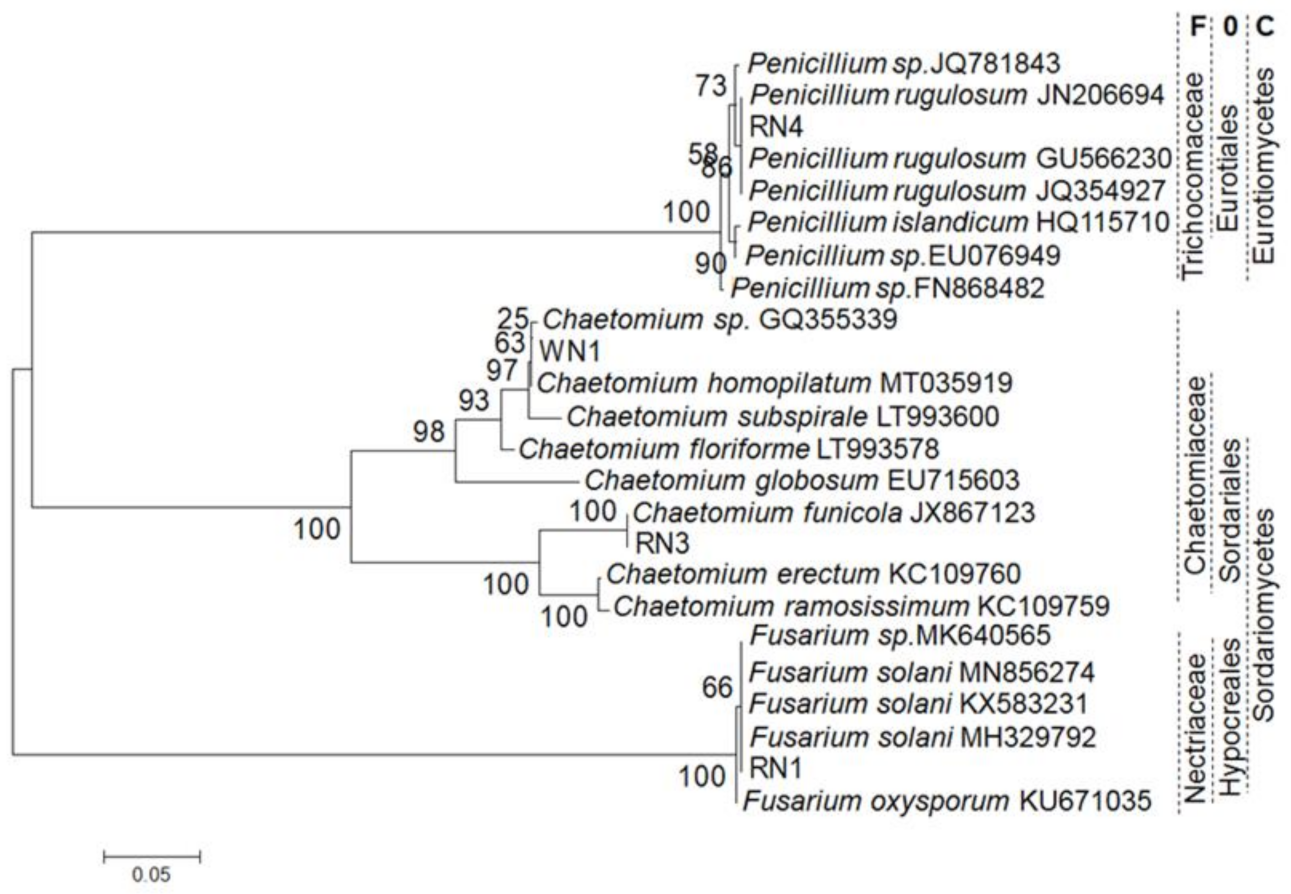
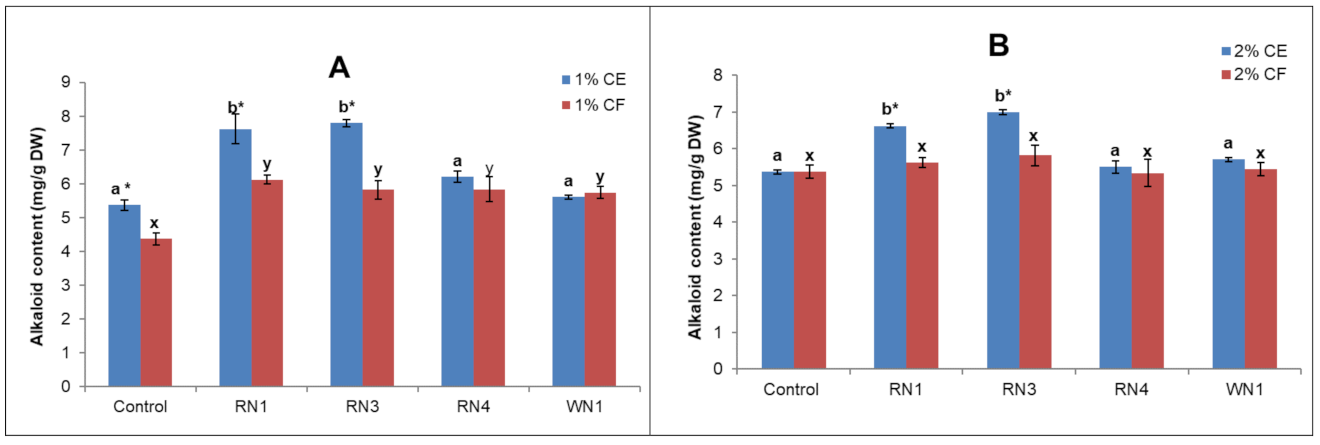
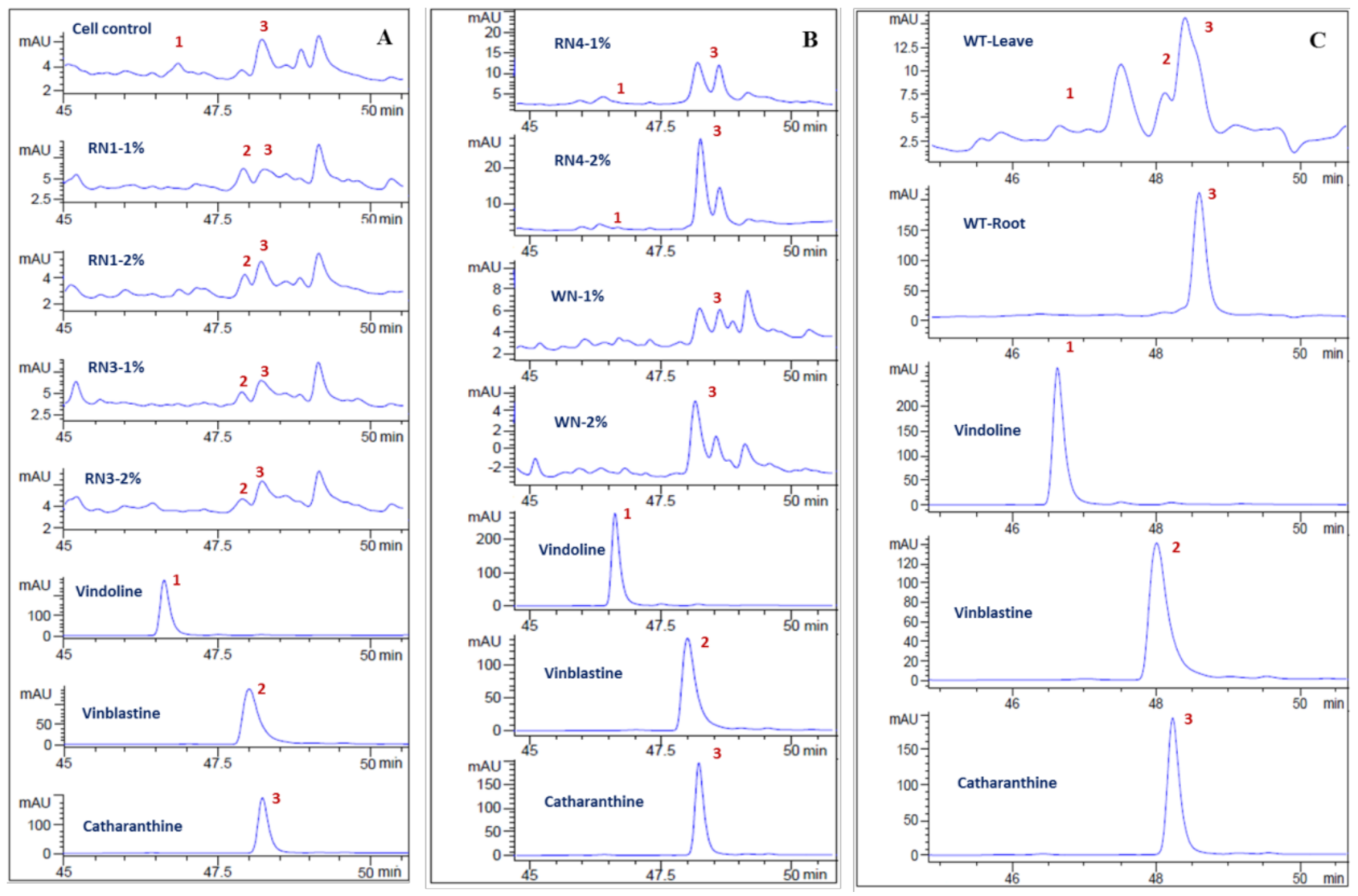
2.3. Endophytic Fungus Isolation and Elicitation
3. Discussion
4. Materials and Methods
4.1. Explant Preparation, Callus Initiation and Induction
4.2. Establishing Cell Suspension Cultures
4.3. Isolation of Endophytic Fungi and Taxonomy Identification
4.4. Elicitor Preparation and Application
4.5. Extraction and Determination of Total Alkaloids
4.6. HPLC Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noble, R.L. The discovery of the vinca alkaloids—Chemotherapeutic agents against cancer. Biochem. Cell Biol. 1990, 68, 1344–1351. [Google Scholar] [CrossRef]
- Shams, K.A.; Nazif, N.M.; Azim, N.; Shafeek, K.; Missiry, M.; Ismail, S.; Nasr, M. Isolation and characterization of antineoplastic alkaloids from Catharanthus roseus L. Don. cultivated in Egypt. Afr. J. Tradit. Complement. Altem. Med. 2009, 6, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, R.; Jacobs, D.; Snoeijer, W.; Hallard, D.; Verpoorte, R. The Catharanthus Alkaloids: Pharmacognosy and Biotechnology. Curr. Med. Chem. 2004, 11, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Barrales-Cureño, H.J. Pharmacological applications and in vitro biotechnological production of anticancer alkaloids of Catharanthus roseus. Biotechnol. Apl. 2015, 32, 1101–1110. [Google Scholar]
- Hellwig, S.; Drossard, J.; Twyman, R.M.; Fischer, R. Plant cell cultures for the production of recombinant proteins. Nat. Biotechnol. 2004, 22, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.Y.; Liang, K.Y.; Lin, W.C.; Kitanaka, S.; Wu, J.B. Regulation and improvement of triterpene formation in plant cultured cells of Eriobotrya japonica Lindl. J. Biosci. Bioeng. 2010, 110, 588–592. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, W.H.; Hu, Q.; He, X.W. Enhanced indole alkaloid production in suspension compact callus clusters of Catharanthus roseus: Impacts of plant growth regulators and sucrose. Plant Growth Regul. 2001, 33, 33–41. [Google Scholar] [CrossRef]
- Mishra, M.R.; Srivastava, R.K.; Akhtar, N. Enhanced alkaloid production from cell culture system of Catharanthus roseus in combined effect of nutrient salts, sucrose and plant growth regulators. J. Biotechnol. Biomed. Sci. 2018, 1, 14–34. [Google Scholar] [CrossRef]
- El-Sayed, M.; Verpoorte, R. Effect of phytohormones on growth and alkaloid accumulation by a Catharanthus roseus cell suspension cultures fed with alkaloid precursors tryptamine and loganin. Plant Cell Tiss. Organ. Cult. 2002, 68, 265–270. [Google Scholar] [CrossRef]
- Lee, C.W.T.; Shuler, M.L. The effect of inoculum density and conditioned medium on the production of ajmalicine and catharanthine from immobilized Catharanthus roseus cells. Biotechnol. Bioeng. 2000, 67, 61–71. [Google Scholar] [CrossRef]
- Haida, Z.; Syahida, A.; Ariff, S.M.; Maziah, M.; Hakiman, M. Factors affecting cell biomass and flavonoid production of Ficus deltoidea var. kunstleri in cell suspension culture system. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Yahia, A.; Kevers, C.; Gaspar, T.; Chénieux, J.C.; Rideau, M.; Crèche, J. Cytokinins and ethylene stimulate indole alkaloid accumulation in cell suspension cultures of Catharanthus roseus by two distinct mechanisms. Plant Sci. 1998, 133, 9–15. [Google Scholar] [CrossRef]
- Nagella, P.; Murthy, H.N. Establishment of cell suspension cultures of Withania somnifera for the production of withanolide A. Bioresour. Technol. 2010, 101, 6735–6739. [Google Scholar] [CrossRef]
- Hoopen, H.J.G.; Vinke, J.L.; Moreno, P.; Verpoorte, R.; Heijnen, S. Influence of temperature on growth and ajmalicine production by Catharanthus roseus suspension cultures. Enzyme Microb. Technol. 2002, 30, 56–65. [Google Scholar] [CrossRef]
- Christen, A.; Gibson, D.; Bland, T. Production of Taxol or Taxol-Like Compounds in cell Culture. US Patent 5019504A, 28 May 1991. [Google Scholar]
- Strobel, G.A.; Stierle, A.; van Kuijk, F.J.G.M. Factors influencing the in vitro production of radiolabeled taxol by Pacific yew, Taxus brevifolia. Plant Sci. 1992, 84, 65–74. [Google Scholar] [CrossRef]
- Tonk, D.; Mujib, A.; Maqsood, M.; Ali, M.; Zafar, N. Aspergillus flavus fungus elicitation improves vincristine and vinblastine yield by augmenting callus biomass growth in Catharanthus roseus. Plant Cell Tiss. Organ Cult. 2016, 126, 291–303. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Q.; Guo, Y.Q.; Zhu, W.H. Effects of stress factors, bioregulators, and synthetic precursors on indole alkaloid production in compact callus clusters cultures of Catharanthus roseus. Appl. Microbiol. Biotechnol. 2001, 55, 693–698. [Google Scholar] [CrossRef]
- Namdeo, A.; Patil, S.; Fulzele, D.P. Influence of fungal elicitors on production of ajmalicine by cell cultures of Catharanthus roseus. Biotechnol. Prog. 2002, 18, 159–162. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Z.; Lu, L.; Jin, H.; Sun, L.; Yu, Q.; Xu, H.; Yang, F.; Fu, M.; Li, S.; et al. Interaction between abscisic acid and nitric oxide in PB90-induced catharanthine biosynthesis of Catharanthus roseus cell suspension cultures. Biotechnol. Prog. 2013, 29, 994–1001. [Google Scholar] [CrossRef]
- Liang, C.; Chen, C.; Zhou, P.; Xu, L.; Zhu, J.; Liang, J.; Zi, J.; Yu, R. Effect of Aspergillus flavus fungal elicitor on the production of terpenoid indole alkaloids in Catharanthus roseus cambial meristematic cells. Molecules 2018, 23, 3276. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.M.; Abdel-Azeem, M.A.; Khalil, W.F. Endophytic fungi as a new source of antirheumatoid metabolites. In Bioactive Food as Dietary Interventions for Arthritis and Related Diseases, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 355–384. [Google Scholar] [CrossRef]
- Kaul, S.; Ahmed, M.; Sharma, T.; Dhar, M.K. Unlocking the myriad benefits of endophytes: An overview. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R.N., Upadhyay, R., Dubey, N., Raghuwanshi, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 41–57. [Google Scholar] [CrossRef]
- Boller, T. Chemoperception of microbial signals in plant cells. Annu. Rev. Plant Biol. 1995, 46, 189–214. [Google Scholar] [CrossRef]
- Tang, Z.; Rao, L.; Peng, G.; Zhou, M.; Guorong, S.; Liang, Y. Effects of endophytic fungus and its elicitors on cell status and alkaloid synthesis in cell suspension cultures of Catharanthus roseus. J. Med. Plants Res. 2011, 5, 2192–2200. [Google Scholar]
- Barrett, L.; Heil, M. Unifying concepts and mechanisms in the specificity of plant-enemy interactions. Trends Plant Sci. 2012, 17, 282–292. [Google Scholar] [CrossRef]
- Van Der Heijden, R.; Verpoorte, R.; Ten Hoopen, H.J.G. Cell and tissue cultures of Catharanthus roseus (L.) G. Don: A literature survey. Plant Cell Tiss. Organ. Cult. 1989, 18, 231–280. [Google Scholar] [CrossRef]
- Smetanska, I. Production of secondary metabolites using plant cell cultures. Food Biotechnol. 2008, 111, 187–228. [Google Scholar] [CrossRef]
- Gantet, P.; Imbault, N.; Thiersault, M.; Doireau, P. Inhibition of alkaloid accumulation by 2,4-D in Catharanthus roseus cell suspension is overcome by methyl jasmonate. Acta Bot. Gall. 1997, 144, 501–508. [Google Scholar] [CrossRef]
- Hernández-Restrepo, M.; Gené, J.; Castañeda-Ruiz, R.; Mena-Portales, J.; Crous, P.; Guarro, J. Phylogeny of saprobic microfungi from Southern Europe. Stud. Mycol. 2017, 86, 53–97. [Google Scholar] [CrossRef]
- Momsia, P.; Momsia, T. Isolation, frequency distribution and diversity of novel fungal endophytes inhabiting leaves of Catharanthus roseus. Int. J. Life Sci. Biotechnol. Pharm. Res. 2013, 2, 82–87. [Google Scholar]
- Palem, P.P.C.; Kuriakose, G.C.; Jayabaskaran, C. An endophytic fungus, Talaromyces radicus, isolated from Catharanthus roseus, produces vincristine and vinblastine, which induce apoptotic cell death. PLoS ONE 2015, 10, e0144476. [Google Scholar] [CrossRef]
- Pandey, S.S.; Singh, S.; Babu, C.S.V.; Shanker, K.; Srivastava, N.K.; Shukla, A.K.; Kalra, A. Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ayob, F.; Simarani, K. Endophytic filamentous fungi from a Catharanthus roseus: Identification and its hydrolytic enzymes. Saudi Pharm. J. 2016, 24, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Dhayanithy, G.; Subban, K.; Chelliah, J. Diversity and biological activities of endophytic fungi associated with Catharanthus roseus. BMC Microbiol. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Pham, H.N.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Phytochemicals derived from Catharanthus roseus and their health benefits. Technologies 2020, 8, 80. [Google Scholar] [CrossRef]
- Tikhomiroff, C.; Jolicoeur, M. Screening of Catharanthus roseus secondary metabolites by high-performance liquid chromatography. J. Chromatogr. A 2002, 955, 87–93. [Google Scholar] [CrossRef]
- St-Pierre, B.; De Luca, V.A. Cytochrome P-450 monooxygenase catalyzes the first step in the conversion of tabersonine to vindoline in Catharanthus roseus. Plant Physiol. 1995, 109, 131–139. [Google Scholar] [CrossRef]
- Vazquez-Flota, F.; De Carolis, E.; Alarco, A.M.; De Luca, V. Molecular cloning and characterization of desacetoxyvindoline-4-hydroxylase, a 2-oxoglutarate dependent-dioxygenase involved in the biosynthesis of vindoline in Catharanthus roseus (L.) G. Don. Plant Mol. Biol. 1997, 34, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell wall glucans of fungi. A review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and receptor molecules: Orchestrators of plant defense and immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Baldi, A.; Srivastava, A.; Bisaria, V.S. Fungal elicitors for enhanced production of secondary metabolites in plant cell suspension cultures. In Symbiotic Fungi Soil Biology; Varma, A., Kharkwal, A.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 373–380. [Google Scholar] [CrossRef]
- Gomaa, N.H.; Hassan, M.O.; Fahmy, G.M.; González, L.; Hammouda, O.; Atteya, A.M. Allelopathic effects of Sonchus oleraceus L. on the germination and seedling growth of crop and weed species. Acta Bot. Bras. 2014, 28, 408–416. [Google Scholar] [CrossRef]


| Plant Growth Regulators (mg/L) | Percentage of Explants Forming Callus (%) | ||||
|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | Day 28 | ||
| 2.4-D | 0.5 | 16.7 ± 1.87 a | 46.3 ± 2.12 a | 83.0 ± 1.88 a,b | 83 ± 2.34 b |
| 1 | 53.0 ± 2.00 b | 90.3 ± 3.02 b,c | 93.0 ± 2.19 b,c | 100.0 ± 0.0 c | |
| 1.5 | 58.7 ± 1.26 b | 97.7 ± 1.76 c | 100.0 ± 0.0 c | 100.0 ± 0.0 c | |
| 2 | 65.3 ± 2.73 b,c | 97.3 ± 4.44 c | 100.0 ± 0.0 c | 100.0 ± 0.0 c | |
| 2.5 | 70.0 ± 1.76 c | 86.7 ± 1.30 b | 77.7 ± 3.35 a | 68.9 ± 2.17 a | |
| NAA | 0.5 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | |
| 1.5 | 0 | 0 | 3.3 ± 0.53 | 3.3 ± 0.12 | |
| 2 | 0 | 0 | 2.2 ± 0.21 | 2.2 ± 0.39 | |
| 2.5 | 0 | 0 | 0 | 0 | |
| # | Taxa | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| Genetic Distance (0–1) | |||||||||
| 1 | C. funicola JX867123 | 0.104 | 0.239 | 0.361 | 0.239 | 0.000 | 0.361 | 0.104 | |
| 2 | C. homopilatum MT035919 | 89.6 | 0.232 | 0.355 | 0.232 | 0.104 | 0.355 | 0.000 | |
| 3 | F. solani MH329792 | 76.1 | 76.8 | 0.313 | 0.000 | 0.239 | 0.313 | 0.232 | |
| 4 | P. rugulosum JN206694 | 63.9 | 64.5 | 68.7 | 0.313 | 0.361 | 0.000 | 0.355 | |
| 5 | RN1 | 76.1 | 76.8 | 100.0 | 68.7 | 0.239 | 0.313 | 0.232 | |
| 6 | RN3 | 100.0 | 89.6 | 76.1 | 63.9 | 76.1 | 0.361 | 0.104 | |
| 7 | RN4 | 63.9 | 64.5 | 68.7 | 100.0 | 68.7 | 63.9 | 0.355 | |
| 8 | WN1 | 89.6 | 100.0 | 76.8 | 64.5 | 76.8 | 89.6 | 64.5 | |
| Homology level (%) | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linh, T.M.; Mai, N.C.; Hoe, P.T.; Ngoc, N.T.; Thao, P.T.H.; Ban, N.K.; Van, N.T. Development of a Cell Suspension Culture System for Promoting Alkaloid and Vinca Alkaloid Biosynthesis Using Endophytic Fungi Isolated from Local Catharanthus roseus. Plants 2021, 10, 672. https://doi.org/10.3390/plants10040672
Linh TM, Mai NC, Hoe PT, Ngoc NT, Thao PTH, Ban NK, Van NT. Development of a Cell Suspension Culture System for Promoting Alkaloid and Vinca Alkaloid Biosynthesis Using Endophytic Fungi Isolated from Local Catharanthus roseus. Plants. 2021; 10(4):672. https://doi.org/10.3390/plants10040672
Chicago/Turabian StyleLinh, Tran My, Nguyen Chi Mai, Pham Thi Hoe, Ninh Thi Ngoc, Phan Thi Hong Thao, Ninh Khac Ban, and Nguyen Tuong Van. 2021. "Development of a Cell Suspension Culture System for Promoting Alkaloid and Vinca Alkaloid Biosynthesis Using Endophytic Fungi Isolated from Local Catharanthus roseus" Plants 10, no. 4: 672. https://doi.org/10.3390/plants10040672
APA StyleLinh, T. M., Mai, N. C., Hoe, P. T., Ngoc, N. T., Thao, P. T. H., Ban, N. K., & Van, N. T. (2021). Development of a Cell Suspension Culture System for Promoting Alkaloid and Vinca Alkaloid Biosynthesis Using Endophytic Fungi Isolated from Local Catharanthus roseus. Plants, 10(4), 672. https://doi.org/10.3390/plants10040672






