Physiological Responses of Salinized Fenugreek (Trigonellafoenum-graecum L.) Plants to Foliar Application of Salicylic Acid
Abstract
1. Introduction
2. Results
2.1. Chlorophyll Content Index
2.2. Total Soluble Carbohydrates
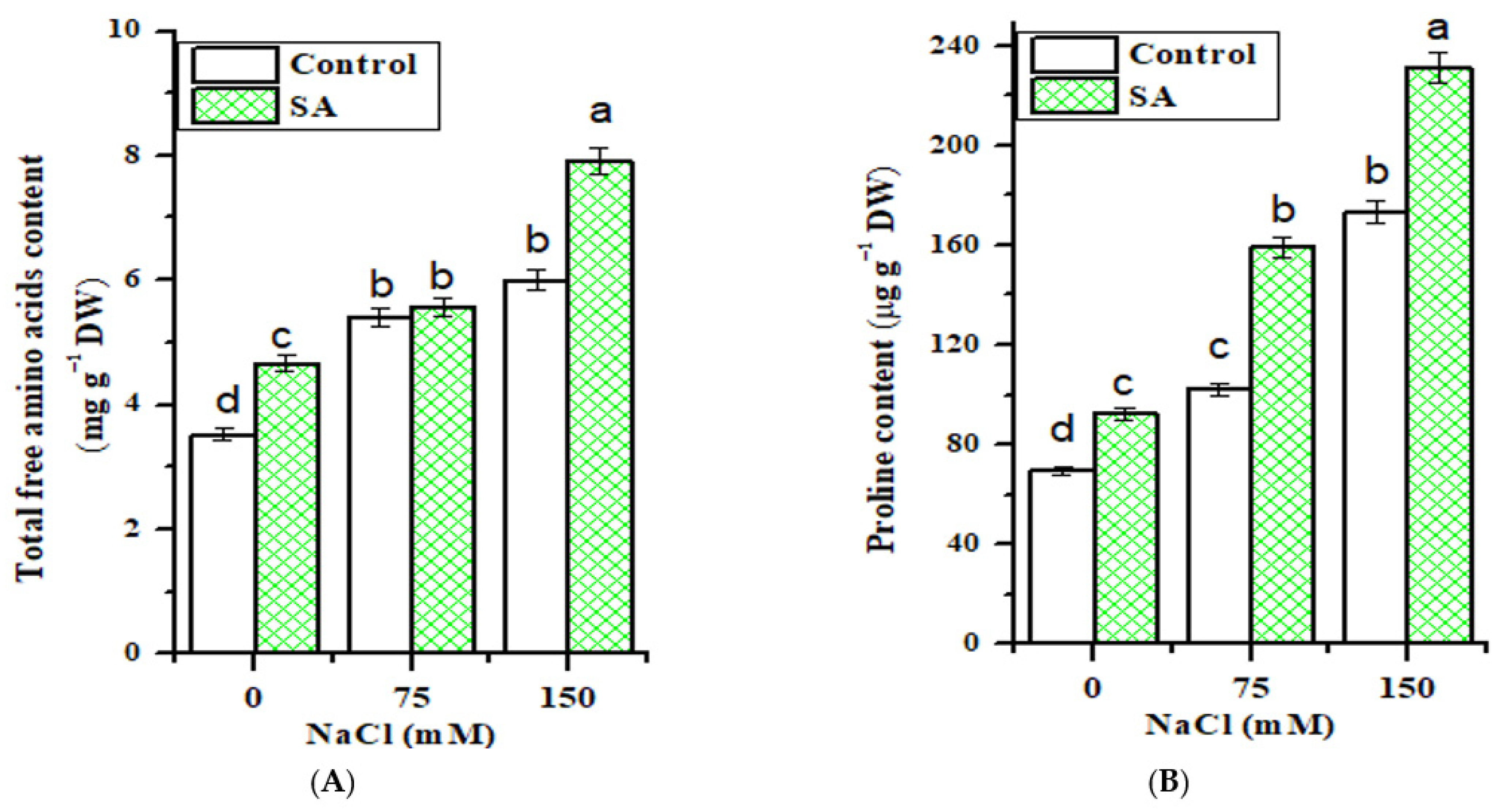
2.3. Total Free Amino Acid Content
2.4. Proline Content
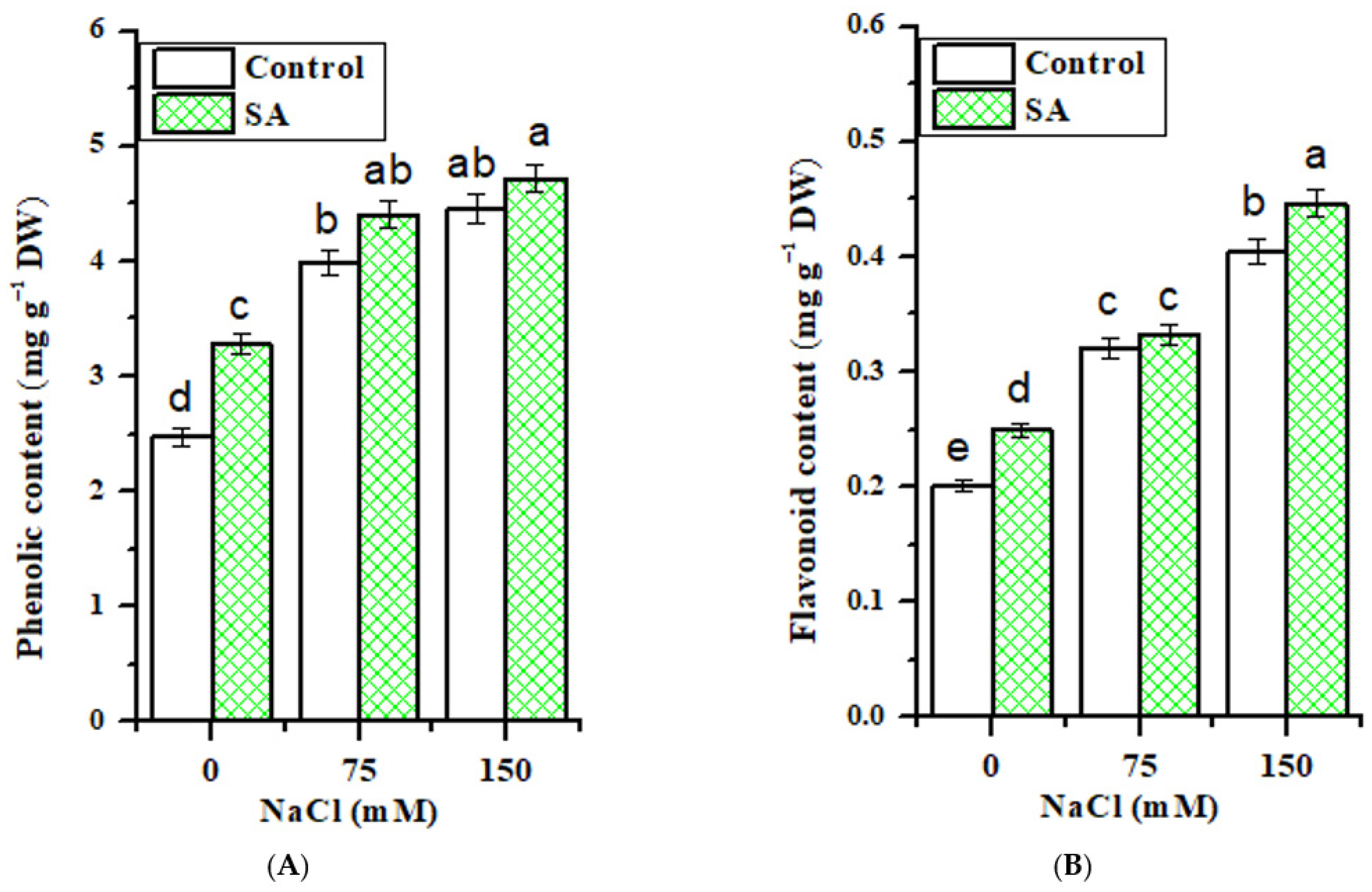
2.5. Total Phenolics and Flavonoids
2.6. Lipid Peroxidation
2.7. Shikimic Acid Content
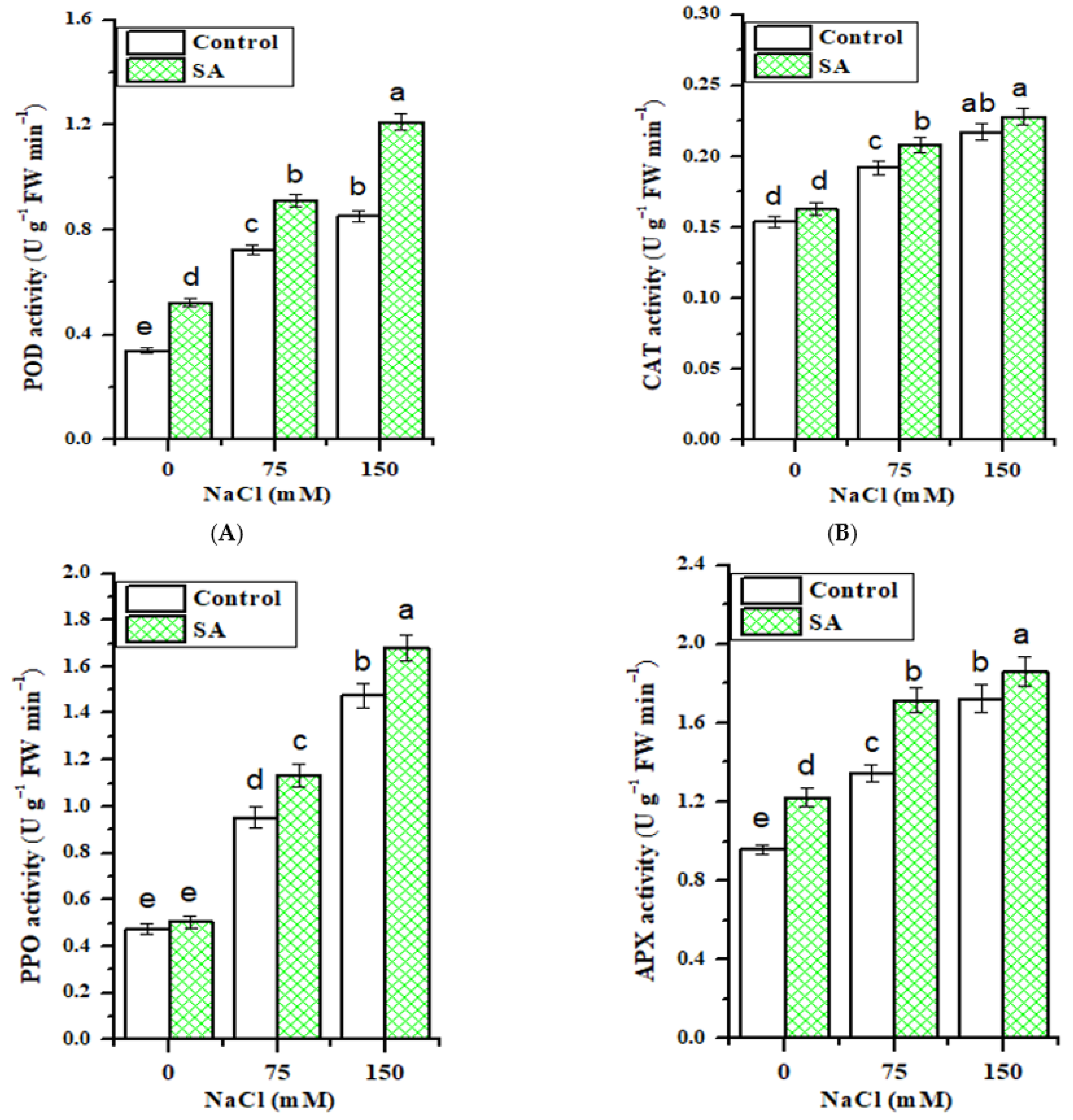
2.8. Antioxidative Enzymes
3. Discussion
4. Materials and Methods
4.1. Soil, Seed, and Growth Conditions
4.2. Salt and Salicylic Acid Application
4.3. Physiological Evaluation
4.3.1. Estimation of Chlorophyll
4.3.2. Estimation of Carbohydrate Content
4.3.3. Determination of Total Free Amino Acids and Proline Content
4.3.4. Determination of Total Phenolic and Flavonoid Contents
4.3.5. Determination of Malondialdehyde (MDA) Content
4.3.6. Shikimic Acid Content
4.3.7. Estimation of the Antioxidant Enzymes
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel Latef, A.A.; Zaid, A.; Abo-Baker, A.B.E.; Salem, W.; Abu Alhmad, M.F. Mitigation of copper stress in maize by inoculation with Paenibacillus polymyxa and Bacillus circulans. Plants 2020, 9, 1513. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.S.; Badawy, A.A.; Osman, A.I.; Abdel Latef, A.A. Ameliorative impact of an extract of the halophyte Arthrocnemum macrostachyum on growth and biochemical parameters of soybean under salinity stress. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Qi, Z.Y.; Ahammed, G.J.; Jiang, C.Y.; Li, C.X.; Zhou, J. The E3 ubiquitin ligase gene SlRING1 is essential for plant tolerance to cadmium stress in Solanum lycopersicum. J. Biotechnol. 2020, 324, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.F.A.; Tahjib-Ul-Arif, M.; Sohag, A.A.; Abdel Latef, A.A.; Ragaey, M.M. Mechanistic insight of allantoin in protecting tomato plants against ultraviolet C stress. Plants 2021, 10, 11. [Google Scholar] [CrossRef]
- Mantri, N.; Patade, V.; Penna, S.; Ford, R.; Peng, E. Abiotic stress responses in plants: Present and future. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 1–19. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.-K. Understanding and improving salt tolerance in plants. Crop. Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Alhmad, M.F.A.; Kordrostami, M.; Abo–Baker, A.B.A.E.; Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J. Plant Growth Regul. 2020, 39, 1293–1306. [Google Scholar] [CrossRef]
- Al-Elwany, O.A.A.I.; Mohamed, G.F.; Abdurrahman, H.A.; Rady, M.M.; Abdel Latef, A.A. Exogenous glutathione-mediated tolerance to deficit irrigation in salt-affected Capsicum frutescence (L.) plants is connected with higher antioxidant content and ionic homeostasis. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 1957–1979. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanism of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Srivastava, A.K.; Abdel-sadek, M.S.; Kordrostam, M.; Tran, L.S.P. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline conditions. Land Degrad. Dev. 2018, 29, 1065–1073. [Google Scholar] [CrossRef]
- Dief, H.; Hashem, E.A.; Fawzan, S.; El-Sayed, A.S. Alleviation of salt stress in Triticum aestivum by biopriming with Phanerochaete chrysosporium. J. Crop. Sci. Biotechnol. 2021, 24, 103–116. [Google Scholar] [CrossRef]
- Chandrakar, V.; Dubey, A.; Keshavkant, S. Modulation of antioxidant enzymes by salicylic acid in arsenic exposed Glycine max L. J. Soil Sci. Plant Nutr. 2016, 16, 662–676. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Kordrostami, M.; Zakir, A.; Zaki, H.; Saleh, O.M. Eustress with H2O2 facilitates plant growth by improving tolerance to salt stress in two wheat cultivars. Plants 2019, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.E.; Metwally, R.A. Mitigation of salt stress by dual application of arbuscular mycorrhizal fungi and salicylic acid. Agrochimica 2018, 62, 353–366. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, Y.; Li, X.; Han, W.-Y.; Chen, S. Epigallocatechin-3-Gallate Alleviates Salinity-Retarded Seed Germination and Oxidative Stress in Tomato. J. Plant Growth Regul. 2018, 37, 1349–1356. [Google Scholar] [CrossRef]
- Per, T.S.; Fatma, M.; Asgher, M.; Javied, S.; Khan, N.A. Salicylic Acid and Nutrients Interplay in Abiotic Stress. In Salicylic Acid: A Multifaceted Hormone; Nazar, R., Iqbal, N., Khan, N., Eds.; Springer Nature: Singapore, 2017. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic Acid as a Safe Plant Protector and Growth Regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Ahammed, G.J.; Li, Y.-T.; Wei, J.-P.; Yan, P.; Zhang, L.-P.; Han, X.; Han, W.-Y. Salicylic acid acts upstream of nitric oxide in elevated carbon dioxide-induced flavonoid biosynthesis in tea plant (Camellia sinensis L.). Environ. Exp. Bot. 2019, 161, 367–374. [Google Scholar] [CrossRef]
- Palma, F.; López-Gómez, M.; Tejera, N.A.; Lluch, C. Salicylic acid improves the salinity tolerance of Medicago sativa in symbiosis with Sinorhizobium meliloti by preventing nitrogen fixation inhibition. Plant Sci. 2013, 208, 75–82. [Google Scholar] [CrossRef]
- Babar, S.; Siddiqi, E.H.; Hussain, I.; Bhatti, K.H.; Rasheed, R. Mitigating the Effects of Salinity by Foliar Application of Salicylic Acid in Fenugreek. Physiol. J. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Yang, S.; Chen, Y. Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in two melon cultivars (Cucumis melo L.). Protoplasma 2015, 252, 911–924. [Google Scholar] [CrossRef]
- Fayez, K.A.; Bazaid, S.A. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J. Saudi Soc. Agric. Sci. 2014, 13, 45–55. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013, 8, e26374. [Google Scholar] [CrossRef]
- Alsahli, A.; Mohamed, A.-K.; Alaraidh, I.; Al-Ghamdi, A.; Al-Watban, A.; El-Zaidy, M.; Alzahrani, S.M. Salicylic acid alleviates salinity stress through the modulation of biochemical attributes and some key antioxidants in wheat seedlings. Pak. J. Bot. 2019, 51, 1551–1559. [Google Scholar] [CrossRef]
- Noreen, S.; Ashraf, M. Alleviation of adverse effects of salt stress on sunflower (Helianthus annuus L.) by exogenous application of salicylic acid: Growth and photosynthesis. Pak. J. Bot. 2008, 40, 1657–1663. [Google Scholar]
- El-khodary, S.F.A. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agric. Biol. 2004, 6, 5–8. [Google Scholar]
- Shim, I.S.; Momose, Y.; Yamamoto, A.H.; Kim, D.W.A.; Usui, K. Inhibition of catalase activity by oxidative stress and its relationship to salicylic acid accumulation in plants. Plant Growth Regul. 2003, 39, 285–292. [Google Scholar] [CrossRef]
- Petropoulos, G.A. Fenugreek—The Genus Trigonella, 1st ed.; Taylor and Francis: London, UK; New York, NY, USA, 2002; pp. 1–127. [Google Scholar]
- Ahmad, A.; Alghamdi, S.; Mahmood, K.; Afzal, M. Fenugreek a multipurpose crop: Potentialities and improvements. Saudi J. Biol. Sci. 2016, 23, 300–310. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Abu Alhmad, M.F.A.; Ahmad, S. Foliar application of fresh moringa leaf extract overcomes salt stress in fenugreek (Trigonella foenum-graecum) plants. Egypt. J. Bot. 2017, 57, 157–179. [Google Scholar] [CrossRef]
- Metwally, R.A.; Abdelhameed, R.E. Synergistic effect of arbuscular mycorrhizal fungi in growth and physiology of salt-stressed Trigonella foenum-graecum plants. Biocatal. Agric. Biotechnol. 2018, 16, 538–544. [Google Scholar] [CrossRef]
- Selem, E.E.; Abdelhameed, R.E.; Kamel, H.A.; Hegazy, S.H. Physiological and Biochemical Response of Gamma Irradiated Sesamum indicum L. Seed Grown in Heavy Metal Contaminated Soil. Biosci. Res. 2018, 15, 1063–1072. [Google Scholar]
- Shi, Q.; Bao, Z.; Zhu, Z.; Ying, Q.; Qian, Q. Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul. 2006, 48, 127–135. [Google Scholar] [CrossRef]
- Abdel-Fattah, G.M.; Rabie, G.H.; Lamis, D.S.; Metwally, R.A. The impact of arbuscular mycorrhizal fungi on growth and physiological parameters of cowpea plants grown under salt stress conditions. Int. J. Appl. Sci. Biotechnol. 2016, 4, 372–379. [Google Scholar] [CrossRef][Green Version]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.; Abu Alhmad, M.F.A.; Abdelfattah, K.E. The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Fahad, S.; Bano, A. Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pak. J. Bot. 2012, 44, 1433–1438. [Google Scholar]
- Jini, D.; Joseph, B. Physiological Mechanism of Salicylic Acid for Alleviation of Salt Stress in Rice. Rice Sci. 2017, 24, 97–108. [Google Scholar] [CrossRef]
- Hernandez-Montes, G.; Diaz-Mejia, J.J.; Perez-Rueda, E.; Segovia, L. The hidden universal distribution of amino acid biosynthetic networks: A genomic perspective on their origins and evolution. Genome Biol. 2008, 9, R95. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Srivastava, A.K.; Saber, H.; Alwaleed, E.A.; Tran, L.S.P. Sargassum muticum and Jania rubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Sci. Rep. 2017, 7, 10537. [Google Scholar] [CrossRef]
- Mansour, M.M. Nitrogen containing compounds and adaptation of plants to salinity stress. Biol. Plant. 2000, 43, 491–500. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino acid-derived molecules in plant responces and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M.; Khan, M.M.A. Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol. Plant. 2010, 32, 121–132. [Google Scholar] [CrossRef]
- Asadi, M.; Heidari, M.A.; Kazemi, M.; Filinejad, A.R. Salicylic acid induced changes in some physiological parameters in chickpea (Cicer arietinum L.) under salt stress. J. Agric. Technol. 2013, 9, 311–316. [Google Scholar]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Trotel, P.; Bouchereau, A.; Niogret, M.F.; Larher, F. The fate of osmo-accumulated proline in leaf discs of Rape (Brassica napus L.) incubated in a medium of low osmolarity. Plant Sci. 1996, 118, 31–45. [Google Scholar] [CrossRef]
- Woodward, A.J.; Bennett, I.J. The effect of salt stress and abscisic acid on proline production, chlorophyll content and growth of in vitro propagated shoots of Eucalyptus camaldulensis. Plant Cell Tissue Organ Cult. 2005, 82, 189–200. [Google Scholar] [CrossRef]
- Zlatić, N.; Jakovljević, D.; Stanković, M. Temporal, Plant Part, and Interpopulation Variability of Secondary Metabolites and Antioxidant Activity of Inula helenium L. Plants 2019, 8, 179. [Google Scholar] [CrossRef]
- Metwally, R.A.; Abdelhameed, R.E. Impact of Ridomil, Bavistin and Agrothoate on arbuscular mycorrhizal fungal colonization, biochemical changes and potassium content of cucumber plants. Ecotoxicology 2019, 28, 487–498. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Ghasemnezhad, A.; Barani, M.; Telmadarrehei, T. Effect of Salinity on Phenolic Composition and Antioxidant Activity of Artichoke (Cynara scolymus L.) Leaves. Res. J. Med. Plants 2012, 6, 245–252. [Google Scholar] [CrossRef]
- Li, J.; Ou-Lee, T.M.; Raba, R.; Amundson, R.G.; Last, R.L. Arabidopsis flavonoid mutants are hypersensitive to UV-B radiation. Plant Cell 1993, 5, 171–179. [Google Scholar] [CrossRef]
- Ismail, H.; Maksimovic, J.D.; Maksimovic, V.; Shabala, L.; Zivanovic, B.D.; Tian, Y.; Jacobsen, S.-E.; Shabala, S. Rutin, a flavonoid with antioxidant activity, improves plant salinity tolerance by regulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans. Funct. Plant Biol. 2016, 43, 75–86. [Google Scholar] [CrossRef]
- Neffati, M.; Sriti, J.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem. 2011, 124, 221–225. [Google Scholar] [CrossRef]
- Qureshi, M.I.; Abdin, M.Z.; Qadir, S.; Iqbal, M. Lead induced oxidative stress and metabolic alterations in Cassia angustifolia Vahl. Biol. Plant. 2007, 51, 121–128. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Mostofa, M.G.; Rahman, M.M.; Abdel-Farid, I.B.; Tran, L.S.P. Extracts from yeast and carrot roots enhance maize performance under seawater-induced salt stress by altering physio-biochemical characteristics of stressed plants. J. Plant Growth Regul. 2019, 38, 966–979. [Google Scholar] [CrossRef]
- Sicher, C.; Barnaby, J.Y. Impact of carbon dioxide enrichment on the responses of maize leaf transcripts and metabolites to water stress. Physiol. Plant. 2012, 144, 238–253. [Google Scholar] [CrossRef]
- Warren, C.; Aranda, I.; Cano, F.J. Metabolomics demonstrates divergent responses of two Eucalyptus species to water stress. Metabolomics 2012, 8, 186–200. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Redondo-Gil, M.; Benavides, J.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front. Plant Sci. 2015, 6, 837. [Google Scholar] [CrossRef]
- Matallo, M.B.; Almeida, S.D.B.; Franco, D.A.S.; Cerdeira, A.L.; Gazzeiro, D.L.P. Glyphosate as a tool to produce shikimic acid in plants. Planta Daninha 2014, 32, 601–608. [Google Scholar] [CrossRef][Green Version]
- Herrmann, K.M.; Weaver, L.M. The shikmate pathway. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Correlations of antioxidant activity against phenolic content revisited: A new approach in data analysis for food and medicinal plants. J. Food Sci. 2009, 74, 107–113. [Google Scholar] [CrossRef]
- Chen, Z.; Silva, H.; Klessig, D.F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 1993, 262, 1883–1886. [Google Scholar] [CrossRef]
- Jaiswal, A.; Pandurangam, V.; Sharma, S.K. Effect of salicylic acid in soybean (Glycine max L. Meril) under salinity stress. Int. J. Life Sci. Res. 2014, 9, 671–676. [Google Scholar]
- Singh, S.; Singh, V.P.; Prasad, S.M.; Sharma, S.; Ramawat, N.; Dubey, N.K.; Tripathi, D.K.; Chauhan, D.K. Interactive Effect of Silicon (Si) and Salicylic Acid (SA) in Maize Seedlings and Their Mechanisms of Cadmium (Cd) Toxicity Alleviation. J. Plant Growth Regul. 2019, 38. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Calorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lee, Y.P.; Takanashi, T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem. 1966, 14, 71–77. [Google Scholar] [CrossRef]
- Bates, I.S.; Waldern, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Jindal, K.K.; Singh, R.N. Phenolic content in male and female Carica papaya: A possible physiological marker sex identification of vegetative seedlings. Physiol. Plant. 1975, 33, 104–107. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of flavonoid-rich extract of Hypericum perforatum L in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Zelya, I.A.; Anderson, J.A.H.; Owen, M.D.K.; Landes, R.D. Evaluation of spectrophotometric and HPLC methods for shikimic acid determination in plants: Models in glyphosate resistant and susceptible crops. J. Agric. Food Chem. 2011, 59, 2202–2212. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H., Ed.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 273–286. [Google Scholar] [CrossRef]
- Maehly, A.C. Assay of catalases and peroxidases. In Methods of Biochemical Analysis; Glick, D., Ed.; Wiley: Hoboken, NJ, USA, 1955; Volume 2, pp. 764–775. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, peroxidase and polyphenol oxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]

| NaCl (mM) | SA (mM) | CCI | % C | Carbohydrates (mg g−1 DW) | % C |
|---|---|---|---|---|---|
| 0 | 0 | 22.03 ± 0.91 ab | 11.3 | 79.2 ± 4.5 c | 4.3 |
| 0.5 | 24.51 ± 3.08 a | 82.6 ± 4.6 bc | |||
| 75 | 0 | 17.87 ± 1.11 b | 35.3 ↑ | 91 ± 5.1 bc | 8.4 ↑ |
| 0.5 | 24.17 ± 4.61 a | 98.6 ± 5.5 ab | |||
| 150 | 0 | 13.27 ± 3.56 c | 32.1 ↑ | 110.4 ± 6.1 a | 7.8 ↑ |
| 0.5 | 17.53 ± 3.65 b | 119 ± 7.3 a |
| Parameters | Salt | SA | Salt × SA |
|---|---|---|---|
| Chlorophyll content index | * | * | * |
| Carbohydrates | * | ns | ns |
| Lipid peroxidation | * | * | * |
| Phenolics | * | * | ns |
| Flavonoids | * | * | ns |
| Shikimic acid | * | * | * |
| Total free amino acids | * | * | * |
| Proline | * | * | * |
| POD | * | * | ns |
| CAT | * | * | ns |
| APX | * | * | * |
| PPO | * | * | * |
| Property | Sand (%) | Silt (%) | Clay (%) | Soil Texture | Saturation Percent | Electric Conductivity | pH | CaCO3 (%) | Mineral Content | Organic Matter | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/kg Soil) | (%) | ||||||||||||
| K | Mg | Ca | Total P | (%) | |||||||||
| Value | 13.9 | 27.4 | 58.7 | clay | 69 | 3.4 | 8.2 | 4.9 | 0.37 | 6.3 | 8.4 | 0.69 | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhameed, R.E.; Abdel Latef, A.A.H.; Shehata, R.S. Physiological Responses of Salinized Fenugreek (Trigonellafoenum-graecum L.) Plants to Foliar Application of Salicylic Acid. Plants 2021, 10, 657. https://doi.org/10.3390/plants10040657
Abdelhameed RE, Abdel Latef AAH, Shehata RS. Physiological Responses of Salinized Fenugreek (Trigonellafoenum-graecum L.) Plants to Foliar Application of Salicylic Acid. Plants. 2021; 10(4):657. https://doi.org/10.3390/plants10040657
Chicago/Turabian StyleAbdelhameed, Reda E., Arafat Abdel Hamed Abdel Latef, and Rania S. Shehata. 2021. "Physiological Responses of Salinized Fenugreek (Trigonellafoenum-graecum L.) Plants to Foliar Application of Salicylic Acid" Plants 10, no. 4: 657. https://doi.org/10.3390/plants10040657
APA StyleAbdelhameed, R. E., Abdel Latef, A. A. H., & Shehata, R. S. (2021). Physiological Responses of Salinized Fenugreek (Trigonellafoenum-graecum L.) Plants to Foliar Application of Salicylic Acid. Plants, 10(4), 657. https://doi.org/10.3390/plants10040657







