Physiological, Biochemical and Yield-Component Responses of Solanum tuberosum L. Group Phureja Genotypes to a Water Deficit
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Water Deficit Conditions
2.2. Measurements of Plant Physiological Variables
2.3. Determination of Soluble Sugars in Potato Leaves Using HPLC
2.4. Yield Measurement
2.5. Drought Tolerance Index (DTI)
2.6. Statistical Analysis
3. Results
3.1. Effect of Water Deficit on Physiological and Biochemical, and Yield-Component Variables in a Genetic Diversity Panel
3.2. Correlation between Physiological and Biochemical Variables and Yield Component
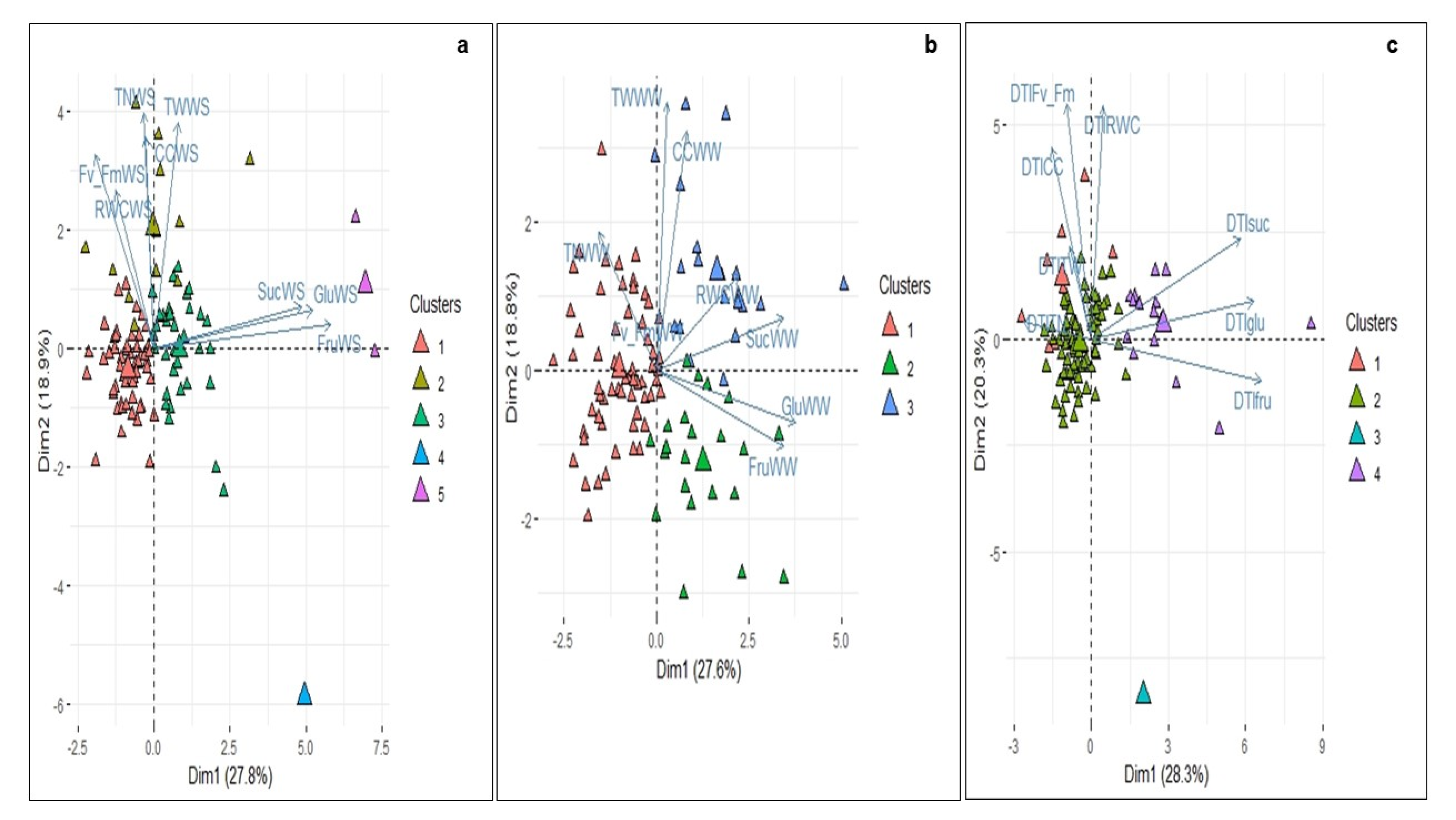
3.3. Principal Component Analysis of Physiological, Biochemical and Yield-Component Variables and Evaluation of the Water Deficit Responses
3.4. Cluster Analysis between the 104 Diploid Genotypes under Well-Watered and Water Deficit Conditions and DTI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolaou, G.; Neocleous, D.; Christou, A.; Kitta, E.; Katsoulas, N. Implementing sustainable irrigation in water-scarce regions under the impact of climate change. Agronomy 2020, 10, 1120. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Niu, Z.; Zhang, M.; Li, C.; Li, J. The effects of projected climate change and extreme climate on maize and rice in the Yangtze River Basin, China. Agric. For. Meteorol. 2020, 282–283, 107867. [Google Scholar] [CrossRef]
- Mukherjee, D. Stochastic model for crop water stress during agricultural droughts. Eng. Rep. 2019, 1, 1–24. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- FAO. World Corp Production Statistics. 2018. Available online: http//faostat.fao.org/ (accessed on 26 May 2020).
- Sprenger, H.; Rudack, K.; Schudoma, C.; Neumann, A.; Seddig, S.; Peter, R.; Zuther, E.; Kopka, J.; Hincha, D.; Walther, D.; et al. Assessment of drought tolerance and its potential yield in potato. Funct. Plant Biol. 2015, 42, 655–667. [Google Scholar] [CrossRef]
- Obidiegwu, J.; Bryan, G.; Jones, H.; Prashar, A. Coping with drought: Stress and adaptive response in potato and perspective for improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R. The effect of climate change on global potato production. Am. J. Potato Res. 2003, 80, 271–279. [Google Scholar] [CrossRef]
- Jones, P.; Lister, D.; Jaggard, K.; Pidgeon, J. Future climate impact on the productivity of sugar beet (Beta vulgaris L.) in Europe. Clim. Chang. 2003, 58, 93–108. [Google Scholar] [CrossRef]
- Monneveux, P.; Ramirez, D.; Pino, M. Drought tolerance in potato (S. tuberosum) Can we learn from drought tolerance research in cereals. Plant Sci. 2013, 205–206, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Hirut, B.; Shimelis, H.; Fentahum, M.; Bonierbale, M.; Gastelo, M.; Asfaw, A. Combining ability of highland tropic adapted potato for tuber and yield components under drought. PLoS ONE 2017, 12, e0181541. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Alarcon, A.; Galeano, C. Physiological assessment of water stress in potato using spectral information. Front. Plant Sci. 2017, 8, 1608. [Google Scholar] [CrossRef]
- Rolando, J.; Ramírez, D.; Yactayo, W.; Monneveux, P.; Quiroz, R. Leaf greenness as a drought tolerance related trait in potato (Solanum tuberosum L.). Environ. Exp. Bot. 2015, 110, 27–35. [Google Scholar] [CrossRef]
- Aliche, E.; Theeuwen, T.; Oortwijn, M.; Visser, R.; Van der Linden, G. Carbon partitioning mechanisms in potato under drought stress. Plant Physiol. Biochem. 2020, 146, 211–219. [Google Scholar] [CrossRef]
- Anithakumari, A.; Nataraja, K.; Visser, R.; Van der Linden, C. Genetic dissection of drought tolerance and recovery potential by quantitative trait locus mapping of a diploid potato population. Mol. Breed. 2012, 30, 1413–1429. [Google Scholar] [CrossRef] [PubMed]
- Schafleitner, R.; Gutiérrez, R.; Espino, R.; Gaudin, A.; Perez, J.; Martínez, M.; Domínguez, A. Field screening for variation of drought tolerance in Solanum tuberosum L. Agronomical, physiological and genetic analysis. Potato Res. 2007, 50, 71–85. [Google Scholar] [CrossRef]
- Dien, D.; Mochizuki, T.; Yamakawa, T. Effect of various drought stress and subsequent recovery on proline, total soluble sugar and starch metabolisms in rice (Oryza sativa L.) varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef]
- Shi, S.; Fan, M.; Iwama, K.; Li, F.; Zhang, Z.; Jia, L. Physiological basis of drought tolerance in potato grown under long-term water deficiency. Int. J. Plant. Prod. 2015, 9, 305–320. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Sun, Y.; Zhang, J.; Li, C. Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J. Integr. Agric. 2018, 17, 336–347. [Google Scholar] [CrossRef]
- Aliche, E.; Oortwijn, R.; Theeuwen, T.; Bachem, C.; Visser, R.; Van der Linden, G. Drought response in field grown potatoes and the interactions between canopy growth and yield. Agric. Water Manag. 2018, 206, 20–30. [Google Scholar] [CrossRef]
- Qin, J.; Bian, C.; Liu, J.; Zhang, J.; Jin, L. An efficient greenhouse method to screen potato genotypes for drought tolerance. Sci. Hortic. 2019, 253, 61–69. [Google Scholar] [CrossRef]
- Ramírez, C.; Rolando, J.; Yactayo, W.; Monneveux, P.; Mares, V.; Quiroz, R. Improving potato drought tolerance through the induction of long-term water stress memory. Plant Sci. 2015, 282, 26–32. [Google Scholar] [CrossRef]
- Boguzsewska-Mankowska, D.; Pieczynski, M.; Wyrzykowska, A.; Kalaji, H.; Sieczko, L.; Szweykowska-kulinska, Z.; Zagdanska, B. Divergent strategies displayed by potato (Solanum tuberosum L.) cultivars to cope with soil drought. J. Agron. Crop. Sci. 2017, 204, 13–30. [Google Scholar] [CrossRef]
- Li, X.; Ramirez, D.; Qin, J.; Dormatey, R.; Bi, Z.; Sun, C.; Wang, H.; Bai, J. Water restriction scenarios and their effects in traits in potato with different degrees of drought stress. Sci. Hortic. 2019, 256, 108525. [Google Scholar] [CrossRef]
- Cabello, R.; Chujoy, E.; Mendiburu, F.; Bonierbale, M.; Monnevuex, P. Large-scale evaluation of potatoes improved varieties, pre-breeding material and landraces for drought tolerance. Am. J. Potato Res. 2012, 89, 400–410. [Google Scholar] [CrossRef]
- Tardieu, F. Any trait or trait-related allele can confer drought tolerance: Just design the right drought scenario. J. Exp. Bot. 2012, 63, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Juyó, D.; Sarmiento, F.; Alvarez, M.; Brochero, H.; Cortes, H.; Gebhart, C. Genetic diversity and population structure in dipliod potatotes. Crop. Sci. 2015, 55, 760–769. [Google Scholar] [CrossRef]
- Spooner, D.; Ñúñez, J.; Trujillo, G.; Herrera, M.; Guzmán, F.; Ghislain, M. Extensive simple sequence repeat genotypes of potato landraces support a major reevaluation of their gene pool structure and classification. Proc. Natl. Acad. Sci. USA 2007, 104, 19398–19403. [Google Scholar] [CrossRef]
- Estrada, N. Los recursos genéticos en el mejoramiento de la papa en los países andinos. In Papas Colombianas con el Mejor Entorno Ambiental; UNIPAPA-ICA-CORPOICA: Bogotá, Colombia, 1996; pp. 1–14. [Google Scholar]
- Mosquera, T.; Del Castillo, S.; Gálvez, D. Breeding differently: Participatory selection and scaling up innovations in Colombia. Potato Res. 2017, 60, 361–381. [Google Scholar] [CrossRef]
- Juyó, D.; Soto, J.; Ballvora, A.; Léon, J.; Mosquera, T. Novel organ-specific genetic factor for quantitative resistance to late blight in potato. PLoS ONE 2019, 14, e0213818. [Google Scholar] [CrossRef]
- Ramírez, L.; Zuluaga, C.; Cotes, J. Evaluación de la resistencia a la sarna polvosa en genotipos bajo condiciones controladas a partir de tubérculos semillas. RFCB 2014, 11, 9–19. [Google Scholar] [CrossRef]
- Peña, C.; Sanchez, L.; Kushalappa, A.; Rodriguez, L.; Mosquera, T.; Nárvaez, C. Nutritional contents of advanced breeding clones of Solanum tuberosum group Phureja. LWT 2015, 62, 76–86. [Google Scholar] [CrossRef]
- Narváez, C.; Peña, C.; Restrepo, L.; Kushalappa, A.; Mosquera, T. Macronutrient contents of potato genotype collections in the Solanum tuberosum Group Phureja. J. Food Compos. Anal. 2018, 66, 179–184. [Google Scholar] [CrossRef]
- Ñúztes, C. Estudios Fenotípicos y Genéticos Asociados a la Calidad de Fritura en Solanum tuberosum Phureja Juz et Buk. [Online]. Ph.D. Thesis, Universidad Nacional de Colombia, 2011. Available online: http://www.bdigital.unal.edu.co/4318/ (accessed on 10 June 2020).
- Duarte, D.; Ñústez, C.; Narváez, C.; Restrepo, L.; Melo, S.; Sarmiento, F.; Kushalappa, A.; Mosquera, T. Natural variation of sucrose, glucose and fructose contents in Colombian genotypes of Solanum tuberosum Group Phureja at harvest. J. Sci. Food Agric. 2016, 96, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Piñeros, C.; Narvaéz, C.; Kushalappa, A.; Mosquera, T. Hydroxycinnamic acids in cooked potato tubers from Solanum tuberosum Group Phureja. Food Sci. Nutr. 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Parra, M.; Piñeros, C.; Soto, J.; Mosquera, T. Chromosomes I and X harbor consistent genetic factors associated with anthocyanin variation in potato. Agronomy 2019, 10, 366. [Google Scholar] [CrossRef]
- Lichtenthaler, H. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembrans. Method. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Lozano, P.; Sarmiento, F.; Mejía, L.; Álvarez, F.; Melgarejo, L. Physiological, biochemical and transcriptional responses of Passiflora edulis f. edulis under progressive drought stress. Sci. Hortic. 2021, 275, 109655. [Google Scholar] [CrossRef]
- Liu, F.; Jensena, C.; Shahanzaria, A.; Andersenb, M.; Jacobsen, S. ABA regulated stomatal control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci. 2005, 168, 831–836. [Google Scholar] [CrossRef]
- Duarte, D.; Narváez, C.; Restrepo, L.; Kushalappa, A.; Mosquera, T. Development and validation of liquid chromatographic method to quantify sucrose, glucose and fructose of Solanum tuberosum Group Phureja. J. Chromatogr. B 2015, 975, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, G. Effective selection criteria for assessing stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress, Taipei, Taiwan, 13–16 August 1992; Kuo, C.G., Ed.; AVRDC Publication: Tainan, Taiwan, 1992; pp. 257–270. [Google Scholar]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Fabregas, N.; Fernie, A. The metabolic response to drought. J. Expt. Bot. 2019, 7010771085. [Google Scholar] [CrossRef]
- Naservafaei, S.; Sohrabi, Y.; Moradi, P.; Sweeny, E.; Mastinu, A. Biological Response of Lallemantia iberica to Brassinolide Treatment under Different Watering Conditions. Plants 2021, 10, 496. [Google Scholar] [CrossRef]
- Batool, T.; Ali, S.; Seleiman, M. Plant growth promoting rhizobacterium alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020, 10, 16975. [Google Scholar] [CrossRef]
- Marcek, T.; Hamow, K.; Végh, B.; Janda, T.; Darko, E. Metabolic response to drought in six winter wheat genotypes. PLoS ONE 2019, 14, e0212411. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Shi, L.; Jiao, Y.; Li, M.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Metabolic response to drought stress in the tissues of drought–tolerant and drought-sensitive wheat genotype seedlings. AoB Plants. 2018, 10, ply016. [Google Scholar] [CrossRef]
- Ronquim, C.; Prado, C.; Souza, J. Growth, photosynthesis and leaf water potential in young plants of Capaifera langsdorffi Desf. (Caesalpiniacea) under contrasting irradiances. Braz. Soc. Plant Phys. 2019, 23, 197–208. [Google Scholar] [CrossRef][Green Version]
- Dos Santos, C.; Verissimo, V.; Wanderley-Filho, H.; Ferreira, V.; Cavalcante, P.; Rolim, E.; Endress, L. Seasonal variations of photosynthesis, gas Exchange, quantum efficiency of photosystem II and biochemical responses of Jatropha curcas L. grown in semi-humid and semi-arid areas subject to water stress. Ind. Crop. Prod. 2013, 41, 203–213. [Google Scholar] [CrossRef]
- Baker, N. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Rodriguez, L.; Ñústez, C.; Moreno, L. Drought stress affect physiological parameters but not tuber yield in three Andean potatoes (Solanum tuberosum L.) cultivars. Agron. Colomb. 2017, 35, 158–170. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishna, M.; Sharma, A. The impact of drought in plants metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Ramírez, D.; Yactayo, W.; Gutiérrez, R.; Mares, V.; De Mendiburu, F.; Posadas, A. Chlorophyll concentration in leaves is an indicator of potato tuber yield in water-shortage conditions. Sci. Hortic. 2014, 168, 202–209. [Google Scholar] [CrossRef]
- Plich, J.; Boguszewska-Mankowska, D.; Marczewski, W. Relations between photosynthetic parameters and drought-induced tuber yield decrease in Katahdin-derived potato cultivars. Potato Res. 2020, 63, 463–477. [Google Scholar] [CrossRef]
- Soltys- Kalina, D.; Plich, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of “Katahdin”- derived potato cultivars. Breed. Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Rudack, K.; Seddig, S.; Sprenger, H.; Kohl, K.; Uptmoor, R.; Ordon, F. Drought stress-induced changes in starch yield and physiological traits in potato. J. Agron. Crop. Sci. 2017, 1–12. [Google Scholar] [CrossRef]
- Cabello, R.; Monneveux, P.; Bonierbale, M.; Khan, M. Heritability of yield components under irrigated and drought conditions in Andigenum potatoes. Am. J. Potato Res. 2014, 91, 492–499. [Google Scholar] [CrossRef]
- Badr, A.; El-Shazly, H.; Tarawneh, R.; Borner, A. Screening for drought tolerance in Maize (Zea mays L.) germplasm using germination and seedling traits under simulated drought conditions. Plants 2020, 9, 565. [Google Scholar] [CrossRef] [PubMed]
| Variables | Mean Square | Overall Means ± SD | RR (%) | |||
|---|---|---|---|---|---|---|
| G | WC | G*WC | Well-Watered | Water Deficit | ||
| Sucrose (mg g−1 fw) | 3.08 * | 312.03 *** | 2.90 * | 1.55 ± 0.76 | 3.01 ± 1.30 | −94.19 |
| Glucose (mg g−1 fw) | 7.58 * | 784.80 *** | 6.52 * | 1.33 ± 0.78 | 3.72 ± 1.97 | −179.70 |
| Fructose (mg g−1 fw) | 27.70 *** | 2446.47 *** | 22.39 ** | 2.08 ± 1.18 | 6.29 ± 4.40 | −202.40 |
| Fv/Fm | 0.01 *** | 0.54 *** | 0.08 *** | 0.82 ± 0.009 | 0.78 ± 0.06 | 4.88 |
| Relative chlorophyll content (SPAD unit) | 281.98 * | 4265.66 *** | 188.66 ns | 40.18 ± 3.66 | 43.94 ± 8.38 | −9.36 |
| Tuber number per plant | 119.80 *** | 124.79 *** | 28.12 *** | 7.98 ± 3.51 | 8.73 ± 4.05 | −9.40 |
| Tuber fresh weight (g per plant) | 9621.55 *** | 312,527.11 *** | 1460.84 *** | 85.12 ± 37.81 | 52.54 ± 27.12 | 38.28 |
| RWC (%) | 346.28 *** | 157,199.67 *** | 289.46 *** | 80.05 ± 4.19 | 55.70 ± 9.97 | 30.42 |
| WC | Var | WD | WW | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suc | Glc | Fru | Fv/Fm | CC | TN | TW | RWC | Suc | Glc | Fru | Fv/Fm | CC | TN | TW | RWC | ||
| WD | Suc | 1 | 0.09 ns | 0.13 ns | 0.15 ns | −0.13 ns | −0.06 ns | −0.29 * | −0.11 ns | 0.03 ns | |||||||
| Glc | 0.4 ** | 1 | −0.01 ns | 0.05 ns | 0.05 ns | 0 ns | 0.03 ns | −0.25 * | −0.11 ns | 0.11 ns | |||||||
| Fru | 0.63 *** | 0.69 *** | 1 | 0.05 ns | 0.16 ns | 0.17 ns | −0.01 ns | −0.07 ns | −0.22 * | −0.15 ns | 0.13 ns | ||||||
| Fv/Fm | −0.09 ns | −0.10 ns | −0.13 ns | 1 | 0.14 ns | 0.03 ns | −0.21 * | −0.04 ns | 0.16 ns | 0.02 ns | −0.13 ns | 0.03 ns | |||||
| CC | 0.06 ns | −0.04 ns | −0.02 ns | 0.16 ns | 1 | −0.02 ns | −0.08 ns | −0.23 * | −0.02 ns | 0.27 * | 0.13 ns | 0.12 ns | −0.05 ns | ||||
| TN | −0.03 ns | −0.07 ns | −0.01 ns | 0.08 ns | 0.13 ns | 1 | −0.02 ns | −0.14 ns | −0.20 ns | −0.18 ns | 0.04 ns | 0.58 *** | 0.29 * | −0.16 ns | |||
| TW | 0 ns | 0.15 ns | 0.04 ns | −0.05 ns | 0.17 ns | 0.37 ** | 1 | 0.14 ns | −0.06 ns | −0.03 ns | −0.01 ns | 0.36 ** | 0.10 ns | 0.74 *** | 0.20 ns | ||
| RWC | 0.02 ns | −0.02 ns | −0.11 ns | 0.43 *** | 0.04 ns | −0.01 ns | −0.02 ns | 1 | 0.20 ns | 0.12 ns | −0.03 ns | 0.07 ns | 0.23 * | −0.03 ns | −0.08 ns | 0.08 ns | |
| WW | Suc | 0.09 ns | −0.01 ns | 0.05 ns | 0.14 ns | −0.02 ns | −0.02 ns | 0.14 ns | 0.02 ns | 1 | 0.51 *** | 0.35 *** | −0.05 ns | 0.26 * | −0.13 ns | 0.04 ns | 0.27 * |
| Glc | 0.13 ns | 0.05 ns | 0.16 ns | 0.03 ns | −0.08 ns | −0.14 ns | −0.06 ns | 0.12 ns | 1 | 0.61 *** | −0.08 ns | 0.03 ns | −0.16 ns | −0.03 ns | 0.18 ns | ||
| Fru | 0.15 ns | 0.05 ns | 0.17 ns | −0.21 * | 0.23 * | −0.20 ns | −0.03 ns | −0.03 ns | 1 | −0.04 ns | −0.10 ns | −0.18 ns | 0.02 ns | 0.21 * | |||
| Fv/Fm | −0.13 ns | 0 ns | −0.01 ns | −0.04 ns | −0.02 ns | −0.18 ns | −0.01 ns | 0.07 ns | 1 | 0.11 ns | −0.09 ns | −0.03 ns | 0.20 * | ||||
| CC | −0.06 ns | 0.03 ns | −0.07 ns | 0.16 ns | 0.27 * | 0.04 ns | 0.36 ** | 0.23 ** | 1 | −0.01 ns | 0.33 ** | 0.08 ns | |||||
| TN | −0.29 * | −0.25 * | −0.22 * | 0.02 ns | 0.13 ns | 0.58 *** | 0.10 ns | −0.03 ns | 1 | 0.28 * | 0.15 ns | ||||||
| TW | −0.11 ns | −0.11 ns | −0.15 ns | −0.13 ns | 0.12 ns | 0.29 * | 0.74 *** | −0.08 ns | 1 | 0.18 ns | |||||||
| RWC | 0.03 ns | 0.11 ns | 0.13 ns | 0.03 ns | −0.05 ns | −0.16 ns | 0.2 ns | 0.02 | 1 | ||||||||
| Group | Number of Genotypes | Sucrose (mg g−1 fw) | Glucose (mg g−1 fw) | Fructose (mg g−1 fw) | Fv/Fm | Relative Chlorophyll Content (SPAD Unit) | Tuber Number | Tuber Fresh Weight (g Per Plant) | Relative Water Content (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Well-Watered Conditions | 1 | 61 | 1.17 ± 0.47 | 0.83 ± 0.31 | 1.50 ± 0.77 | 0.82 ± 0.01 | 39.86 ± 2.85 | 8.61 ± 3.97 | 82.88 ± 32.07 | 78.80 ± 3.99 |
| 2 | 23 | 1.90 ± 0.54 | 2.11 ± 0.60 | 3.18 ± 1.20 | 0.82 ± 0.01 | 37.60 ± 3.22 | 6.52 ± 2.24 | 63.05 ± 23.14 | 80.26 ± 4.01 | |
| 3 | 20 | 2.29 ± 0.96 | 1.96 ± 0.84 | 2.60 ± 1.08 | 0.83 ± 0.01 | 44.13 ± 3.21 | 7.77 ± 2.74 | 117.32 ± 46.76 | 83.63 ± 2.79 | |
| Water Deficit | 1 | 55 | 2.44 ± 0.70 | 2.80 ± 0.98 | 4.09 ± 1.66 | 0.79 ± 0.03 | 42.71 ± 4.24 | 7.97 ± 2.81 | 41.08 ± 15.74 | 56.78 ± 10.28 |
| 2 | 12 | 3.11 ± 1.76 | 3.28 ± 1.18 | 6.21 ± 3.63 | 0.78 ± 0.03 | 52.65 ± 19.88 | 14.97 ± 6.56 | 97.20 ± 38.51 | 55.77 ± 7.39 | |
| 3 | 34 | 3.59 ± 0.98 | 4.77 ± 1.06 | 8.40 ± 2.60 | 0.78 ± 0.03 | 43.29 ± 4.59 | 7.93 ± 2.64 | 54.54 ± 19.20 | 55.17 ± 8.62 | |
| 4 | 1 | 5.13 | 6.66 | 12.20 | 0.22 | 29.53 | 3.75 | 64.60 | 18.35 | |
| 5 | 2 | 7.09 ± 4.17 | 12.15 ± 7.85 | 28.32 ± 7.86 | 0.81 ± 0.05 | 43.75 ± 3.81 | 8.00 ± 4.24 | 59.63 ± 48.97 | 53.16 ± 1.69 | |
| Drought Tolerance Index (DTI) a | 1 | 8 (T) | 1.68 ± 1.31 | 2.40 ± 2.31 | 2.19 ± 1.42 | 0.95 ± 0.05 | 1.52 ± 0.46 | 2.92 ± 1.95 | 2.42 ± 1.64 | 0.71 ± 0.09 |
| 2 | 80 (MS) | 1.57 ± 0.75 | 2.02 ± 1.20 | 2.25 ± 1.64 | 0.95 ± 0.03 | 1.07 ± 0.17 | 1.11 ± 0.73 | 0.59 ± 0.38 | 0.69 ± 0.12 | |
| 3 | 1 (MT) | 1.13 | 3.04 | 10.57 | 0.27 | 0.64 | 0.19 | 1.09 | 0.22 | |
| 4 | 15 (S) | 4.35 ± 2.49 | 7.43 ± 2.88 | 8.43 ± 6.15 | 0.95 ± 0.04 | 1.04 ± 0.19 | 0.98 ± 1.29 | 0.49 ± 0.42 | 0.73 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Valencia, P.; Melgarejo, L.M.; Arcila, I.; Mosquera-Vásquez, T. Physiological, Biochemical and Yield-Component Responses of Solanum tuberosum L. Group Phureja Genotypes to a Water Deficit. Plants 2021, 10, 638. https://doi.org/10.3390/plants10040638
Diaz-Valencia P, Melgarejo LM, Arcila I, Mosquera-Vásquez T. Physiological, Biochemical and Yield-Component Responses of Solanum tuberosum L. Group Phureja Genotypes to a Water Deficit. Plants. 2021; 10(4):638. https://doi.org/10.3390/plants10040638
Chicago/Turabian StyleDiaz-Valencia, Paula, Luz Marina Melgarejo, Ivon Arcila, and Teresa Mosquera-Vásquez. 2021. "Physiological, Biochemical and Yield-Component Responses of Solanum tuberosum L. Group Phureja Genotypes to a Water Deficit" Plants 10, no. 4: 638. https://doi.org/10.3390/plants10040638
APA StyleDiaz-Valencia, P., Melgarejo, L. M., Arcila, I., & Mosquera-Vásquez, T. (2021). Physiological, Biochemical and Yield-Component Responses of Solanum tuberosum L. Group Phureja Genotypes to a Water Deficit. Plants, 10(4), 638. https://doi.org/10.3390/plants10040638






