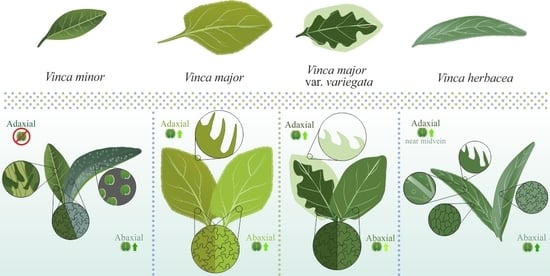
The Morphological and Anatomical Traits of the Leaf in Representative Vinca Species Observed on Indoor- and Outdoor-Grown Plants
Abstract
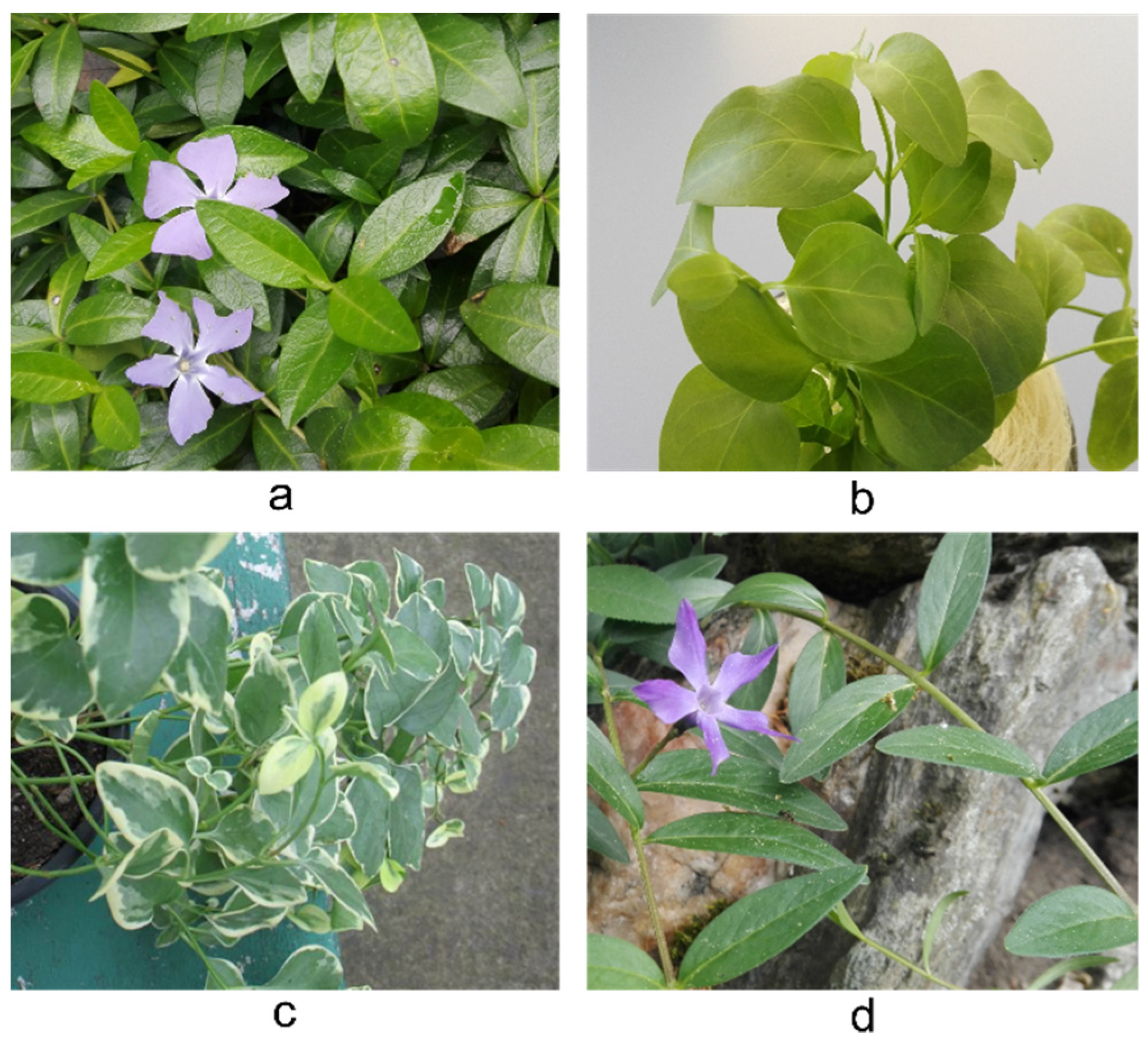
1. Introduction
2. Results
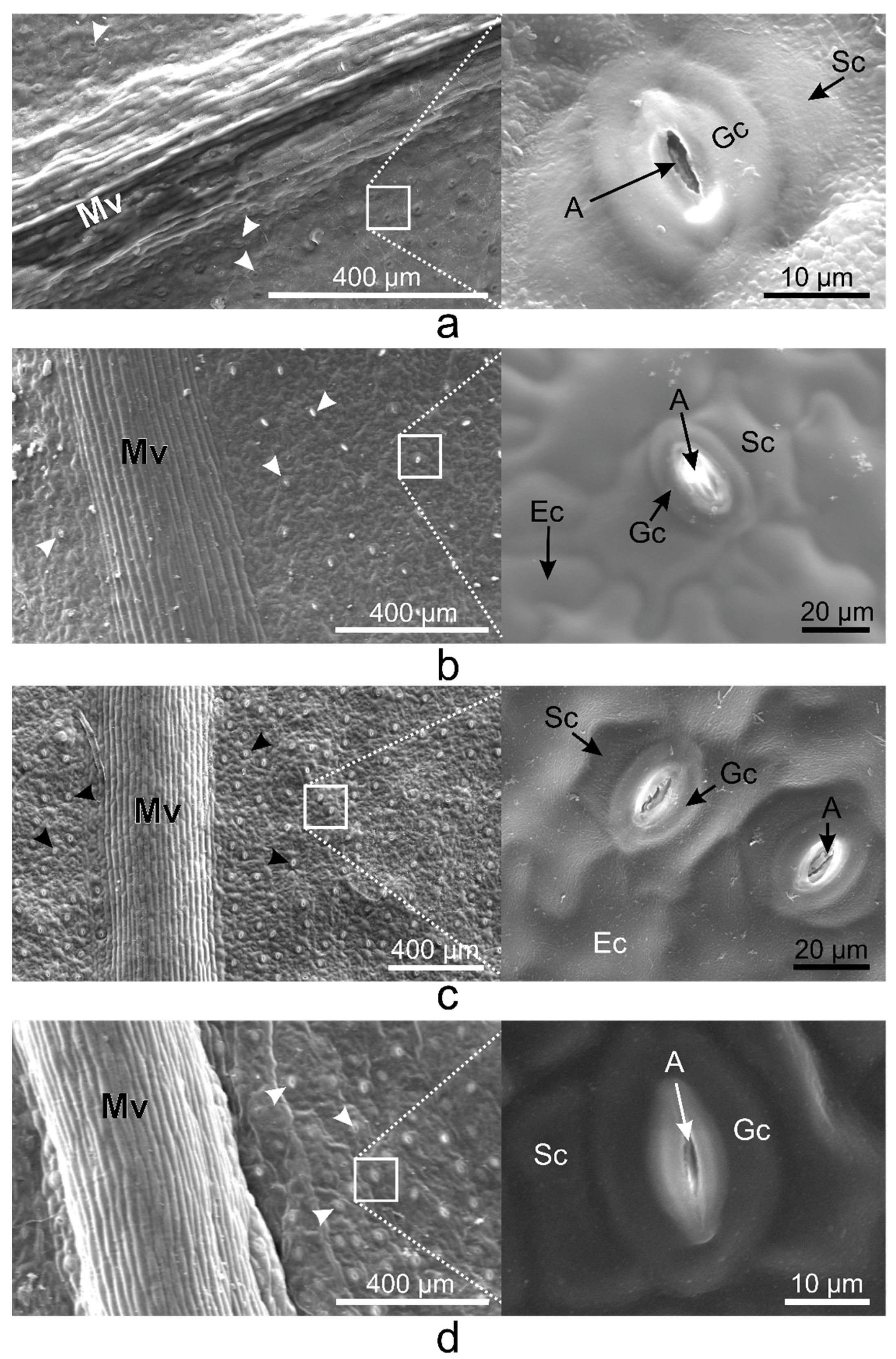
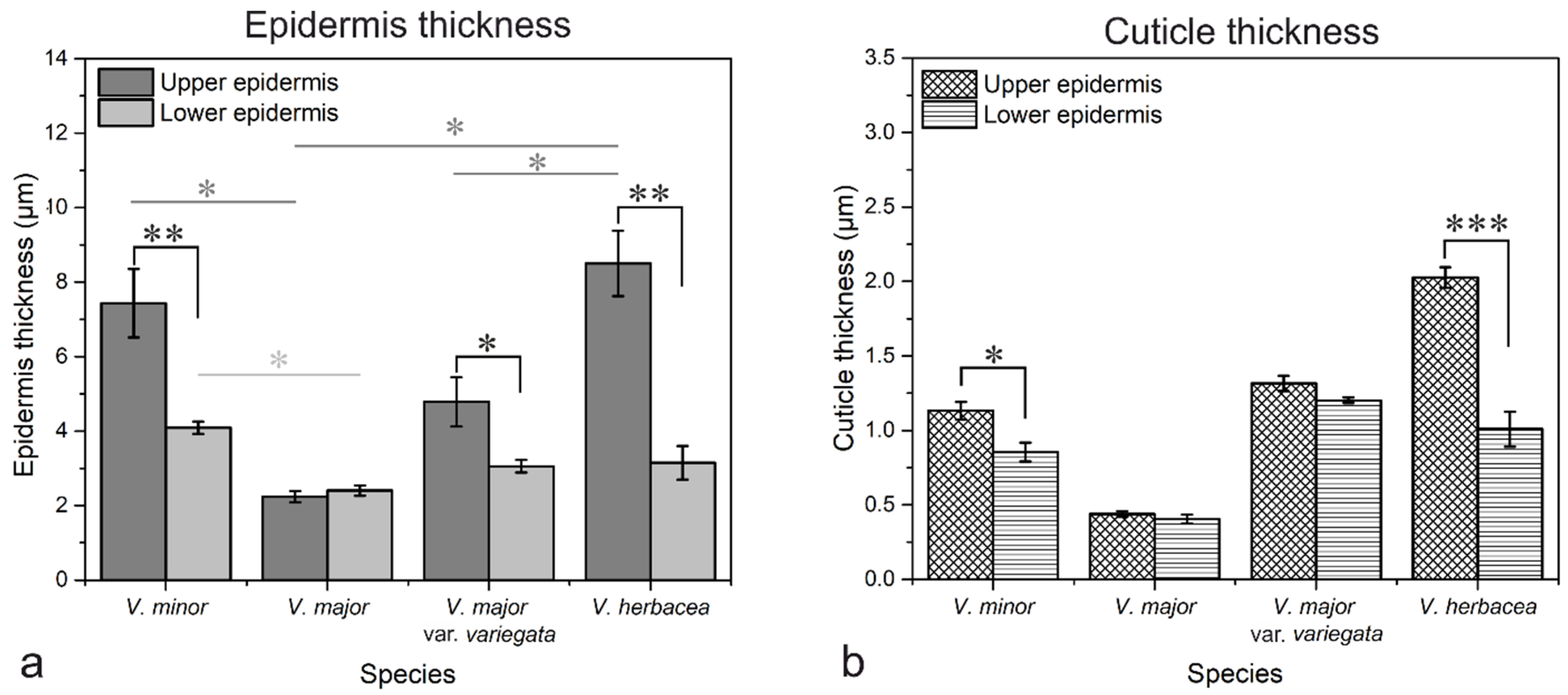
2.1. Vinca Leaf Epidermises
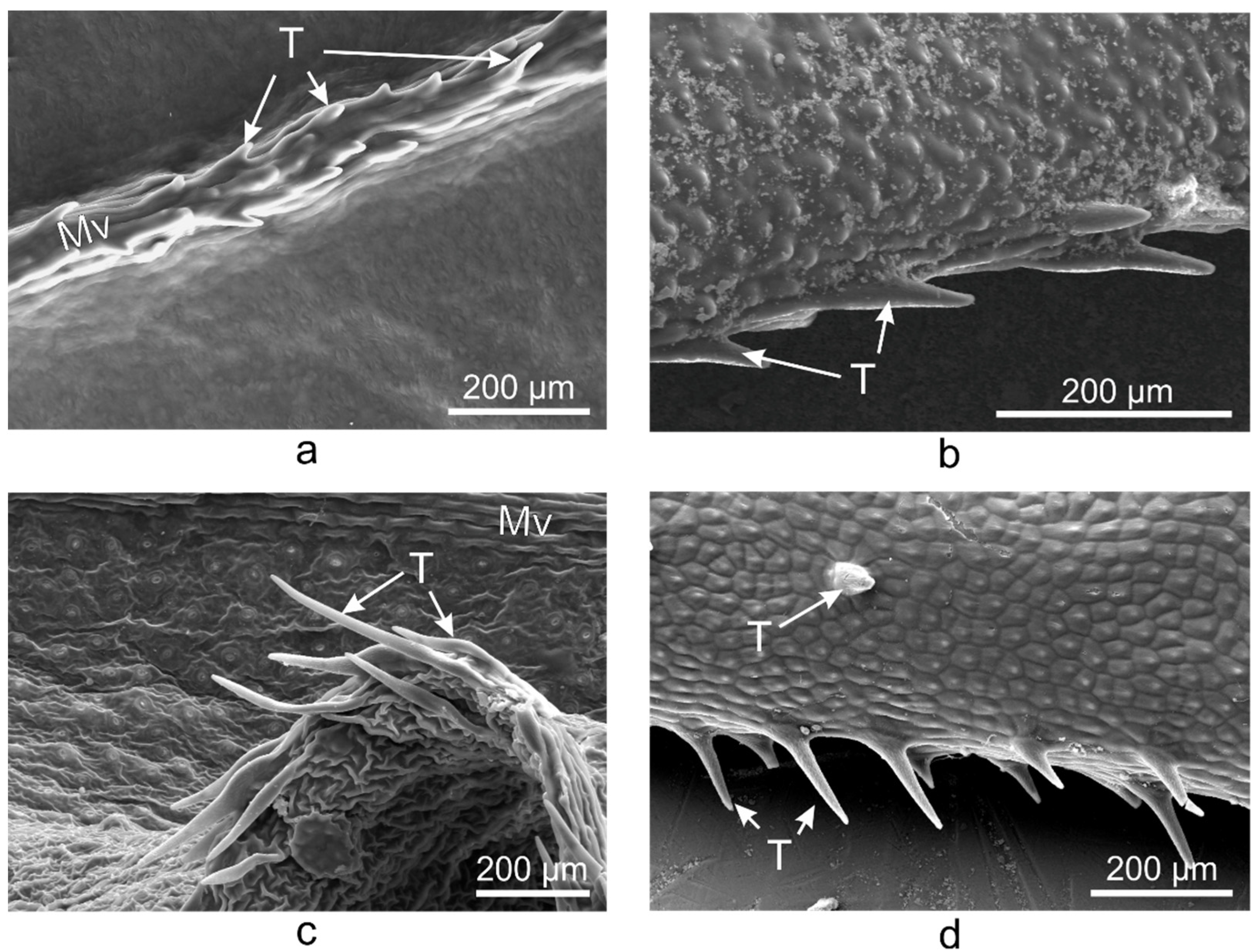
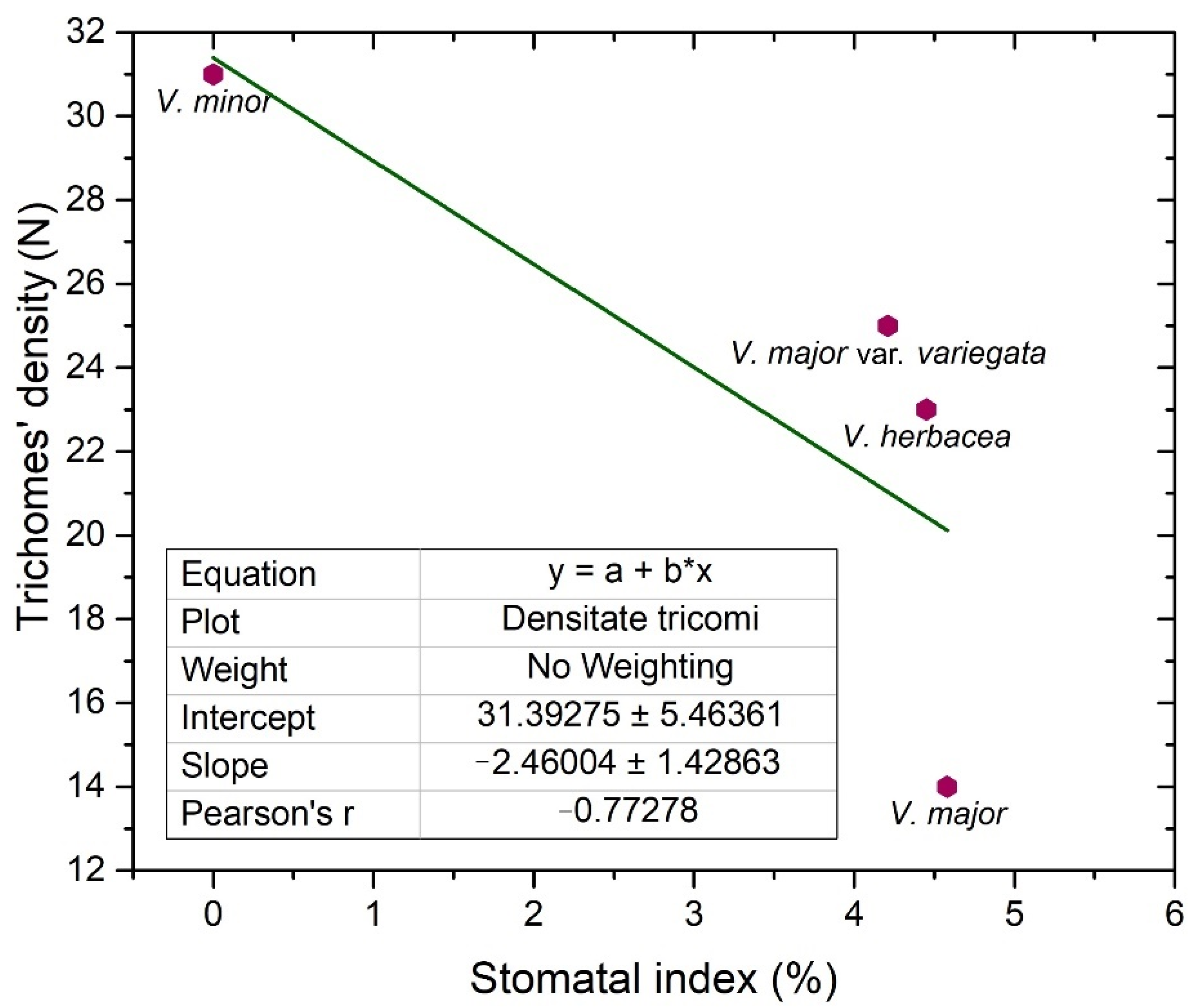
2.2. Vinca Trichomes and Stomata Characteristics
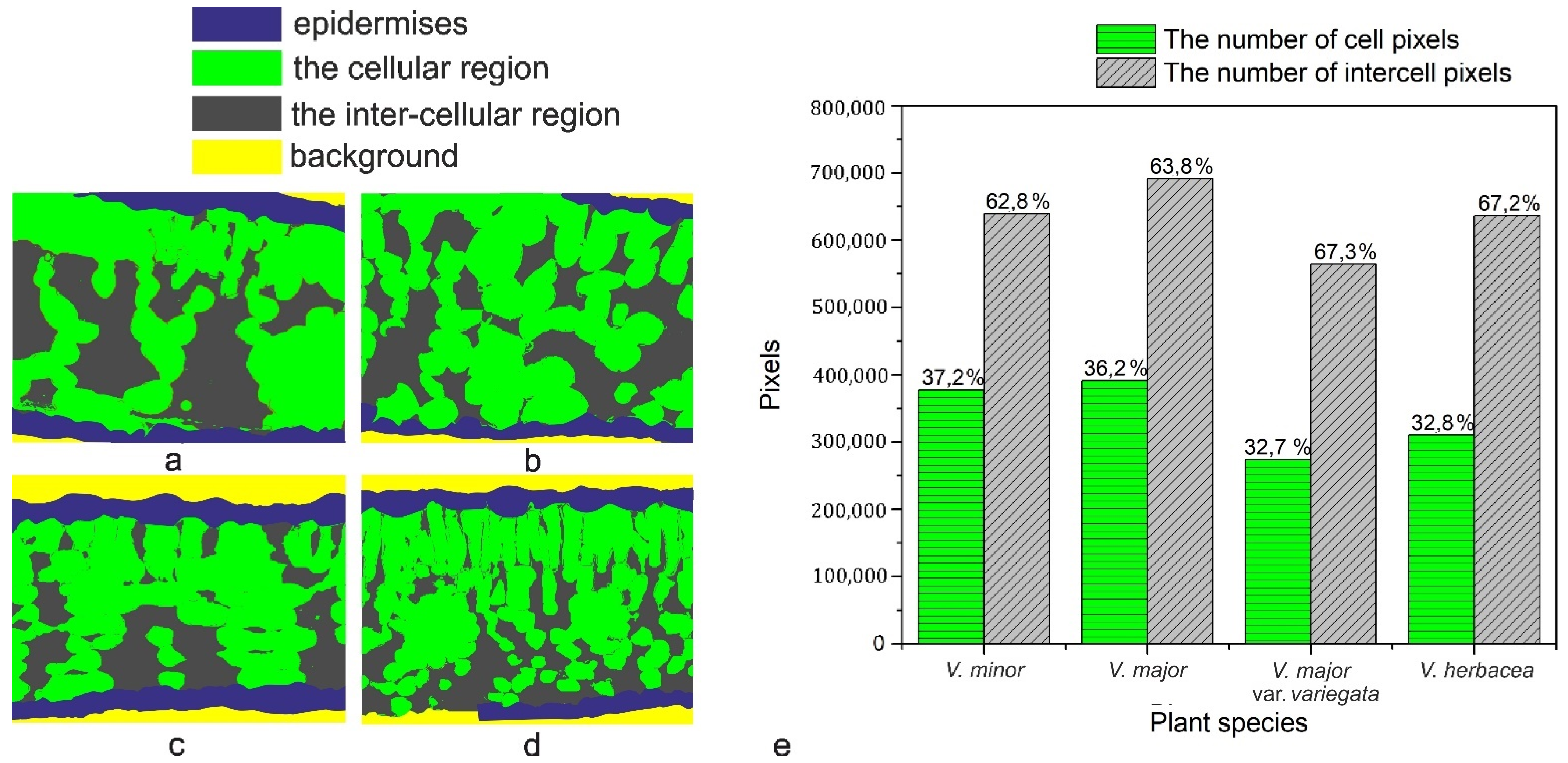
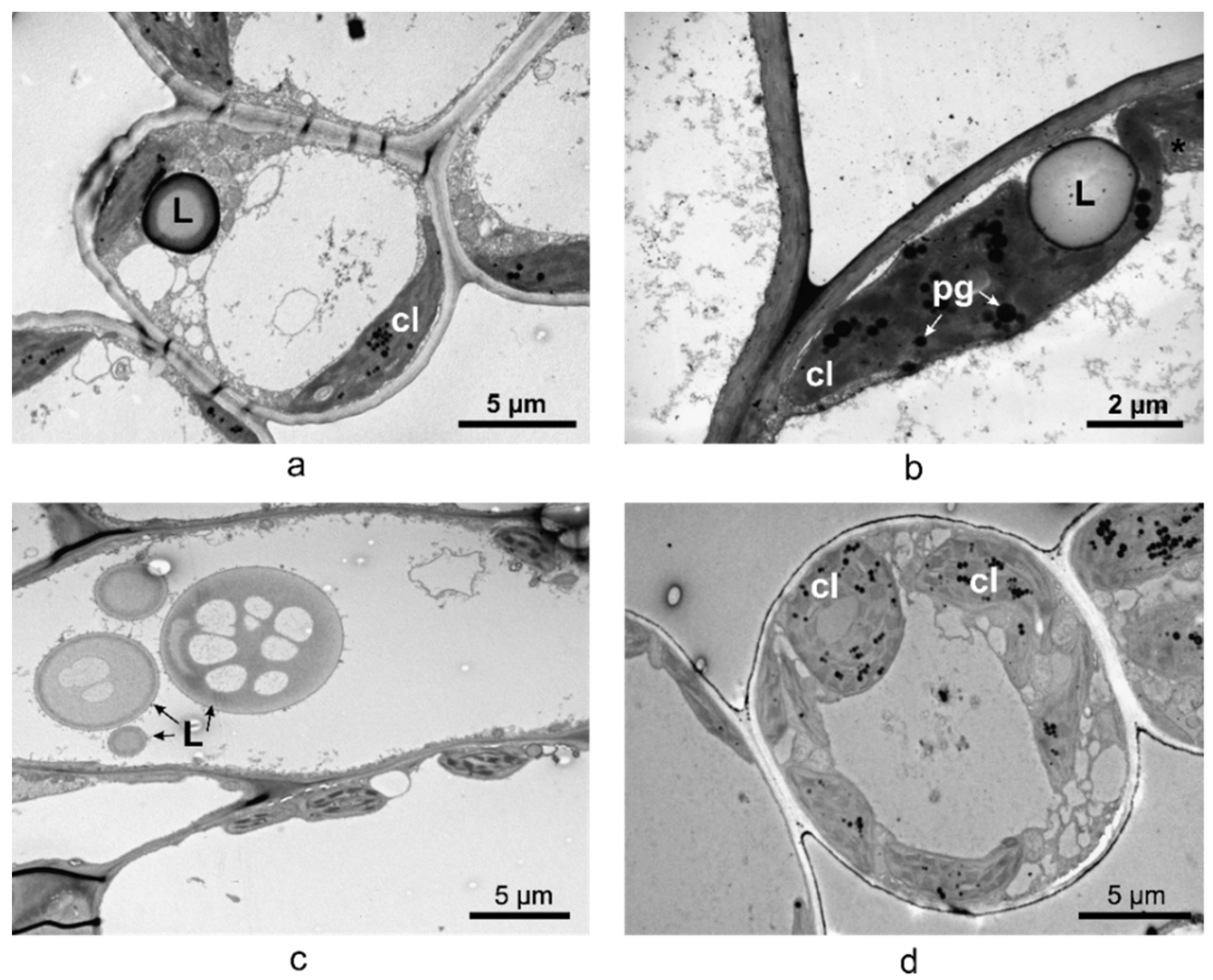
2.3. Vinca Mesophyll
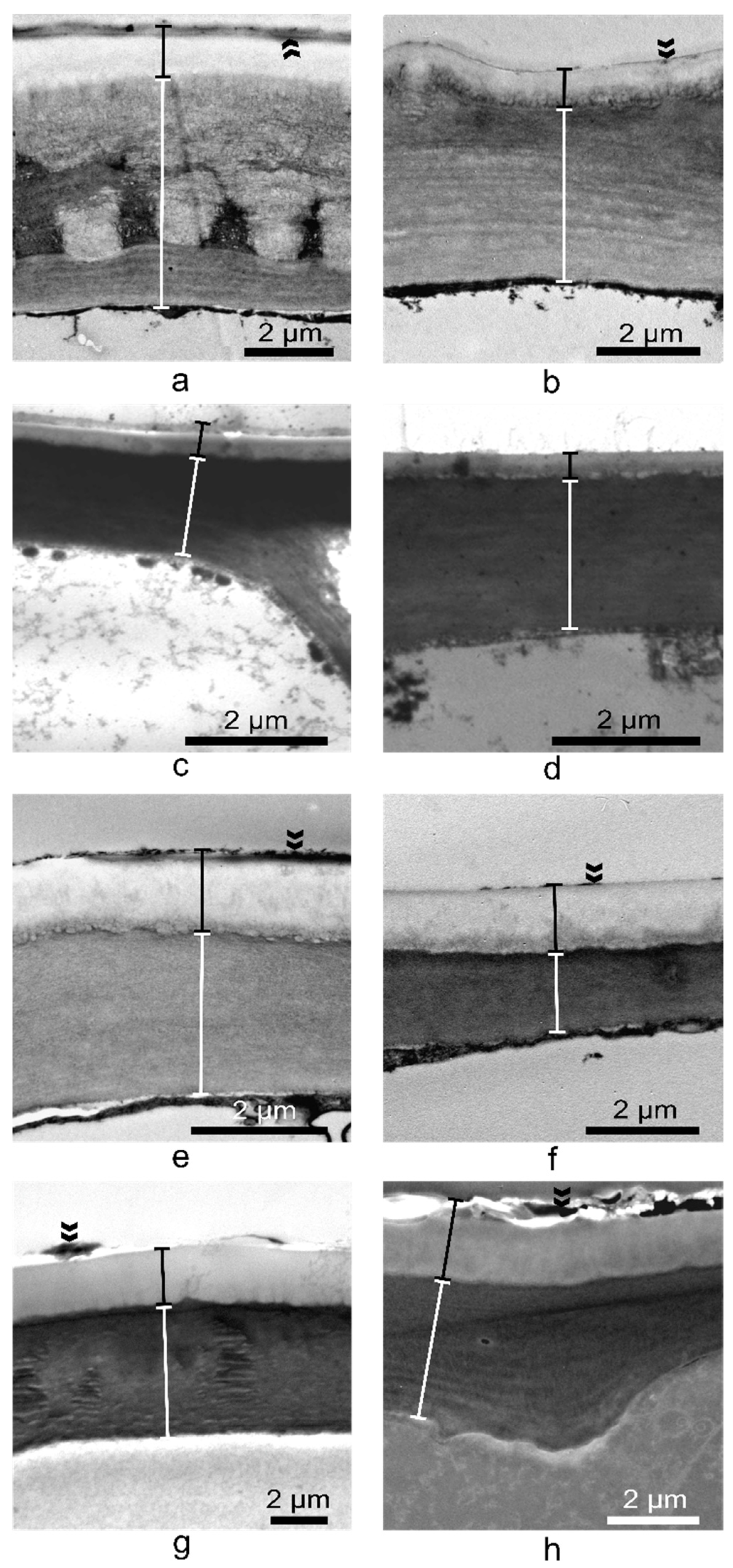
2.4. Vinca Epidermal Cell Walls and Cuticle Layers
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Scanning Electron Microscopy
4.3. Leaf Stomatal Traits
4.4. Light Microscopy
4.5. Leaf Anatomical Traits
4.6. Transmission Electron Microscopy
4.7. Computational Estimation of the Intercellular Space in the Mesophyll
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United States Department of Agriculture. PLANTS Database. Available online: https://plants.sc.egov.usda.gov (accessed on 21 January 2021).
- Plants of the World Online. Available online: http://powo.science.kew.org/ (accessed on 21 January 2021).
- Barrales-Cureño, H.J.; Reyes, C.R.; García, I.V.; Valdez, L.G.L.; De Jesús, A.G.; Cortés Ruíz, J.A.; Sánchez Herrera, L.M.; Calderón Caballero, M.C.; Magallón, J.A.S.; Espinoza Perez, J.; et al. Alkaloids of pharmacological importance in Catharanthus roseus. In Alkaloids—Their Importance in Nature and Human Life; Kurek, J., Ed.; Intech Open Ltd.: London, UK, 2019; Volume 1, p. 18. [Google Scholar]
- Cheng, G.-G.; Zhao, Y.-L.; Zhang, Y.; Lunga, P.-K.; Hu, D.-B.; Li, Y.; Gu, J.; Song, C.-W.; Sun, W.-B.; Liu, Y.-P.; et al. Indole alkaloids from cultivated Vinca major. Tetrahedron 2014, 70, 8723–8729. [Google Scholar] [CrossRef]
- Sukhdev, S.; Shamsher, K.S.; Indu, K. Antilipase activity guided fractionation of Vinca major. J. King. Saud. Univ. Sci. 2017, 30, 433–439. [Google Scholar] [CrossRef]
- Abouzeid, S.; Hijazin, T.; Lewerenz, L.; Hansch, R.; Selmar, D. The genuine localization of indole alkaloids in Vinca minor and Catharanthus roseus. Phytochemistry 2019, 168, 112110. [Google Scholar] [CrossRef]
- Bahadori, F.; Topçu, G.; Boğa, M.; Türkekul, A.; Kolak, U.; Kartal, M. Indole alkaloids from Vinca major and V. minor growing in Turkey. Nat. Prod. Commun. 2012, 7, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Boga, M.; Kolak, U.; Topcu, G.; Bahadori, F.; Kartal, M.; Farnsworth, N.R. Two new indole alkaloids from Vinca herbacea L. Phytochem. Lett. 2011, 4, 399–403. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Pan, Y.-j.; Zu, Y.-G.; Tang, Z.-H. Determination of alkaloids in Catharanthus roseus and Vinca minor by high-performance liquid chromatography—Tandem mass spectrometry. Anal. Lett. 2015, 49, 1143–1153. [Google Scholar] [CrossRef]
- Andrade, E.A.; Folquitto, D.G.; Luz, L.E.C.; Paludo, K.S.; Farago, P.V.; Budel, J.M. Anatomy and histochemistry of leaves and stems of Sapium glandulosum. Revista Brasileira de Farmacognosia 2017, 27, 282–289. [Google Scholar] [CrossRef]
- Verma, P.; Mathur, A.K.; Shanker, K. Enhanced vincamine production in selected tryptophan-overproducing shoots of Vinca minor. Plant Cell Tissue Organ Cult. 2012, 111, 239–245. [Google Scholar] [CrossRef]
- De-la-Cruz, I.M.; Cruz, L.L.; Martínez-García, L.; Valverde, P.L.; Flores-Ortiz, C.M.; Hernández-Portilla, L.B.; Núñez-Farfán, J. Evolutionary response to herbivory: Population differentiation in microsatellite loci, tropane alkaloids and leaf trichome density in Datura stramonium. Arthropod Plant Interact. 2019, 14, 21–30. [Google Scholar] [CrossRef]
- Liu, X.; Vrieling, K.; Klinkhamer, P.G.L. Interactions between plant metabolites affect herbivores: A study with pyrrolizidine alkaloids and chlorogenic acid. Front. Plant Sci. 2017, 8, 903. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, X.; Guo, X.; Guo, Q.; Li, D. Metabolomics characterization of two apocynaceae plants, Catharanthus roseus and Vinca minor, using GC-MS and LC-MS methods in combination. Molecules 2017, 22, 997. [Google Scholar] [CrossRef]
- Verma, P.; Khan, S.A.; Masood, N.; Manika, N.; Sharma, A.; Verma, N.; Luqman, S.; Mathur, A.K. Differential rubisco content and photosynthetic efficiency of rol gene integrated Vinca minor transgenic plant: Correlating factors associated with morpho-anatomical changes, gene expression and alkaloid productivity. J. Plant Physiol. 2017, 219, 12–21. [Google Scholar] [CrossRef]
- Islam, B.; Lustberg, M.; Staff, N.P.; Kolb, N.; Alberti, P.; Argyriou, A.A. Vinca alkaloids, thalidomide and eribulin-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Soc. 2019, 24 (Suppl. 2), S63–S73. [Google Scholar] [CrossRef]
- Liao, F.-Y.; Xie, Y.; Jiang, H. The effect of water stress on the physiology of Vinca major ‘variegata’. Appl. Mech. Mater. 2013, 409, 782–787. [Google Scholar] [CrossRef]
- Cheng, G.-G.; Zhao, H.-Y.; Liu, L.; Zhao, Y.-L.; Song, C.-W.; Gu, J.; Sun, W.-B.; Liu, Y.-P.; Luo, X.-D. Non-alkaloid constituents of Vinca major. Chin. J. Nat. Med. 2016, 14, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D. Antioxidant activity of polyphenolic plant extracts. Antioxidants 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, H.-L.; Li, Y.; Gu, H.-W.; Yin, X.-L.; Xie, L.-X.; Yu, R.-Q. Rapid and simultaneous determination of five Vinca alkaloids in Catharanthus roseus and human serum using trilinear component modeling of liquid chromatography–diode array detection data. J. Chromat. B 2016, 1026, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Foddai, M.; Maldini, M.; Addis, R.; Petretto, G.L.; Chessa, M.; Pintore, G. Profiling of the bioactive compounds in flowers, leaves and roots of Vinca sardoa. Nat. Prod. Commun. 2017, 12, 933–936. [Google Scholar] [CrossRef]
- Christodoulakis, N.S.; Mamoucha, S.; Termentzi, A.; Fokialakis, N. Leaf structure and histochemistry of the hardy evergreen Euphorbia characias L. (Mediterranean spurge). Flora 2015, 210, 13–18. [Google Scholar] [CrossRef]
- Zador, E.; Jones, D. The biosynthesis of a novel nicotine alkaloid in the trichomes of Nicotiana stocktonii. Plant Physiol. 1986, 82, 479–484. [Google Scholar] [CrossRef]
- Jing, H.; Liu, J.; Liu, H.; Xin, H. Histochemical investigation and kinds of alkaloids in leaves of different developmental stages in Thymus quinquecostatus. Sci. World J. 2014, 2014, 839548. [Google Scholar] [CrossRef]
- Giordano, C.; Maleci, L.; Agati, G.; Petruccelli, R. Ficus carica L. leaf anatomy: Trichomes and solid inclusions. Ann. Appl. Biol. 2019, 176, 47–54. [Google Scholar] [CrossRef]
- Mamoucha, S.; Fokialakis, N.; Christodoulakis, N.S. Leaf structure and histochemistry of Ficus carica (Moraceae), the fig tree. Flora 2016, 218, 24–34. [Google Scholar] [CrossRef]
- Boyadzhiev, L.; Yordanov, B. Pertraction of indole alkaloids from Vinca minor L. Sep. Sci. Technol. 2004, 39, 1321–1329. [Google Scholar] [CrossRef]
- Segev, R.; Nannapaneni, R.; Sindurakar, P.; Kim, H.; Read, H.; Lijek, S. The effect of the stomatal index on the net rate of photosynthesis in the leaves of Spinacia oleracea, Vinca minor, Rhododendron spp., Epipremnum aureum, and Hedera spp. J. Emerg. Investig. 2015, 20, 2018. [Google Scholar]
- Gotoh, E.; Suetsugu, N.; Higa, T.; Matsushita, T.; Tsukaya, H.; Wada, M. Palisade cell shape affects the light-induced chloroplast movements and leaf photosynthesis. Sci. Rep. 2018, 8, 1472. [Google Scholar] [CrossRef]
- Esteban, R.; Barrutia, O.; Artetxe, U.; Fernandez-Marin, B.; Hernandez, A.; Garcia-Plazaola, J.I. Internal and external factors affecting photosynthetic pigment composition in plants: A meta-analytical approach. New Phytol. 2015, 206, 268–280. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Environ. 2018, 6, 64. [Google Scholar] [CrossRef]
- Opriş, O.; Ciorîţă, A.; Soran, M.-L.; Lung, I.; Copolovici, D.; Copolovici, L. Evaluation of the photosynthetic parameters, emission of volatile organic compounds and ultrastructure of common green leafy vegetables after exposure to non-steroidal anti-inflammatory drugs (NSAIDs). Ecotoxicology 2019, 28, 631–642. [Google Scholar] [CrossRef]
- Csiky, J.; Purger, D. Herbaceous periwinkle, Vinca herbacea Waldst. et Kit. 1799 (Apocynaceae), a new species of the Croatian flora. Acta Bot. Croat. 2013, 72, 399–406. [Google Scholar] [CrossRef][Green Version]
- Ochirova, K.S.; Ovanova, E.A.; Dordzhieva, V.I. Vinca minor L. leaf anatomical structure. J. Pharm. Sci. Res. 2018, 10, 2528–2530. [Google Scholar]
- Petra, S.A.; Georgescu, M.I.; Manescu, C.R.; Toma, F.; Badea, M.L.; Dobrescu, E.; Popa, V.I. Leaves anatomical and physiological adaptations of Vinca major ‘Variegata’ and Hedera helix L. to specific roof garden conditions. Not. Bot. Horti Agrobot. 2020, 47, 318–328. [Google Scholar] [CrossRef]
- Samiyarsih, S.; Nettyani, N.; Dian, P. Variability of Catharanthus roseus based on morphological and anatomical characters, and chlorophyll contents. Biodiv. J. Biol. Div. 2019, 20, 2986–2993. [Google Scholar] [CrossRef]
- Shi, P.; Li, Y.; Hui, C.; Ratkowsky, D.A.; Yu, X.; Niinemets, Ü. Does the law of diminishing returns in leaf scaling apply to vines?—Evidence from 12 species of climbing plants. Glob. Ecol. Conserv. 2020, 21, e00830. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Kidner, C.A.; Umbreen, S. Why is Leaf Shape so Variable? Int. J. Plant Dev. Biol. 2010, 4, 64–75. [Google Scholar]
- Murata, J.; Roepke, J.; Gordon, H.; De Luca, V. The leaf epidermome of Catharanthus roseus reveals its biochemical specialization. Plant Cell 2008, 20, 524–542. [Google Scholar] [CrossRef] [PubMed]
- Gagua, N.; Mchedlidze, K.; Vachnadze, V.; Bakuridze, A. Structural peculiarities of the vegetative organs of the species of Vinca. PHCOG J. 2012, 4, 49–55. [Google Scholar] [CrossRef]
- Sapala, A.; Runions, A.; Routier-Kierzkowska, A.-L.; Gupta, M.D.; Hong, L.; Hofhuis, H.; Verger, S.; Mosca, G.; Li, C.-B.; Hay, A.; et al. Why plants make puzzle cells, and how their shape emerges. eLife 2018, 7, e32794. [Google Scholar] [CrossRef]
- Sampathkumar, A.; Krupinski, P.; Wightman, R.; Milani, P.; Berquand, A.; Boudaoud, A.; Hamant, O.; Jönsson, H.; Meyerowitz, E.M. Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. eLife 2014, 3, e01967. [Google Scholar] [CrossRef]
- Vofely, R.V.; Gallagher, J.; Pisano, G.D.; Bartlett, M.; Braybrook, S.A. Of puzzles and pavements: A quantitative exploration of leaf epidermal cell shape. New Phytol. 2018, 221, 540–552. [Google Scholar] [CrossRef]
- Royer, D.L. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev. Palaeobot. Palynol. 2001, 114, 1–28. [Google Scholar] [CrossRef]
- Ticha, I. Ontogeny of leaf morphology and anatomy. In Photosynthesis During Leaf Development; Šestăk, Z., Ed.; Springer: Drodrecht, The Netherlands, 1985; Volume 11. [Google Scholar]
- Venkateshwar, C.; Rao, S.G.; Kumar, R.S. Epidermal study of medicinal plants with special reference to identification, adulteration and authentification of crude leaf drugs. Ann. Phytomed. 2013, 2, 115–125. [Google Scholar]
- El-Fiki, M.A.; El-Taher, A.M.; EL-Gendy, A.G.; Lila, M.I. Morphological and anatomical studies on some taxa of family Apocynaceae. Al Azhar J. Agric. Res. 2019, 44, 136–147. [Google Scholar]
- Roepke, J.; Salim, V.; Wu, M.; Thamm, A.M.; Murata, J.; Ploss, K.; Boland, W.; De Luca, V. Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. Proc. Natl. Acad. Sci. USA 2010, 107, 15287–15292. [Google Scholar] [CrossRef]
- Guzman, P.; Fernandez, V.; Garcia, M.L.; Khayet, M.; Fernandez, A.; Gil, L. Localization of polysaccharides in isolated and intact cuticles of eucalypt, poplar and pear leaves by enzyme-gold labelling. Plant Physiol. Biochem. 2014, 76, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, V.; Guzman-Delgado, P.; Graca, J.; Santos, S.; Gil, L. Cuticle Structure in Relation to Chemical Composition: Re-assessing the Prevailing Model. Front. Plant Sci. 2016, 7, 427. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.H. Molecular and Evolutionary Mechanisms of Cuticular Wax for Plant Drought Tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef]
- Fjell, I. Anatomy of the xeromorphic leaves of Allamanda neriifolia, Thevetia peruviana and Vinca minor (Apocynaceae). Nord. J. Bot. 1983, 3, 383–392. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, Q.; Xu, C.; Li, J.; Qin, G. Rapid estimation of stomatal density and stomatal area of plant leaves based on object-oriented classification and its ecological trade-off strategy analysis. Forests 2018, 9, 616. [Google Scholar] [CrossRef]
- Beghin, T.; Cope, J.S.; Remagnino, P.; Barman, S. Shape and Texture Based Plant Leaf Classification. In Advanced Concepts for Intelligent Vision Systems; Blanc-Talon, J., Bone, D., Philips, W., Popescu, D., Scheunders, P., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6475. [Google Scholar]
- Jia, C.; Zhang, L.; Wei, X.; Yu, J.; Li, M. Phenotypic Polymorphism of Litsea mollis Hemsl in West Sichuan Province. For. Res. 2015, 28, 844–850. [Google Scholar] [CrossRef]
- Hong, T.; Lin, H.; He, D. Characteristics and correlations of leaf stomata in different Aleurites montana provenances. PLoS ONE 2018, 13, e0208899. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ratkowsky, D.; Hui, C.; Wang, P.; Su, J.; Shi, P. Leaf Fresh Weight Versus Dry Weight: Which is Better for Describing the Scaling Relationship between Leaf Biomass and Leaf Area for Broad-Leaved Plants? Forests 2019, 10, 256. [Google Scholar] [CrossRef]
- O’Connor, S.E. Alkaloid biosynthesis. In Wiley Encyclopedia of Chemical Biology; Begley, T.P., Ed.; John Wiley & Sons, Inc: Hobokend, NJ, USA, 2008; Volume 1, pp. 17–33. [Google Scholar] [CrossRef]
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef]
- Kulwa, F.; Li, C.; Zhao, X.; Cai, B.; Xu, N.; Qi, S.; Chen, S.; Teng, Y. A State-of-the-art Survey for Microorganism Image Segmentation Methods and Future Potential. IEEE Access 2019, 7, 100243–100269. [Google Scholar] [CrossRef]
- Minaee, S.; Boykov, Y.; Porikli, F.; Plaza, A.; Kehtarnavaz, N.; Terzopoulos, D. Image Segmentation Using Deep Learning: A Survey. arXiv 2020, arXiv:2001.05566. [Google Scholar]
- Xue, Y.; Ray, N. Cell Detection in microscopy images with deep convolutional neural network and compressed sensing. arXiv 2017, arXiv:1708.03307. [Google Scholar]
- Xue, Y.; Bigras, G.; Hugh, J.; Ray, N. Training Convolutional Neural Networks and Compressed Sensing End-to-End for Microscopy Cell Detection. IEEE Trans. Med. Imag. 2019, 38, 2632–2641. [Google Scholar] [CrossRef]
- Lundgren, M.R.; Mathers, A.; Baillie, A.L.; Dunn, J.; Wilson, M.J.; Hunt, L.; Pajor, R.; Fradera-Soler, M.; Rolfe, S.; Osborne, C.P.; et al. Mesophyll porosity is modulated by the presence of functional stomata. Nat. Commun. 2019, 10, 2825. [Google Scholar] [CrossRef]
- Fan, J.; Yu, L.; Xu, C. Dual role for autophagy in lipid metabolism in Arabidopsis. Plant Cell 2019, 31, 1598–1613. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.A. Principles and Techniques of Electron Microscopy. Biological Applications, 4th ed.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Liu, C.; Li, Y.; Xu, L.; Chen, Z.; He, N. Variation in leaf morphological, stomatal, and anatomical traits and their relationships in temperate and subtropical forests. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]


| Species | SD * | SI (%) * | SL (µm) | SPI (%) | TD * | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ad | Ab | Ad | Ab | Ad | Ab | Ad | Ab | Ad | |
| V. minor | 0 | 223 | 0 | 25.3 | 0 | 9.57 ± 0.6 | 0 | 2.04 | 31 |
| V. major | 10 | 49 | 4.5 | 23.2 | 14.84 ± 1.1 | 19.75 ± 0.4 | 0.22 | 1.91 | 14 |
| V. major var. variegata | 12 | 68 | 4.2 | 20.9 | 12.33 ± 1 | 12.24 ± 1.4 | 0.18 | 1.01 | 25 |
| V. herbacea | 16 | 60 | 4.4 | 25 | 12.56 ± 1 | 13.05 ± 1.2 | 0.25 | 1.02 | 23 |
| Species | LT (µm) | PTT (µm) | STT (µm) | CTR (%) | SR (%) |
|---|---|---|---|---|---|
| V. minor | 85.47 ± 2.6 | 12.58 ± 0.7 | 52 ± 0.5 | 14.72 | 60.84 |
| V. major | 136.85 ± 1.4 | 0 | 124 ± 0.5 | 0 | 90.61 |
| V. major var. variegata | 97.09 ± 1.5 | 14.57 ± 4.4 | 67.29 ± 1.7 | 15.01 | 69.31 |
| V. herbacea | 133.38 ± 2.2 | 36.66 ± 2.2 | 68.42 ± 3.7 | 27.48 | 51.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciorîță, A.; Tripon, S.C.; Mircea, I.G.; Podar, D.; Barbu-Tudoran, L.; Mircea, C.; Pârvu, M. The Morphological and Anatomical Traits of the Leaf in Representative Vinca Species Observed on Indoor- and Outdoor-Grown Plants. Plants 2021, 10, 622. https://doi.org/10.3390/plants10040622
Ciorîță A, Tripon SC, Mircea IG, Podar D, Barbu-Tudoran L, Mircea C, Pârvu M. The Morphological and Anatomical Traits of the Leaf in Representative Vinca Species Observed on Indoor- and Outdoor-Grown Plants. Plants. 2021; 10(4):622. https://doi.org/10.3390/plants10040622
Chicago/Turabian StyleCiorîță, Alexandra, Septimiu Cassian Tripon, Ioan Gabriel Mircea, Dorina Podar, Lucian Barbu-Tudoran, Cristina Mircea, and Marcel Pârvu. 2021. "The Morphological and Anatomical Traits of the Leaf in Representative Vinca Species Observed on Indoor- and Outdoor-Grown Plants" Plants 10, no. 4: 622. https://doi.org/10.3390/plants10040622
APA StyleCiorîță, A., Tripon, S. C., Mircea, I. G., Podar, D., Barbu-Tudoran, L., Mircea, C., & Pârvu, M. (2021). The Morphological and Anatomical Traits of the Leaf in Representative Vinca Species Observed on Indoor- and Outdoor-Grown Plants. Plants, 10(4), 622. https://doi.org/10.3390/plants10040622