Towards Conservation of the Remarkably High Number of Daisy Trees (Asteraceae) in Mexico
Abstract
1. Introduction
2. Results
2.1. Species List
2.2. Uses of Daisy Trees
2.3. Diversity per Vegetation Type
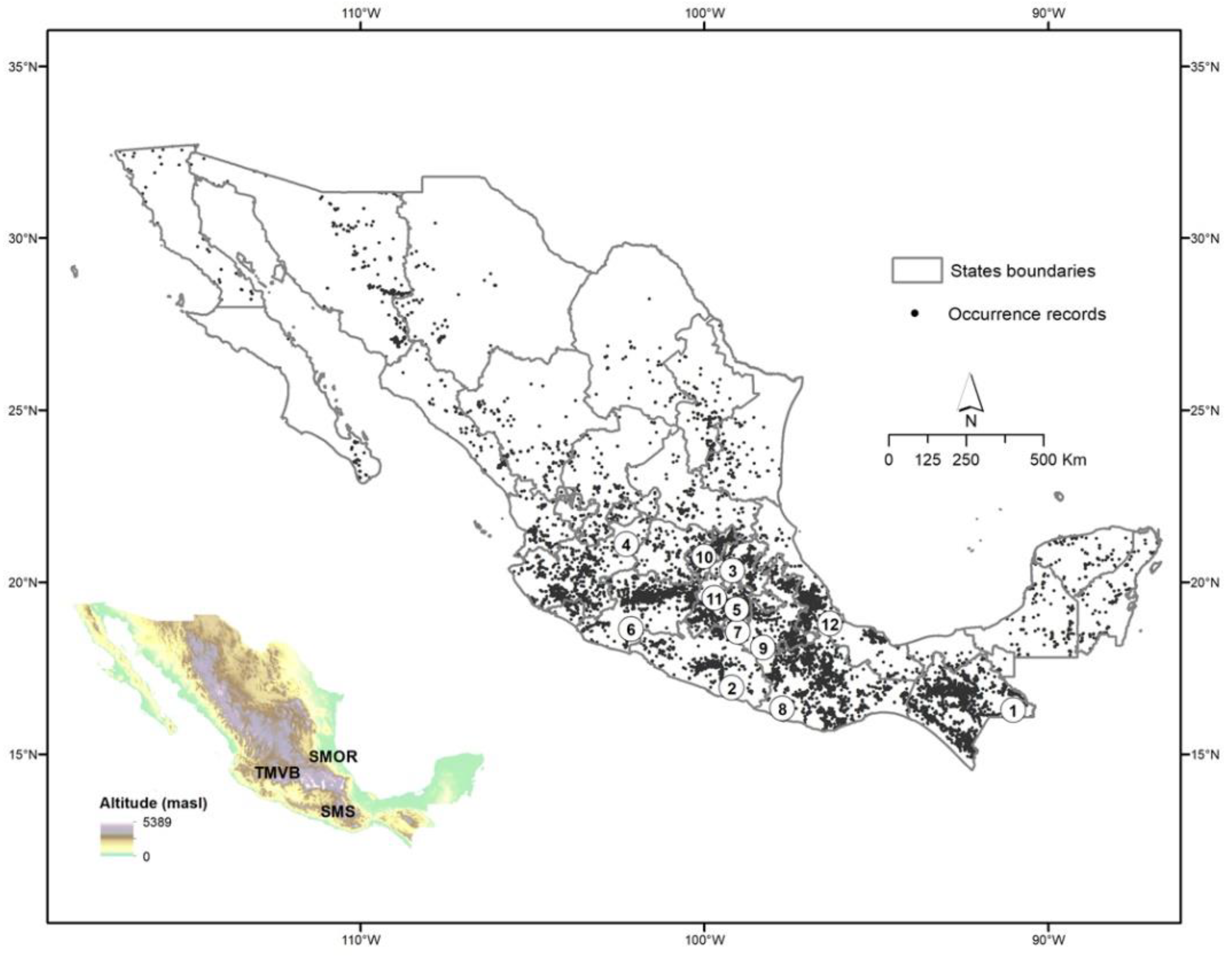
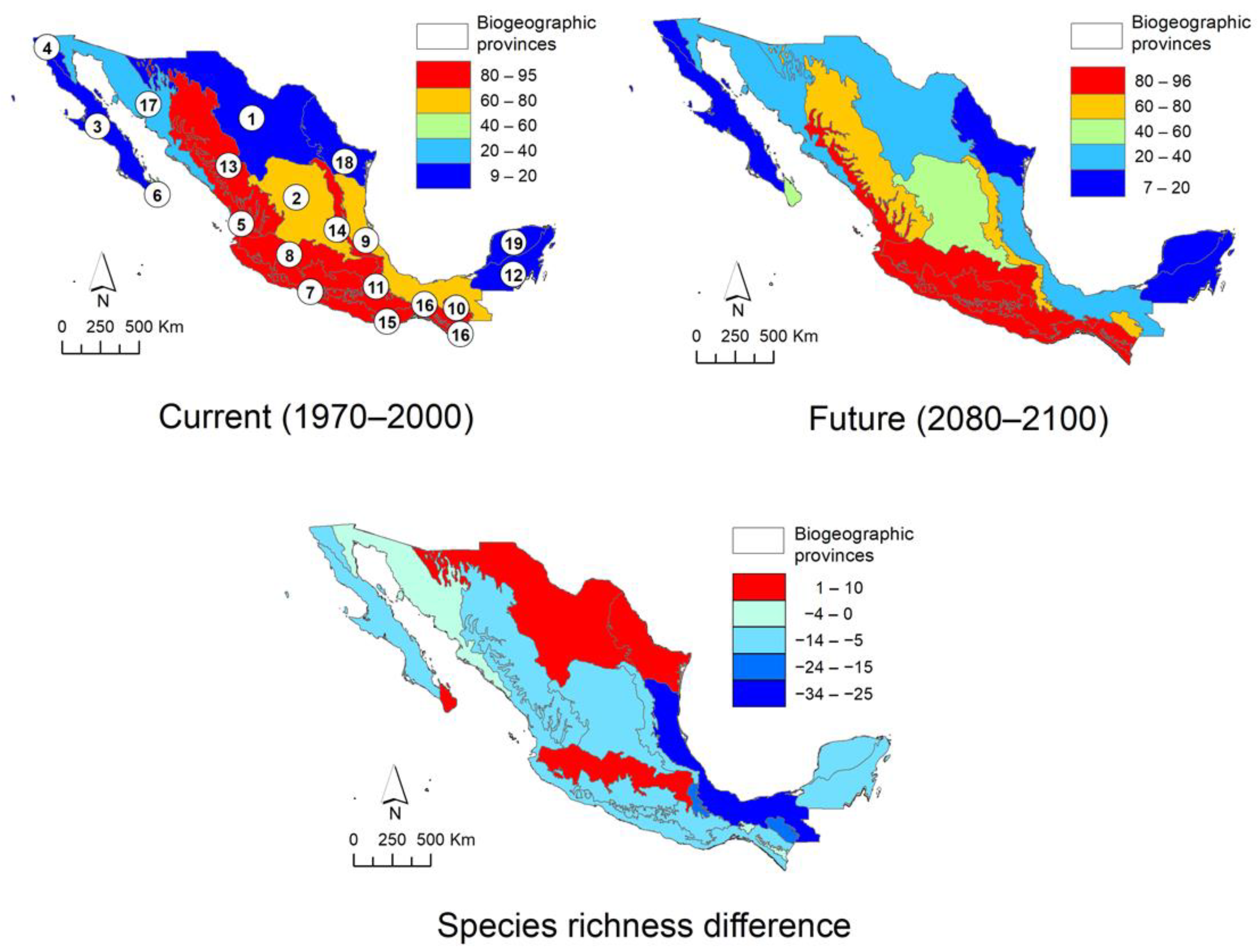
2.4. Distribution in Mexico
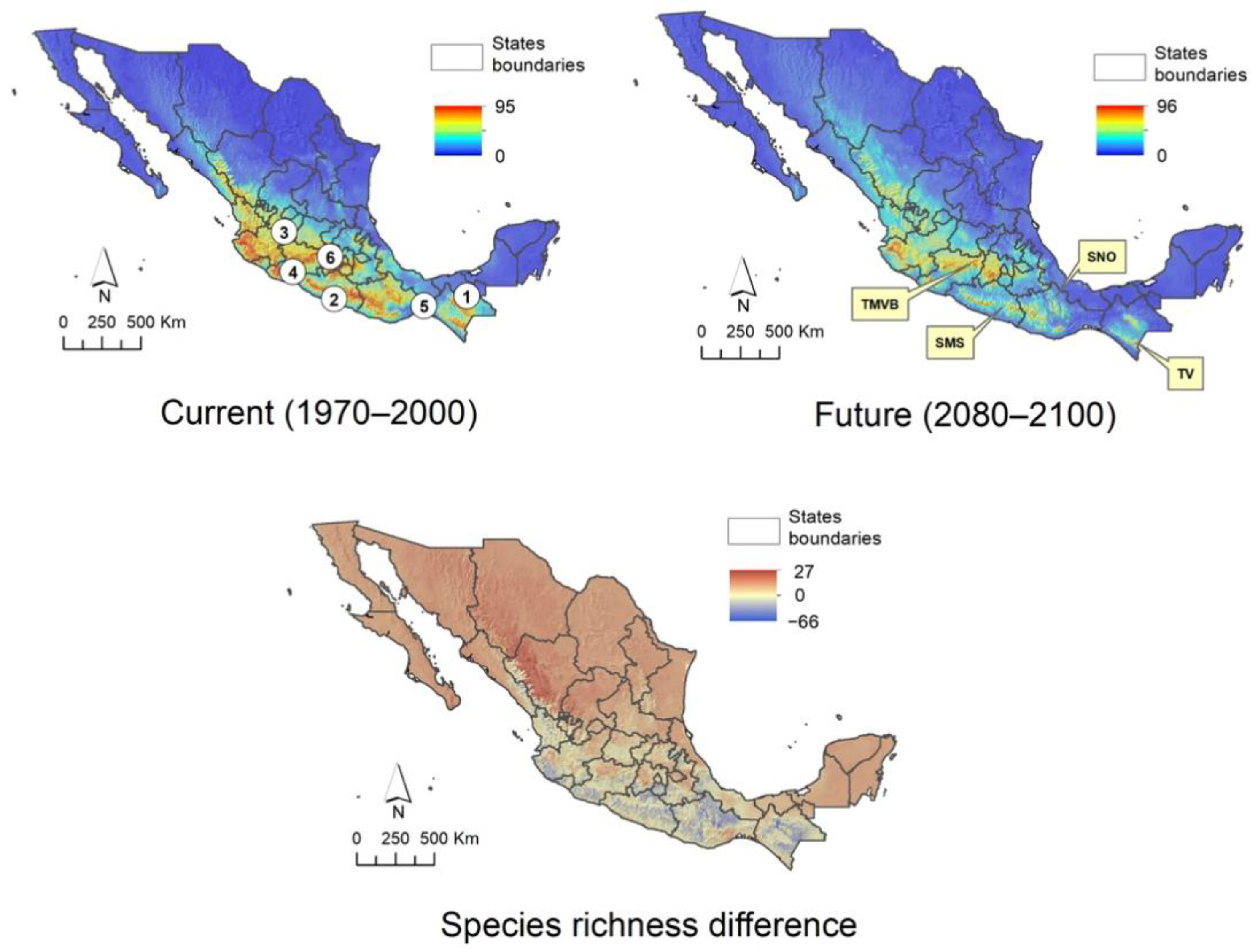
2.5. Climatic Suitability Patterns
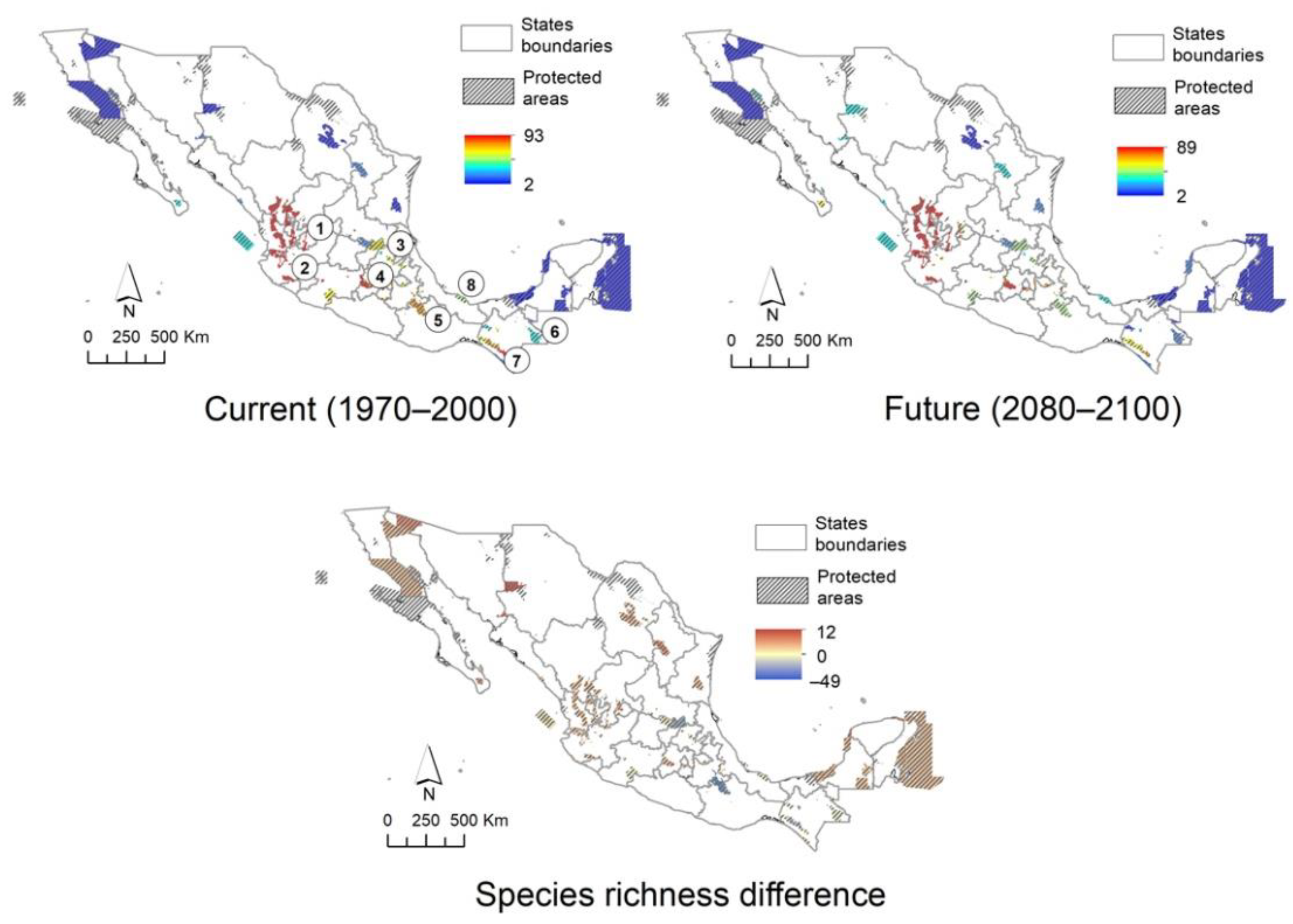
2.6. Protected Area Network and Biogeographic Provinces
2.7. Mexican Daisy Tree Conservation
3. Discussion
3.1. Daisy Tree Diversity in Mexico and Comparison with Other Diverse Areas
3.2. Uses of Mexican Daisy Trees
3.3. Distribution, Including Characterization of Climatic Suitability
3.4. Conservation
4. Materials and Methods
4.1. Study Area
4.2. Compilation of Taxonomic List and Species Information (Use and Habitat)
4.3. Compilation of Species Ocurrence Geographical Data
4.4. Spatial Analyses
4.5. IUCN Red List Assessments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Subfamily | Tribe | Species |
|---|---|---|
| Gochnatioideae | Gochnatieae | Nahuatlea arborescens (Brandegee) V.A. Funk |
| Gochnatieae | Nahuatlea hypoleuca (DC.) V.A. Funk | |
| Gochnatieae | Nahuatlea smithii (B.L. Rob. & Greenm.) V.A. Funk | |
| Vernonioideae | Liabeae | Sinclairia glabra (Hemsl.) Rydb. |
| Vernonieae | Critoniopsis baadii (McVaugh) H. Rob. | |
| Vernonieae | Critoniopsis heydeana (J.M. Coult.) H. Rob. | |
| Vernonieae | Critoniopsis leiocarpa (DC.) H. Rob. | |
| Vernonieae | Critoniopsis macvaughii (S.B. Jones) H. Rob. | |
| Vernonieae | Critoniopsis obtusa (Gleason) H. Rob. | |
| Vernonieae | Critoniopsis salicifolia (DC.) H. Rob. | |
| Vernonieae | Critoniopsis shannonii (J.M. Coult.) H. Rob. | |
| Vernonieae | Critoniopsis tomentosa (Lex.) H. Rob. | |
| Vernonieae | Critoniopsis triflosculosa (Kunth) H. Rob. | |
| Vernonieae | Critoniopsis uniflora (Sch. Bip.) H. Rob. | |
| Vernonieae | Critoniopsis villaregalis (Carvajal) H. Rob. | |
| Vernonieae | Lepidaploa polypleura (S.F. Blake) H. Rob. | |
| Vernonieae | Lepidonia salvinae (Hemsl.) H. Rob. & V.A. Funk | |
| Vernonieae | Lepidonia wendtiana (B.L. Turner) Redonda-Mart. & Villaseñor | |
| Vernonieae | Vernonanthura cordata (Kunth) H. Rob. | |
| Vernonieae | Vernonanthura patens (Kunth) H. Rob. | |
| Asteroideae | Astereae | Baccharis glandulifera G.L. Nesom |
| Astereae | Baccharis heterophylla Kunth | |
| Astereae | Baccharis lancifolia Less. | |
| Astereae | Baccharis salicifolia (Ruiz & Pav.) Pers. subsp. monoica (G.L. Nesom) Joch. Müll. | |
| Bahieae | Peucephyllum schottii A.Gray | |
| Coreopsideae | Dahlia imperialis Roezl ex Ortgies | |
| Coreopsideae | Electranthera mutica (DC.) Mesfin, D.J.Crawford & Pruski | |
| Eupatorieae | Ageratina areolaris (DC.) Gage ex B.L.Turner | |
| Eupatorieae | Ageratina cerifera (McVaugh) R.M. King & H. Rob. | |
| Eupatorieae | Ageratina chiapensis (B.L. Rob.) R.M. King & H. Rob. | |
| Eupatorieae | Ageratina chimalapana B.L. Turner | |
| Eupatorieae | Ageratina cylindrica (McVaugh) R.M. King & H. Rob. | |
| Eupatorieae | Ageratina glabrata (Kunth) R.M. King & H. Rob. | |
| Eupatorieae | Ageratina grandifolia (Regel) R.M. King & H. Rob. | |
| Eupatorieae | Ageratina havanensis (Kunth) R.M. King & H. Rob. | |
| Eupatorieae | Ageratina ligustrina (DC.) R.M. King & H. Rob. | |
| Eupatorieae | Ageratina mairetiana (DC.) R.M.King & H.Rob. | |
| Eupatorieae | Ageratina vernalis (Vatke & Kurtz) R.M. King & H. Rob. | |
| Asteroideae | Eupatorieae | Amolinia heydeana (B.L.Rob.) R.M.King & H.Rob. |
| Eupatorieae | Bartlettina luxii (B.L. Rob.) R.M. King & H. Rob. | |
| Eupatorieae | Bartlettina pansamalensis (B.L. Rob.) R.M. King & H. Rob. | |
| Eupatorieae | Bartlettina pinabetensis (B.L. Rob.) R.M. King & H. Rob. | |
| Eupatorieae | Bartlettina platyphylla (B.L. Rob.) R.M. King & H. Rob. | |
| Eupatorieae | Bartlettina prionophylla (B.L. Rob.) R.M. King & H. Rob. | |
| Eupatorieae | Bartlettina sordida (Less.) R.M. King & H. Rob. | |
| Eupatorieae | Bartlettina tuerckheimii (Klatt) R.M. King & H. Rob. | |
| Eupatorieae | Bartlettina williamsii R.M. King & H. Rob. | |
| Eupatorieae | Chromolaena collina (DC.) R.M. King & H. Rob. | |
| Eupatorieae | Chromolaena glaberrima (DC.) R.M.King & H.Rob. | |
| Eupatorieae | Critonia breedlovei R.M. King & H. Rob. | |
| Eupatorieae | Critonia conzatti (Greenm.) R.M. King & H. Rob. | |
| Eupatorieae | Critonia daleoides DC. | |
| Eupatorieae | Critonia hebebotrya DC. | |
| Eupatorieae | Critonia hospitalis (B.L. Rob.) R.M. King & H. Rob. | |
| Eupatorieae | Critonia iltisii R.M. King & H. Rob. | |
| Eupatorieae | Critonia morifolia (Mill.) R.M. King & H. Rob. | |
| Eupatorieae | Critonia paneroi B.L.Turner | |
| Eupatorieae | Critonia quadrangularis (DC.) R.M. King & H. Rob. | |
| Eupatorieae | Critonia sexangularis (Klatt) R.M. King & H. Rob. | |
| Eupatorieae | Critonia tuxtlae R.M. King & H. Rob. | |
| Eupatorieae | Critoniadelphus microdon (B.L. Rob.) R.M. King & H. Rob. | |
| Eupatorieae | Critoniadelphus nubigenus (Benth.) R.M. King & H. Rob. | |
| Eupatorieae | Koanophyllon albicaule (Sch. Bip. ex Klatt) R.M. King & H. Rob. | |
| Eupatorieae | Koanophyllon galeottii (B.L. Rob.) R.M. King & H. Rob. | |
| Eupatorieae | Koanophyllon palmeri (A. Gray) R.M. King & H. Rob. | |
| Eupatorieae | Koanophyllon pittieri (Klatt) R.M. King & H. Rob. | |
| Eupatorieae | Koanophyllon revealii B.L. Turner | |
| Eupatorieae | Kyrsteniopsis nelsonii (B.L. Rob.) R.M. King & H. Rob. | |
| Eupatorieae | Pachythamnus crassirameus (B.L. Rob.) R.M. King & H.Rob. | |
| Heliantheae | Clibadium arboreum Donn. Sm. | |
| Heliantheae | Clibadium leiocarpum Steetz in Seemann | |
| Heliantheae | Clibadium surinamense L. | |
| Heliantheae | Dendroviguiera puruana (Paray) E.E. Schill. & Panero | |
| Heliantheae | Dendroviguiera quinqueradiata (Cav.) E.E. Schill. & Panero | |
| Heliantheae | Dendroviguiera sphaerocephala (DC.) E.E. Schill. & Panero | |
| Heliantheae | Dendroviguiera splendens (Panero & E.E. Schill.) E.E. Schill. & Panero | |
| Heliantheae | Lagascea palmeri (B.L. Rob.) B.L. Rob. | |
| Heliantheae | Lasianthaea fruticosa (L.) K.M. Becker | |
| Heliantheae | Montanoa andersonii McVaugh | |
| Asteroideae | Heliantheae | Montanoa bipinnatifida (Kunth) K. Koch |
| Heliantheae | Montanoa frutescens (Mairet ex DC.) Hemsl. | |
| Heliantheae | Montanoa grandiflora Alamán ex DC. | |
| Heliantheae | Montanoa hexagona B.L. Rob. & Greenm. | |
| Heliantheae | Montanoa imbricata V.A. Funk | |
| Heliantheae | Montanoa karwinskii DC. | |
| Heliantheae | Montanoa leucantha (Lag.) S.F. Blake | |
| Heliantheae | Montanoa revealii H. Rob. | |
| Heliantheae | Montanoa speciosa DC. | |
| Heliantheae | Montanoa tomentosa Cerv. | |
| Heliantheae | Parthenium fruticosum Less. ex Schltdl. & Cham. | |
| Heliantheae | Parthenium schottii Greenm. ex Millsp. & Chase | |
| Heliantheae | Parthenium tomentosum DC. | |
| Heliantheae | Perymenium grande Hemsl. | |
| Heliantheae | Perymenium hintonii McVaugh | |
| Heliantheae | Podachaenium chiapanum B.L. Turner & Panero | |
| Heliantheae | Podachaenium eminens (Lag.) Sch. Bip. ex Sch. Bip. | |
| Heliantheae | Podachaenium standleyi (Steyerm.) B.L.Turner & Panero | |
| Heliantheae | Rensonia salvadorica S.F. Blake | |
| Heliantheae | Rojasianthe superba Standl. & Steyerm. | |
| Heliantheae | Squamopappus skutchii (S.F. Blake) R.K. Jansen, N.A. Harriman & Urbatsch | |
| Heliantheae | Tetrachyron orizabaensis (Klatt) Wussow & Urbatsch | |
| Heliantheae | Tithonia koelzii McVaugh | |
| Heliantheae | Tithonia longiradiata (Bertol.) S.F. Blake | |
| Heliantheae | Verbesina apleura S.F. Blake | |
| Heliantheae | Verbesina breedlovei B.L. Turner | |
| Heliantheae | Verbesina culminicola McVaugh | |
| Heliantheae | Verbesina fastigiata B.L. Rob. & Greenm. | |
| Heliantheae | Verbesina furfuracea McVaugh | |
| Heliantheae | Verbesina guatemalensis B.L. Rob. & Greenm. | |
| Heliantheae | Verbesina hypargyrea B.L. Rob. & Greenm. | |
| Heliantheae | Verbesina hypoglauca Sch. Bip. ex Klatt | |
| Heliantheae | Verbesina klattii B.L. Rob. & Greenm. | |
| Heliantheae | Verbesina lanata B.L. Rob. & Greenm. | |
| Heliantheae | Verbesina montanoifolia B.L. Rob. & Greenm. | |
| Heliantheae | Verbesina oncophora B.L. Rob. & Seaton | |
| Heliantheae | Verbesina oligantha B.L. Rob. | |
| Heliantheae | Verbesina ovatifolia A. Gray | |
| Heliantheae | Verbesina perymenioides Sch. Bip. ex Klatt | |
| Heliantheae | Verbesina platyptera Sch. Bip. ex Klatt | |
| Heliantheae | Verbesina sousae J.J. Fay | |
| Asteroideae | Heliantheae | Verbesina sphaerocephala A. Gray |
| Heliantheae | Verbesina turbacensis Kunth | |
| Heliantheae | Verbesina villaregalis McVuagh | |
| Heliantheae | Wamalchitamia aurantiaca (Klatt) Strother | |
| Inuleae | Pluchea sericea (Nutt.) Coville | |
| Millerieae | Desmanthodium perfoliatum Benth. | |
| Millerieae | Rumfordia floribunda DC. | |
| Millerieae | Schistocarpha longiligula Rydb. | |
| Neurolaeneae | Neurolaena macrophylla Greenm. | |
| Senecioneae | Barkleyanthus salicifolius (Kunth) H. Rob. & Brettell | |
| Senecioneae | Lepidospartum squamatum (A. Gray) A. Gray | |
| Senecioneae | Mixtecalia teitaensis Redonda-Mart., García-Mend., & D. Sandoval | |
| Senecioneae | Pittocaulon filare (McVaugh) H. Rob. & Brettell | |
| Senecioneae | Pittocaulon praecox (Cav.) H. Rob. & Brettell | |
| Senecioneae | Pittocaulon velatum (Greenm.) H. Rob. & Brettell | |
| Senecioneae | Roldana albonervia (Greenm.) H. Rob. & Brettell | |
| Senecioneae | Roldana angulifolia (DC.) H. Rob. & Brettell | |
| Senecioneae | Roldana barba-johannis (DC.) H. Rob. & Brettell | |
| Senecioneae | Roldana eriophylla (Greenm.) H. Rob. & Brettell | |
| Senecioneae | Roldana gentryi H. Rob. & Brettell | |
| Senecioneae | Roldana greenmanii H. Rob. & Brettell | |
| Senecioneae | Roldana neogibsonii (B.L. Turner) B.L. Turner | |
| Senecioneae | Roldana oaxacana (Hemsl.) H.Rob. & Brettell | |
| Senecioneae | Roldana schaffneri (Sch. Bip. ex Klatt) H. Rob. & Brettell | |
| Senecioneae | Telanthophora cobanensis (J.M. Coult.) H. Rob. & Brettell | |
| Senecioneae | Telanthophora grandifolia (Less.) H.Rob. & Brettell | |
| Senecioneae | Telanthophora jaliscana H. Rob. & Brettell | |
| Senecioneae | Telanthophora standleyi (Greenm.) H. Rob. & Brettell | |
| Senecioneae | Telanthophora uspantanensis (J.M. Coult.) H. Rob. & Brettell |
Appendix B
| ID | Species | I | II | III | IV | V | VI | VII | VIII | IX | X |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ageratina areolaris (DC.) Gage ex B.L.Turner | 552 | 538 | 439,361 | 258,128 | 37,770 | 167,455 | 184,977 | 9.0 | 39.8 | 43.9 |
| 2 | Ageratina chiapensis (B.L. Rob.) R.M. King & H. Rob. | 97 | 94 | 364,784 | 246,875 | 3687 | 10,875 | 237,846 | 1.0 | 3.0 | 65.2 |
| 3 | Ageratina grandifolia (Regel) R.M. King & H. Rob. | 68 | 68 | 412,400 | 139,148 | 1401 | 110,475 | 221,618 | 0.3 | 26.8 | 53.7 |
| 4 | Ageratina ligustrina (DC.) R.M. King & H. Rob. | 824 | 716 | 625,473 | 241,772 | 2746 | 191,062 | 309,808 | 0.4 | 30.5 | 49.5 |
| 5 | Ageratina mairetiana (DC.) R.M.King & H.Rob. | 577 | 567 | 387,878 | 198,528 | 12,833 | 146,160 | 165,799 | 3.3 | 37.7 | 42.7 |
| 6 | Ageratina vernalis (Vatke & Kurtz) R.M. King & H. Rob. | 106 | 102 | 557,923 | 183,660 | 3409 | 142,534 | 301,187 | 0.6 | 25.5 | 54.0 |
| 7 | Baccharis heterophylla Kunth | 502 | 499 | 650,100 | 444,872 | 45,738 | 307,814 | 211,270 | 7.0 | 47.3 | 32.5 |
| 8 | Baccharis lancifolia Less. | 71 | 58 | 372,699 | 119,928 | 4076 | 92,403 | 206,874 | 1.1 | 24.8 | 55.5 |
| 9 | Baccharis monoica G.L. Nesom | 146 | 55 | 336,151 | 128,384 | 3697 | 101,109 | 172,325 | 1.1 | 30.1 | 51.3 |
| 10 | Barkleyanthus salicifolius (Kunth) H. Rob. & Brettell | 416 | 411 | 871,464 | 563,600 | 41,211 | 402,173 | 283,498 | 4.7 | 46.1 | 32.5 |
| 11 | Bartlettina platyphylla (B.L. Rob.) R.M. King & H. Rob. | 77 | 37 | 279,330 | 118,849 | 3467 | 92,476 | 132,519 | 1.2 | 33.1 | 47.4 |
| 12 | Bartlettina sordida (Less.) R.M. King & H. Rob. | 179 | 176 | 311,664 | 64,849 | 258 | 51,960 | 199,004 | 0.1 | 16.7 | 63.9 |
| 13 | Bartlettina tuerckheimii (Klatt) R.M. King & H. Rob. | 146 | 132 | 420,782 | 100,855 | 95 | 81,591 | 258,139 | 0.0 | 19.4 | 61.3 |
| 14 | Chromolaena collina (DC.) R.M. King & H. Rob. | 742 | 608 | 862,983 | 918,322 | 189,840 | 531,576 | 156,402 | 22.0 | 61.6 | 18.1 |
| 15 | Chromolaena glaberrima (DC.) R.M.King & H.Rob. | 227 | 97 | 386,145 | 174,246 | 5085 | 136,090 | 176,815 | 1.3 | 35.2 | 45.8 |
| 16 | Clibadium arboreum Donn. Sm. | 509 | 443 | 312,076 | 118,778 | 9241 | 87,285 | 164,964 | 3.0 | 28.0 | 52.9 |
| 17 | Clibadium surinamense L. | 359 | 9 | 17,074 | 2561 | 1116 | 978 | 12,947 | 6.5 | 5.7 | 75.8 |
| 18 | Critonia daleoides DC. | 417 | 228 | 534,699 | 215,084 | 11,819 | 160,473 | 270,312 | 2.2 | 30.0 | 50.6 |
| 19 | Critonia hebebotrya DC. | 121 | 95 | 725,959 | 587,158 | 37,854 | 430,086 | 153,176 | 5.2 | 59.2 | 21.1 |
| 20 | Critonia hospitalis (B.L. Rob.) R.M. King & H. Rob. | 78 | 75 | 300,071 | 64,605 | 241 | 52,128 | 190,791 | 0.1 | 17.4 | 63.6 |
| 21 | Critonia morifolia (Mill.) R.M. King & H. Rob. | 802 | 301 | 532,910 | 281,050 | 36,494 | 188,731 | 241,060 | 6.8 | 35.4 | 45.2 |
| 22 | Critonia quadrangularis (DC.) R.M. King & H. Rob. | 147 | 112 | 823,247 | 1,190,328 | 288,440 | 648,289 | 5097 | 35.0 | 78.7 | 0.6 |
| 23 | Critonia sexangularis (Klatt) R.M. King & H. Rob. | 178 | 31 | 77,991 | 11,474 | 4005 | 5362 | 58,177 | 5.1 | 6.9 | 74.6 |
| 24 | Critoniadelphus nubigenus (Benth.) R.M. King & H. Rob. | 57 | 37 | 313,638 | 89,891 | 0 | 73,133 | 182,011 | 0.0 | 23.3 | 58.0 |
| 25 | Critoniopsis leiocarpa (DC.) H. Rob. | 315 | 221 | 311,807 | 103,670 | 1117 | 82,904 | 170,099 | 0.4 | 26.6 | 54.6 |
| 26 | Critoniopsis obtusa (Gleason) H. Rob. | 118 | 118 | 461,220 | 360,251 | 54,851 | 230,966 | 133,515 | 11.9 | 50.1 | 28.9 |
| 27 | Critoniopsis salicifolia (DC.) H. Rob. | 115 | 115 | 587,539 | 563,699 | 46,612 | 404,981 | 68,241 | 7.9 | 68.9 | 11.6 |
| 28 | Critoniopsis tomentosa (Lex.) H. Rob. | 225 | 225 | 426,071 | 293,206 | 27,519 | 206,722 | 136,001 | 6.5 | 48.5 | 31.9 |
| 29 | Critoniopsis uniflora (Sch. Bip.) H. Rob. | 170 | 267 | 686,446 | 717,520 | 121,094 | 443,992 | 103,775 | 17.6 | 64.7 | 15.1 |
| 30 | Dahlia imperialis Roezl ex Ortgies | 143 | 197 | 536,126 | 473,635 | 33,319 | 345,751 | 84,478 | 6.2 | 64.5 | 15.8 |
| 31 | Dendroviguiera quinqueradiata (Cav.) E.E. Schill. & Panero | 114 | 42 | 218,168 | 33,681 | 0 | 27,472 | 150,172 | 0.0 | 12.6 | 68.8 |
| 32 | Dendroviguiera sphaerocephala (DC.) E.E. Schill. & Panero | 110 | 114 | 182,080 | 173,000 | 36,608 | 101,219 | 44,420 | 20.1 | 55.6 | 24.4 |
| 33 | Desmanthodium perfoliatum Benth. | 94 | 110 | 328,888 | 198,100 | 5352 | 154,402 | 111,546 | 1.6 | 46.9 | 33.9 |
| 34 | Electranthera mutica (DC.) Mesfin, D.J.Crawford & Pruski | 730 | 94 | 264,289 | 105,968 | 4255 | 81,480 | 132,739 | 1.6 | 30.8 | 50.2 |
| 35 | Critoniopsis triflosculosa (Kunth) H. Rob. | 364 | 679 | 540,285 | 336,732 | 18,823 | 252,324 | 183,402 | 3.5 | 46.7 | 33.9 |
| 36 | Koanophyllon albicaule (Sch. Bip. ex Klatt) R.M. King & H. Rob. | 501 | 407 | 694,526 | 919,970 | 189,819 | 548,328 | 9384 | 27.3 | 78.9 | 1.4 |
| 37 | Koanophyllon galeottii (B.L. Rob.) R.M. King & H. Rob. | 78 | 67 | 481,665 | 331,545 | 7205 | 262,540 | 128,231 | 1.5 | 54.5 | 26.6 |
| 38 | Koanophyllon palmeri (A. Gray) R.M. King & H. Rob. | 93 | 93 | 1,131,471 | 1,447,606 | 263,411 | 867,251 | 17,763 | 23.3 | 76.6 | 1.6 |
| 39 | Koanophyllon pittieri (Klatt) R.M. King & H. Rob. | 406 | 127 | 333,021 | 152,754 | 10,319 | 113,018 | 157,329 | 3.1 | 33.9 | 47.2 |
| 40 | Lasianthaea fruticosa (L.) K.M. Becker | 878 | 467 | 972,478 | 984,137 | 227,609 | 550,550 | 228,008 | 23.4 | 56.6 | 23.4 |
| 41 | Lepidaploa polypleura (S.F. Blake) H. Rob. | 122 | 121 | 247,682 | 36,979 | 0 | 30,114 | 171,540 | 0.0 | 12.2 | 69.3 |
| 42 | Lepidospartum squamatum (A. Gray) A. Gray | 334 | 27 | 132,657 | 74,277 | 0 | 54,872 | 43,738 | 0.0 | 41.4 | 33.0 |
| 43 | Montanoa frutescens (Mairet ex DC.) Hemsl. | 254 | 254 | 565,398 | 375,408 | 39,691 | 259,895 | 195,086 | 7.0 | 46.0 | 34.5 |
| 44 | Montanoa hexagona B.L. Rob. & Greenm. | 50 | 45 | 294,889 | 68,068 | 0 | 55,131 | 184,037 | 0.0 | 18.7 | 62.4 |
| 45 | Montanoa karwinskii DC. | 67 | 67 | 579,350 | 565,442 | 99,152 | 349,899 | 115,859 | 17.1 | 60.4 | 20.0 |
| 46 | Montanoa leucantha (Lag.) S.F. Blake | 826 | 826 | 1,004,151 | 833,182 | 63,699 | 588,544 | 202,978 | 6.3 | 58.6 | 20.2 |
| 47 | Montanoa revealii H. Rob. | 51 | 51 | 90,690 | 55,06 | 0 | 4477 | 69,263 | 0.0 | 4.9 | 76.4 |
| 48 | Montanoa speciosa DC. | 93 | 93 | 1,142,963 | 1,139,462 | 70,460 | 839,795 | 74,223 | 6.2 | 73.5 | 6.5 |
| 49 | Montanoa tomentosa Cerv. | 714 | 611 | 741,882 | 692,138 | 59,744 | 493,406 | 102,033 | 8.1 | 66.5 | 13.8 |
| 50 | Nahuatlea arborescens (Brandegee) V.A. Funk | 64 | 59 | 14,120 | 36,404 | 17,276 | 11,072 | 0 | 122.4 | 78.4 | 0.0 |
| 51 | Nahuatlea hypoleuca (DC.) V.A. Funk | 185 | 179 | 695,788 | 568,631 | 100,275 | 341,675 | 196,511 | 14.4 | 49.1 | 28.2 |
| 52 | Parthenium fruticosum Less. ex Schltdl. & Cham. | 58 | 58 | 268,619 | 625,789 | 309,243 | 174,803 | 36,718 | 115.1 | 65.1 | 13.7 |
| 53 | Parthenium tomentosum DC. | 302 | 302 | 619,554 | 1,277,564 | 529,608 | 468,614 | 19,320 | 85.5 | 75.6 | 3.1 |
| 54 | Perymenium grande Hemsl. | 329 | 176 | 454,542 | 224,368 | 743 | 181,786 | 187,402 | 0.2 | 40.0 | 41.2 |
| 55 | Peucephyllum schottii A. Gray | 237 | 15 | 164,105 | 182,993 | 17,727 | 118,339 | 3981 | 10.8 | 72.1 | 2.4 |
| 56 | Pittocaulon praecox (Cav.) H. Rob. & Brettell | 496 | 496 | 592,825 | 470,700 | 22,228 | 353,993 | 120,575 | 3.7 | 59.7 | 20.3 |
| 57 | Pittocaulon velatum (Greenm.) H. Rob. & Brettell | 163 | 162 | 509,460 | 637,622 | 138,987 | 369,228 | 40,493 | 27.3 | 72.5 | 7.9 |
| 58 | Pluchea sericea (Nutt.) Coville | 138 | 46 | 231,437 | 225,132 | 9077 | 158,609 | 14,129 | 3.9 | 68.5 | 6.1 |
| 59 | Podachaenium eminens (Lag.) Sch. Bip. ex Sch. Bip. | 683 | 564 | 619,757 | 294,788 | 7450 | 229,798 | 270,875 | 1.2 | 37.1 | 43.7 |
| 60 | Rensonia salvadorica S.F. Blake | 96 | 52 | 126,496 | 22,543 | 0 | 18,456 | 84,920 | 0.0 | 14.6 | 67.1 |
| 61 | Roldana albonervia (Greenm.) H. Rob. & Brettell | 441 | 441 | 448,148 | 251,806 | 10,237 | 191,290 | 168,160 | 2.3 | 42.7 | 37.5 |
| 62 | Roldana angulifolia (DC.) H. Rob. & Brettell | 767 | 767 | 587,170 | 258,071 | 1712 | 204,876 | 264,237 | 0.3 | 34.9 | 45.0 |
| 63 | Roldana barba-johannis (DC.) H. Rob. & Brettell | 821 | 817 | 550,253 | 228,428 | 1797 | 181,755 | 260,052 | 0.3 | 33.0 | 47.3 |
| 64 | Roldana eriophylla (Greenm.) H. Rob. & Brettell | 138 | 138 | 500,630 | 608,966 | 94,727 | 396,654 | 7695 | 18.9 | 79.2 | 1.5 |
| 65 | Roldana gentryi H. Rob. & Brettell | 56 | 56 | 690,226 | 475,248 | 27,835 | 344,349 | 200,325 | 4.0 | 49.9 | 29.0 |
| 66 | Roldana schaffneri (Sch. Bip. ex Klatt) H. Rob. & Brettell | 264 | 209 | 436,186 | 118,254 | 1102 | 94,159 | 257,213 | 0.3 | 21.6 | 59.0 |
| 67 | Rumfordia floribunda DC. | 434 | 434 | 376,503 | 147,338 | 5695 | 112,434 | 190,713 | 1.5 | 29.9 | 50.7 |
| 68 | Schistocarpha longiligula Rydb. | 101 | 74 | 81,642 | 7568 | 494 | 5677 | 60,918 | 0.6 | 7.0 | 74.6 |
| 69 | Sinclairia glabra (Hemsl.) Rydb. | 495 | 390 | 522,077 | 402,878 | 53,658 | 267,867 | 153,473 | 10.3 | 51.3 | 29.4 |
| 70 | Telanthophora cobanensis (J.M. Coult.) H. Rob. & Brettell | 179 | 156 | 224,845 | 41,074 | 0 | 33,393 | 149,708 | 0.0 | 14.9 | 66.6 |
| 71 | Telanthophora grandifolia (Less.) H.Rob. & Brettell | 801 | 602 | 521,994 | 217,796 | 4899 | 169,326 | 249,901 | 0.9 | 32.4 | 47.9 |
| 72 | Telanthophora uspantanensis (J.M. Coult.) H. Rob. & Brettell | 160 | 157 | 375,618 | 68,252 | 0 | 55,137 | 248,274 | 0.0 | 14.7 | 66.1 |
| 73 | Tithonia longiradiata (Bertol.) S.F. Blake | 341 | 256 | 187,591 | 35,500 | 78 | 28,755 | 123,464 | 0.0 | 15.3 | 65.8 |
| 74 | Verbesina fastigiata B.L. Rob. & Greenm. | 343 | 343 | 526,821 | 363,759 | 47,946 | 242,226 | 182,200 | 9.1 | 46.0 | 34.6 |
| 75 | Verbesina guatemalensis B.L. Rob. & Greenm. | 88 | 9 | 144,376 | 43,261 | 0 | 35,374 | 82,631 | 0.0 | 24.5 | 57.2 |
| 76 | Verbesina hypargyrea B.L. Rob. & Greenm. | 58 | 50 | 298,605 | 131,918 | 22,497 | 84,905 | 157,747 | 7.5 | 28.4 | 52.8 |
| 77 | Verbesina hypoglauca Sch. Bip. ex Klatt | 149 | 141 | 488,252 | 171,872 | 12,271 | 125,163 | 265,497 | 2.5 | 25.6 | 54.4 |
| 78 | Verbesina klattii B.L. Rob. & Greenm. | 155 | 155 | 266,302 | 108,800 | 9019 | 77,940 | 136,101 | 3.4 | 29.3 | 51.1 |
| 79 | Verbesina lanata B.L. Rob. & Greenm. | 63 | 32 | 155,973 | 24,034 | 4323 | 15,346 | 111,813 | 2.8 | 9.8 | 71.7 |
| 80 | Verbesina montanoifolia B.L. Rob. & Greenm. | 80 | 80 | 504,621 | 339,200 | 24,377 | 244,898 | 157,276 | 4.8 | 48.5 | 31.2 |
| 81 | Verbesina oligantha B.L. Rob. | 91 | 90 | 437,165 | 277,890 | 5494 | 218,217 | 135,095 | 1.3 | 49.9 | 30.9 |
| 82 | Verbesina oncophora B.L. Rob. & Seaton | 292 | 292 | 431,477 | 196,816 | 6150 | 151,754 | 195,615 | 1.4 | 35.2 | 45.3 |
| 83 | Verbesina perymenioides Sch. Bip. ex Klatt | 215 | 203 | 463,276 | 279,876 | 2385 | 224,930 | 151,418 | 0.5 | 48.6 | 32.7 |
| 84 | Verbesina turbacensis Kunth | 658 | 396 | 482,089 | 157,618 | 6000 | 121,700 | 269,119 | 1.2 | 25.2 | 55.8 |
| 85 | Vernonanthura cordata (Kunth) H. Rob. | 221 | 220 | 472,583 | 382,946 | 25,378 | 281,877 | 99,033 | 5.4 | 59.6 | 21.0 |
| 86 | Vernonanthura patens (Kunth) H. Rob. | 748 | 206 | 360,749 | 56,005 | 786 | 44,745 | 248,219 | 0.2 | 12.4 | 68.8 |
References
- Villaseñor, J.L. Checklist of the native vascular plants of Mexico. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Ulloa Ulloa, C.; Acevedo-Rodríguez, P.; Beck, S.; Belgrano, M.J.; Bernal, R.; Berry, P.E.; Brako, L.; Celis, M.; Davidse, G.; Forzza, R.C.; et al. An integrated assessment of the vascular plant species of the Americas. Science 2017, 358, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, J.L. Diversidad y distribución de la familia Asteraceae en México. Bot. Sci. 2018, 96, 332–358. [Google Scholar] [CrossRef]
- FNA, Flora of North America. Asteraceae. Available online: http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=10074#:~:text=With%20418%20genera%20and%202413,alpine%20habitats%20to%20salt%20marshes (accessed on 1 February 2021).
- Roque, N.; Magalhães Teles, A.; Naoki Nakajima, J. (Eds.) A Família Asteraceae no Brasil Clasifição e Diversidade; Editora da Universidade Federal da Bahia: Salvador, Brazil, 2017; p. 260. [Google Scholar] [CrossRef]
- Flora of China. Asteraceae. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=10074 (accessed on 28 January 2021).
- Avila, F.; Funk, V.A.; Diazgranados, M.; Díaz-Piedrahíta, S.; Vargas, O. Asteraceae. In Catálogo de Plantas y Líquenes de Colombia; Bernal, R., Gradstein, S.R., Celis, M., Eds.; Instituto de Ciencias Naturales, Universidad Nacional de Colombia: Bogotá, Colombia; Available online: http://catalogoplantasdecolombia.unal.edu.co/es/resultados/familia/Asteraceae/ (accessed on 2 February 2021).
- Barriga, P.; Toasa, G.; Montúfar, R.; Tye, A. Asteraceae. In Libro Rojo de Plantas Endémicas del Ecuador; León-Yáñez, S., Valencia, R., Pitmam, N., Endara, L., Ulloa Ulloa, C., Navarrete, H., Eds.; Publicaciones del Herbario QCA, Pontificia Universidad Católica del Ecuador: Quito, Ecuador; Available online: https://bioweb.bio/floraweb/librorojo/ListaEspeciesPorFamilia/500047 (accessed on 29 January 2021).
- Funk, V.A.; Randall, J.B.; Keeley, S.C.; Chan, R.; Watson, L.; Gemeinholzer, B.; Schilling, E.; Panero, J.L.; Baldwin, B.G.; García-Jacas, N.; et al. Everywhere but Antarctica: Using a supertree to understand the diversity and distribution of the Compositae. Biol. Skrif. 2005, 55, 343–373. [Google Scholar]
- Funk, V.A.; Susanna, A.; Stuessy, T.F.; Robinson, H. Classification of Compositae. In Systematics, Evolution and Biogeography of the Compositae; Funk, V.A., Susanna, A., Stuessy, T.F., Bayer, R.J., Eds.; IAPT: Vienna, Austria, 2009; pp. 171–189. [Google Scholar]
- Crawford, D.J.; Lowrey, T.K.; Anderson, G.J.; Bernardello, G.; Santos-Guerra, A.; Stuessy, T.F. Genetic diversity in Asteraceae endemic to oceanic islands: Baker’s Law and polyploidy. In Systematics, Evolution and Biogeography of the Compositae; Funk, V.A., Susanna, A., Stuessy, T.F., Bayer, R.J., Eds.; IAPT: Vienna, Austria, 2009; pp. 139–151. [Google Scholar]
- Standley, P.C. Trees and shrubs of Mexico (Bignoniaceae-Asteraceae). Constr. U. S. Natl. Herb. 1926, 23, 1401–1641. [Google Scholar]
- Turner, B.L. The comps of Mexico. A systematic account of the Family Asteraceae, Vol. 1 Eupatorieae. Phytol. Mem. 1997, 11, 1–272. [Google Scholar]
- Strother, J.L. Compositae-Heliantheae s.l. In Flora of Chiapas; Daniel, T.F., Ed.; California Academy of Sciences: San Francisco, CA, USA, 1999; Volume 5, pp. 1–232. [Google Scholar]
- Cabrera, A.L. Revisión del género Gochnatia (Compositae). Rev. Mus. La Plata Nueva Ser. 1971, 12, 1–160. [Google Scholar]
- Robinson, H.; Brettell, R.D. Studies in the Senecioneae (Asteraceae). I. A new genus Pittocaulon. Phytologia 1973, 26, 451–453. [Google Scholar]
- Robinson, H.; Brettell, R.D. Studies in the Senecioneae (Asteraceae). V. The genera Psacaliopsis, Barkleyanthus, Telantophora and Roldana. Phytologia 1974, 27, 402–439. [Google Scholar]
- Funk, V.A. The Systematics of Montanoa (Asteraceae, Heliantheae). Mem. N. Y. Bot. Gard. 1982, 36, 1–133. [Google Scholar]
- Turner, B.L. A recension of Mexican species of Roldana (Asteraceae: Senecioneae). Phytologia 2005, 87, 204–249. [Google Scholar]
- Funston, A.M. Taxonomic revision of Roldana (Asteraceae: Senecioneae), a genus of Southwestern U.S.A., Mexico, and Central America. Ann. Missouri Bot. Gard. 2008, 95, 282–337. [Google Scholar] [CrossRef]
- Funk, V.A.; Sancho, G.; Roque, N. Nahuatlea: A new genus of Compositae (Gochnatieae) from North America. Phytokeys 2017, 91, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.B. Revision of Vernonia section Eremosis (Compositae) in North America. Brittonia 1973, 25, 86–115. [Google Scholar] [CrossRef]
- Turner, B.L. Revision of Verbesina sect. Pseudomontanoa (Asteraceae). Plant Syst. Evol. 1985, 150, 237–262. [Google Scholar] [CrossRef]
- Ricker, M.; Hernández, H.M.; Sousa, M.; Ochoterena, H. Tree and tree-like species of Mexico: Asteraceae, Leguminosae, and Rubiaceae. Rev. Mex. Biodivers. 2013, 84, 439–470. [Google Scholar] [CrossRef]
- Beech, E.; Rivers, M.; Oldfield, S.; Smith, P. GlobalTreeSearch: The first complete global database of tree species and country distribution. J. Sustain. For. 2017, 36, 454–489. [Google Scholar] [CrossRef]
- Téllez, O.; Mattana, E.; Diazgranados, M.; Kühn, N.; Castillo-Lorenzo, E.; Lira, R.; Montes-Leyva, L.; Flores Ortiz, C.M.; Way, M.; Dávila, P.; et al. Native trees of Mexico: Diversity, distribution, uses and conservation. PeerJ 2020, 8, e9898. [Google Scholar] [CrossRef]
- Sistema Nacional de Información Sobre la Biodiversidad de México (SNIB), Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad (CONABIO). Available online: https://www.snib.mx/ (accessed on 19 January 2021).
- Susanna, A.; Baldwin, B.G.; Bayer, R.J.; Bonifacio, J.M.; García-Jacas, N.; Keeley, S.C.; Mandel, J.R.; Ortiz, S.; Robinson, H.; Stuessy, T.F. The classification of the Compositae: A tribute to Vicki Ann Funk (1947–2019). Taxon 2020, 69, 807–814. [Google Scholar] [CrossRef]
- DGRU, Dirección General de Repositorios Universitartios, Universidad Nacional Autónoma de México. Portal de Datos Abiertos UNAM, Colecciones Universitarias, Herbario Nacional de México (MEXU). Available online: https://datosabiertos.unam.mx/biodiversidad/ (accessed on 27 November 2020).
- Diario Oficial de la Federación, Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección Ambiental-Especies Nativas de México de Flora y Faina Silvestres-Categorías de Riesgo y Especificaciones para su Inclusion, Exclusion o Cambio-Lista de Especies en Riesgo. Available online: https://dof.gob.mx/nota_detalle_popup.php?codigo=5173091 (accessed on 26 January 2021).
- CITES, Convención Sobre el Comercio Internacional de Especies Amenazadas de Fauna y Flora Silvestres. Apéndices I, II y III en vigor a Partir del 26 de Noviembre de 2019. Available online: https://cites.org/sites/default/files/esp/app/2019/S-Appendices-2019-11-26.pdf (accessed on 26 January 2021).
- Hempen, C.H.; Fischer, T. A Materia Medica for Chinese Medicine; Churchill Livingstone: London, UK, 2009; pp. 466–513. [Google Scholar] [CrossRef]
- BGCI. GlobalTreeSearch Online Database; Botanic Gardens Conservation International: Richmond, UK, 2017. [Google Scholar]
- CASTUERA-OLIVEIRA, L.; OLIVEIRA-FILHO, A.T.; EISENLOHR, P.V. Emerging hotspots of tree in Brazil. Acta Bot. Bras. 2020, 34, 117–134. [Google Scholar] [CrossRef]
- Ter Steege, H.; Vaessen, R.W.; Cárdenas-López, D.; Sabtier, D.; Antonelli, A.; Mota de Oliveira, S.; Pitman, N.C.A.; Møller Jørgensen, P. The discovery of the Amazonian tree flora with an updated checklist of all know tree taxa. Sci. Rep. 2016, 6, 29549. [Google Scholar] [CrossRef]
- Návar, J. Modeling tree diversity, stand structure and productivity of northern temperate coniferous forests of Mexico. PeerJ 2019, 7, e7051. [Google Scholar] [CrossRef] [PubMed]
- Alaniz-Gutiérrez, L.; Ail-Catzim, C.E.; Villanueva-Gutiérrez, R.; Delgadillo-Rodríguez, J.; Ortiz-Acosta, M.E.; García-Moya, E.; Medina-Cervantes, T.S. Caracterización palinológica de mieles del Valle de Mexicali, Baja California, México. Polibotánica 2017, 43, 255–283. [Google Scholar] [CrossRef]
- Araujo-Mondragón, F.; Redonda-Martínez, R. Flora melífera de la región centro-este del municipio de Pátzcuaro, Michoacán, México. Acta Bot. Mex. 2019, 126, e1444. [Google Scholar] [CrossRef]
- Andrada, A.C. Flora utilizada por Apis mellifera L. en el sur del Caldenal (Provincia Fitogeográfica del Espinal), Argentina. Rev. Mus. Argent. Cienci. Nat. 2003, 5, 329–336. [Google Scholar] [CrossRef]
- Santana-Michel, F.J.; Cervantes-Aceves, N.; Jiménez-Reyes, N. Flora melífera del estado de Colima, México. Ibugana 1998, 6, 251–277. [Google Scholar]
- Calabria, L.M.; Emerenciano, V.P.; Scotti, M.T.; Mabry, T.J. Secondary chemistry of Compositae. In Systematics, Evolution and Biogeography of the Compositae; Funk, V.A., Susanna, A., Stuessy, T.F., Bayer, R.J., Eds.; IAPT: Vienna, Austria, 2009; pp. 73–88. [Google Scholar]
- Torres, C.; Galetto, L. Are nectar sugar composition and corolla tube length related to the diversity of insects than visit Asteraceae flowers? Plant Biol. 2002, 4, 360–366. [Google Scholar] [CrossRef]
- Torres, C.; Galetto, L. Importancia de los polinizadores en la reproducción de Asteraceae de Argentina Central. Acta Bot. Venez. 2008, 31, 473–494. [Google Scholar]
- Camina, J.L.; Tourn, E.; Andrada, A.C.; Pellegrini, C.; Ashworth, L. Spatial and temporal distribution of floral rewards within the capitula: The case of Hyalis argentea (Asteraceae). Bol. Soc. Argent. Bot. 2019, 54, 17–27. [Google Scholar] [CrossRef]
- Arroyo, M.T.; Robles, V.; Tamburrino, I.; Martínez-Harms, J.; Garreaud, R.D.; Jara-Arancio, P.; Pliscoff, P.; Copier, A.; Arenas, J.; Keymer, J.; et al. Extreme Drought Affects Visitation and Seed Set in a Plant Species in the Central Chilean Andes Heavily Dependent on Hummingbird Pollination. Plants 2020, 9, 1553. [Google Scholar] [CrossRef]
- Moreira-Muñoz, A.; Scherson, R.A.; Luebert, F.; Román, M.J.; Monge, M.; Diazgranados, M.; Silva, H. Biogeography, phylogenetic relationships and morphological analyses of the South American genus Mutisia L.f. (Asteraceae) shows early connections of two disjunct biodiversity hotspots. Org. Divers. Evol. 2020, 20, 639–656. [Google Scholar] [CrossRef]
- Secretaría del Medio Ambiente y Recursos Naturales. Reserva de la Biosfera Mariposa Monarca. Available online: https://www.gob.mx/semarnat/articulos/reserva-de-la-biosfera-mariposa-monarca-79228 (accessed on 15 January 2021).
- Cornejo-Tenorio, G.; Casas, A.; Farfán, B.; Villaseñor, J.L.; Ibarra-Manríquez, G. Flora y vegetación de las zonas núcleo de la Reserva de la Biosfera Mariposa Monarca, México. Bol. Soc. Bot. Mex. 2003, 73, 43–62. [Google Scholar] [CrossRef][Green Version]
- Cornejo-Tenorio, G.; Ibarra-Manríquez, G. Flora Ilustrada de la Reserva de la Biosfera Mariposa Monarca; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO), Universidad Nacional Autónoma de México(UNAM): Mexico City, Mexico, 2008; p. 441. [Google Scholar]
- Secretaría de Agricultura y Desarrollo Rural. La miel Mexicana va Endulzando al Mundo. Available online: https://www.gob.mx/agricultura/articulos/la-miel-mexicana-va-endulzando-el-mundo?idiom=es (accessed on 15 January 2021).
- Secretaría de Agricultura y Desarrollo Rural. Yucatán se Encuentra entre los Principales Productores de miel del país. Available online: https://www.gob.mx/agricultura/yucatan/articulos/yucatan-se-encuentra-entre-los-principales-productores-de-miel-del-pais?idiom=es (accessed on 15 January 2021).
- Simpson, B.B. Economic importance of Compositae. In Systematics, Evolution and Biogeography of the Compositae; Funk, V.A., Susanna, A., Stuessy, T.F., Bayer, R.J., Eds.; IAPT: Vienna, Austria, 2009; pp. 45–58. [Google Scholar]
- Ruiz-Reyes, E.; Suárez, M. Lactonas sesquiterpénicas. Diversidad estructural y sus actividades biológicas. Rev. CENIC Cienc. Biol. 2015, 46, 9–24. [Google Scholar]
- Diario Oficial de la Federación. Secretaría de Agricultura y Ganadería. Decreto por el que se Declara Símbolo de la Floricultura Nacional la Flor de Dalia en todas sus Especies y Variedades. Available online: http://www.dof.gob.mx/nota_to_imagen_fs.php?codnota=4720563&fecha=13/05/1963&cod_diario=203468 (accessed on 16 January 2021).
- Secretaría de Medio Ambiente y Recursos Naturales. Dalia, flor Representativa de México. Available online: https://www.gob.mx/semarnat/articulos/dalia-flor-representativa-de-mexico?idiom=es (accessed on 16 January 2021).
- McVaugh, R. Compositae. In Flora Novo-Galiciana; Anderson, W.R., Ed.; The University of Muchigan Press: Ann Arbor, MI, USA, 1984; Volume 12, pp. 1–1157. [Google Scholar]
- Rzedowski, J.; Calderón de Rzedowski, G.; Carrillo-Reyes, P. Compositae Tribu Helientheae II (géneros Lagascea-Zinnia). In Flora del Bajío y de Regiones Adyacentes; Rzedowski, J., Calderón de Rzedowski, G., Eds.; Instituto de Ecología, A.C., Centro Regional del Bajío: Pátzcuaro, Michoacán, Mexico, 2011; Volume 172, pp. 1–100. [Google Scholar]
- Flora Ornamental de Barcelona. Available online: https://floraornamentaldebarcelona.com/2020/01/18/flora-del-parc-de-la-guineueta-3-arbustos-2-margaritero-montanoa-bipinnatifida/ (accessed on 8 October 2020).
- Plant This. Available online: http://plantthis.co.nz/plant-information.asp?gardener=18906&tabview=features&plantSpot=1 (accessed on 8 October 2020).
- Auckland Museum. Available online: https://www.aucklandmuseum.com/collections-research/collections/record/am_naturalsciences-object-780123 (accessed on 8 October 2020).
- VivoPlant. Available online: https://vivoplant.com/comprar-plantas-arbustivas-exterior/69-257-especies-arbustivas-bartlettina-sordida.html (accessed on 16 January 2021).
- Toledo, V.M. Los Cambios Climáticos del Pleistoceno y sus Efectos Sobre la Vegetación Tropical Cálida y Húmeda de México. Master’s Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico City, Mexico, 29 April 1976. [Google Scholar]
- Torrescano-Valle, N.; Islebe, G.A. Holocene paleoecology, climate history and human influence in the southwestern Yucatan Peninsula. Rev. Palaeobot. Palynol. 2015, 217, 1–8. [Google Scholar] [CrossRef]
- Escobar García, P.; Winkler, M.; Flatscher, R.; Sonnleitner, M.; Krejcikova, J.; Suda, J.; Hülber, K.; Schneeweiss, G.; Schönswetter, P. Extensive range persistence in peripheral and interior refugia characterizes Pleistocene range dynamics in a widespread Alpine plant species (Senecio carniolicus, Asteraceae). Mol. Ecol. 2012, 21, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Robayo, A.P.; Ureta, C.; Gómez-Albores, M.A.; Meneses-Mosquera, A.K.; Téllez-Valdés, O.; Martínez-Meyer, E. One hundred years of climate change in Mexico. PLoS ONE 2020, 15, e0209808. [Google Scholar] [CrossRef] [PubMed]
- Comisión Nacional de Áreas Naturales Protegidas. Áreas Naturales Protegidas Decretadas. Available online: http://sig.conanp.gob.mx/website/pagsig/datos_anp.htm (accessed on 19 February 2021).
- Secretaría de Medio Ambiente y Desarrollo Territorial de Jalisco. 15 Cuenca Alimentadora del Distrito Nacional de Riego 043, Nayarit. Available online: https://semadet.jalisco.gob.mx/medio-ambiente/biodiversidad/areas-naturales-protegidas/142 (accessed on 19 February 2021).
- Sistema de Información y Monitoreo para la Conservación, Comisión Nacional de Áreas Naturales Protegidas. CADNR043 Estado de Nayarit. Available online: https://simec.conanp.gob.mx/ficha_pdf.php?anp=4®=11 (accessed on 19 February 2021).
- Secretaría de Medio Ambiente y Recursos Naturales. Reserva de la Biosfera Sierra de Manantlán. Available online: https://www.gob.mx/semarnat/articulos/reserva-de-la-biosfera-sierra-de-manantlan (accessed on 19 February 2021).
- Secretaría de Medio Ambiente y Recursos Naturales. Reserva de la Biosfera Sierra Gorda. Available online: https://www.gob.mx/semarnat/articulos/reserva-de-la-biosfera-sierra-gorda-celebra-su-19-aniversario (accessed on 19 February 2021).
- Centro de Investigación en Ciencias de Información Geoespacial. Selva Lacandona, Mosaico del Paisaje, Paisaje Ecológico. Available online: http://mapas.centrogeo.org.mx/ciberatlas/lacandona/mosaico/clima.htm (accessed on 20 February 2021).
- Instituto Nacional de Ecología, Plan de manejo de la Reserva de la Biosfera El Triunfo. Available online: http://www.paot.org.mx/centro/ine-semarnat/anp/AN14.PDF (accessed on 20 February 2021).
- Secretaría de Medio Ambiente y Recursos Naturales. Reserva de la Biosfera Montes Azules. Available online: https://www.gob.mx/semarnat/articulos/reserva-de-la-biosfera-montes-azules-selva-lancandona-chiapas?idiom=es (accessed on 20 February 2021).
- Secretaría de Medio Ambiente y Recursos Naturales. Reserva de la Biosfera El Triunfo. Available online: https://www.gob.mx/semarnat/articulos/reserva-de-la-biosfera-el-triunfo?idiom=es (accessed on 20 February 2021).
- Secretaría de Medio Ambiente y Recursos Naturales. Reserva de la Biosfera Los Tuxtlas. Available online: https://www.gob.mx/semarnat/articulos/reserva-de-la-biosfera-los-tuxtlas?idiom=es (accessed on 21 February 2021).
- Comisión Nacional de Áreas Naturales Protegidas. Programa de Conservación y Manejo Reserva de la Biosfera Los Tuxtlas, México. Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/tuxtla_final.pdf (accessed on 21 February 2021).
- Montsarrat, S.; Jarvie, S.; Svenning, J.C. Anthropocene refugia: Integrating history and predictive modelling to assess the space available for biodiversity in a human-dominated world. Philos. Trans. R. Soc. B 2019, 374, 20190219. [Google Scholar] [CrossRef]
- Bennett, K.D.; Provan, J. What do we mean by ‘refugia’? Quat. Scient. Rev. 2008, 27, 2449–2455. [Google Scholar] [CrossRef]
- Suárez-Mota, E.; Villaseñor, J.L.; Ramírez-Aguirre, M.B. Sitios prioritarios para la conservación de la riqueza y el endemismo de la Sierra Norte de Oaxaca, México. Acta Bot. Mex. 2018, 124, 49–74. [Google Scholar] [CrossRef]
- Aschentrupp Toledo, R. Las Comunidades Indígenas de la Sierra Norte de Oaxaca; Centro de Estudios Sociales y Opinión Pública (CESOP): Mexico City, Mexico, 2015; p. 15. [Google Scholar]
- Ceballos Pérez, S.G. Manejo Forestal Comunitario Sustentable en el Sierra Norte de Oaxaca; Published by the Autor: Mexico City, Mexico, 2017; p. 310. [Google Scholar]
- Sastre Merino, S. Análisis de la gestión forestal comunitaria y sus implicaciones sociales en Ixtlán de Juárez, Oaxaca (México). Bachelor’s Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2008. [Google Scholar]
- Gasca Zamora, J. Gobernanza y gestión comunitaria de recursos naturales en la Sierra Norte de Oaxaca. Reg. Soc. 2014, 60, 89–120. [Google Scholar] [CrossRef]
- Villaseñor, J.L.; Delgadillo, C.; Ortiz, E. Hostspots from a multigroup perspective: Mosses and Senecios in the Transmexican Volcaniv Belt. Biodivers. Conserv. 2006, 15, 4045–4058. [Google Scholar] [CrossRef]
- Suárez-Mota, M.E.; Téllez-Valdés, O. Red de áreas prioritarias para la conservación de la biodiversidad del Eje Volvánico Transmexicano analizando su riqueza florística y variabilidad climática. Polibotánica 2014, 38, 67–93. [Google Scholar]
- Critical Ecosystem Partnership Fund. Región Norte del Hotspot de Biodiversidad de Mesoamérica, Belice, Guatemala, México. Available online: https://www.cepf.net/sites/default/files/final.spanish.mesoamerica.northernmesoamerica.ep_.pdf (accessed on 23 February 2021).
- Instituto Nacional de Estadística y Geografía, Territorio de México. Available online: http://www.cuentame.inegi.org.mx/territorio/extension/default.aspx?tema=T (accessed on 6 February 2021).
- Pruski, J.F. Asteraceae. In Flora Mesoamericana; Davidse, G., Sousa, S.M., Knapp, S., Chiang, F., Eds.; Misssouri Botanical Garden Press: St. Louis, MO, USA, 2018; Volume 5, Part 2, pp. 1–608. [Google Scholar]
- Redonda-Martínez, R.; Villaseñor, J.L. Asteraceae, Vernonieae. In Flora del Valle de Tehuacán-Cuicatlán; Medina Lemos, R., Sánchez Ken, J.G., García Mendoza, A., Arias Montes, S., Eds.; Instituto de Biología, Universidad Nacional Autónoma de México: Mexico City, Mexico, 2009; Volume 72, pp. 1–23. [Google Scholar]
- Redonda-Martínez, R.; Villaseñor, J.L. Asteraceae, Senecioneae. In Flora del Valle de Tehuacán-Cuicatlán; Medina Lemos, R., Sánchez Ken, J.G., García Mendoza, A., Arias Montes, S., Eds.; Instituto de Biología, Universidad Nacional Autónoma de México: Mexico City, Mexico, 2011; Volume 89, pp. 1–64. [Google Scholar]
- Redonda-Martínez, R. Asteraceae, Gochnatieae. In Flora del Valle de Tehuacán-Cuicatlán; Medina Lemos, R., García Mendoza, A., Arias Montes, S., Grether González, R., Fonseca Juárez, R.M., Eds.; Instituto de Biología, Universidad Nacional Autónoma de México: Mexico City, Mexico, 2019; Volume 155, pp. 1–16. [Google Scholar]
- Turner, B.L. Taxonomy of Neurolaena (Asteraceae-Heliantheae). Plant Syst. Evol. 1982, 140, 119–139. [Google Scholar] [CrossRef]
- Redonda-Martínez, R.; Villaseñor, J.L. El género Lepidaploa (Familia Asteraceae, Tribu Vernonieae) en México. Rev. Mex. Biodivers. 2011, 82, 782–797. [Google Scholar] [CrossRef]
- Botany Collection Search, Smithsonian National Museum of Natural History. Available online: http://collections.nmnh.si.edu/search/botany/?v=s1#new-search (accessed on 24 November 2020).
- Tropicos.org. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 28 November 2020).
- SEinet Arizona-New Mexico Chapter. Available online: https://swbiodiversity.org/seinet/collections/harvestparams.php (accessed on 6 December 2020).
- Hinojosa-Espinosa, O.; Villaseñor, J.L.; Ortiz, E. On the identity of two Mexican species of Ageratina (Eupatorieae, Asteraceae): A. grandifolia and A. rivalis. Bot. Sci. 2019, 97, 250–259. [Google Scholar] [CrossRef]
- Robinson, H. Generic and subtribal clasification of American Vernonieae. Smithsonian Contr. Bot. 1999, 89, 1–116. [Google Scholar] [CrossRef]
- Schilling, E.; Panero, J.L. The Eupatorieae Website. Available online: http://schillinglab.utk.edu/Danielweb/Eup/genusindex.html (accessed on 22 September 2020).
- GBIF, Global Biodiversity Information Facility. Available online: https://www.gbif.org/ (accessed on 21 September 2020).
- Bachmann, S.; Moat, J.; Hill, A.W.; de la Torre, J.; Scott, B. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. e-Infrastructures for data publishing in biodiversity science. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Google Earth Pro. Available online: https://www.google.com/intl/es/earth/download/gep/agree.html (accessed on 4 December 2020).
- Instituto Nacional de Estadística y Geografía, Archivo Histórico de Localidades Geoestadísticas. Available online: https://www.inegi.org.mx/app/geo2/ahl/ (accessed on 2 December 2020).
- Mapcarta, El Mapa Libre. Available online: https://mapcarta.com/es/ (accessed on 2 December 2020).
- PueblosAmerica.com. Pueblos de México. Available online: https://mexico.pueblosamerica.com/ (accessed on 28 November 2020).
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Van Proosdij, A.S.J.; Sosef, M.S.M.; Wieringa, J.J.; Raes, N. Minimum required number of specimen records to develop accurate species distribution models. Ecography 2016, 39, 542–552. [Google Scholar] [CrossRef]
- WorldClim, Maps, Graphs, Tables, and Data of the Global Climate. Available online: https://www.worldclim.org/ (accessed on 5 January 2021).
- Mendes, P.; Elías Velasco, S.J.; Alves de Andrade, A.F.; De Marco Júnior, P. Dealing with overprediction in species distribution models: How adding distance constraints can improve model accuracy. Ecol. Model. 2020, 431, 109180. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either-or presence-absence. Acta Oecol. 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Tatebe, H.; Ogura, T.; Nitta, T.; Komuro, Y.; Ogochi, K.; Takemura, T.; Sudo, K.; Sekiguchi, M.; Manabu, A.; Saito, F.; et al. Description and basic evaluation of simulated mean state, internal variability, and climate sensitivity in MIROC6. Geosci. Model Dev. 2019, 12, 2727–2765. [Google Scholar] [CrossRef]
- Fasullo, J.T. Evaluating Simulated Climate Patterns from the CMIP Archives Using Satellite and Reanalysis Datasets. Geosci. Model Dev. 2020, 13, 3627–3642. [Google Scholar] [CrossRef]
- Provincias Biogeográficas de México, Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). 1997. Available online: http://conabio.gob.mx/informacion/metadata/gis/rbiog4mgw.xml?_xsl=/db/metadata/xsl/fgdc_html.xsl&_indent=no (accessed on 25 January 2021).
- International Union for Conservation of Nature. Red List Categories and Criteria, Version 3.1, 2nd ed.; IUCN: Gland, Switzerland; Cambridge, UK, 2012; p. 32. [Google Scholar]

| Subfamily | Tribe | Species | Percentage |
|---|---|---|---|
| Gochnatioideae | Gochnatieae | 3 | 2.01% |
| Vernonioideae | Liabeae | 1 | 0.67% |
| Vernonieae | 16 | 10.73% | |
| Senecioneae | 20 | 13.42% | |
| Astereae | 4 | 2.68% | |
| Inuleae | 1 | 0.67% | |
| Neurolaeneae | 1 | 0.67% | |
| Asteroideae | Millerieae | 3 | 2.01% |
| Coreopsideae | 2 | 1.34% | |
| Bahieae | 1 | 0.67% | |
| Heliantheae | 55 | 36.9% | |
| Eupatorieae | 42 | 28.18% |
| Use | Category | Species |
|---|---|---|
| Medicinal | Oral diseases | 3 |
| Heart diseases | 1 | |
| Stomach diseases | 11 | |
| Skin diseases | 10 | |
| Gynecological diseases | 2 | |
| Anticonceptive | 2 | |
| Anti-inflammatory | 12 | |
| Antiseptic | 5 | |
| Diuretic | 2 | |
| Fever reducer | 5 | |
| Reuma | 4 | |
| Vertigo | 1 | |
| Various | 8 | |
| Nectariferous | Honeybees | 17 |
| Butterflies | 3 | |
| Hummingbirds | 1 | |
| Ornamental | Live fence | 2 |
| Cut flower | 2 | |
| Decoration | 8 | |
| Others | Artesanal | 2 |
| Ceremonial | 3 | |
| Fuel | 8 | |
| Construction | 3 | |
| Forage | 6 | |
| Insecticide | 1 | |
| Ritual | 8 | |
| Shade for coffee | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redonda-Martínez, R.; Pliscoff, P.; Moreira-Muñoz, A.; Martínez Salas, E.M.; Samain, M.-S. Towards Conservation of the Remarkably High Number of Daisy Trees (Asteraceae) in Mexico. Plants 2021, 10, 534. https://doi.org/10.3390/plants10030534
Redonda-Martínez R, Pliscoff P, Moreira-Muñoz A, Martínez Salas EM, Samain M-S. Towards Conservation of the Remarkably High Number of Daisy Trees (Asteraceae) in Mexico. Plants. 2021; 10(3):534. https://doi.org/10.3390/plants10030534
Chicago/Turabian StyleRedonda-Martínez, Rosario, Patricio Pliscoff, Andrés Moreira-Muñoz, Esteban Manuel Martínez Salas, and Marie-Stéphanie Samain. 2021. "Towards Conservation of the Remarkably High Number of Daisy Trees (Asteraceae) in Mexico" Plants 10, no. 3: 534. https://doi.org/10.3390/plants10030534
APA StyleRedonda-Martínez, R., Pliscoff, P., Moreira-Muñoz, A., Martínez Salas, E. M., & Samain, M.-S. (2021). Towards Conservation of the Remarkably High Number of Daisy Trees (Asteraceae) in Mexico. Plants, 10(3), 534. https://doi.org/10.3390/plants10030534