Abstract
An organic greenhouse crop of tomato was established in February following cultivation of cowpea (CP) or common bean (CB) for green pod production, or faba bean (FB) for green manuring. The vegetative residues of CP and CB were incorporated to the soil together with farmyard manure (FYM), prior to establishing the tomato crop. The FB plants were incorporated to the soil at anthesis together with either FYM or composted olive-mill waste (CO). Green manuring with FB resulted in higher soil mineral N levels during the subsequent tomato crop and higher tomato fruit yield when combined with FYM, compared to compost. The level of soil mineral N was the main restrictive factor for yield in organic greenhouse tomato. FB for green manuring as preceding crop to tomato increased significantly the level of soil mineral N and tomato yield compared to CB or CP aiming to produce green pods. The lowest tomato yield was obtained when the preceding crop was CB cultivated for green pod production. The soil mineral N was significantly higher when FYM was applied as base dressing compared with CO, despite the higher total N concentration in CO, pointing to slower mineralization rates of CO during tomato cultivation.
1. Introduction
The global market of organic products has increased more than three times during the recent 20 years due to commensurate increases in consumer demand for these products [1]. However, according to most researchers and practical experience, there is a yield gap of more than 20% between organic and conventional farming [2,3,4]. This difference in yield performance probably represents the most important limitation to the further expansion of organic farming [5].
Nitrogen (N) availability is of paramount importance for successful cultivation in any form of agriculture [6,7]. In organic farming, the use of industrially produced fertilizers of inorganic N is prohibited [3]. In addition, in many countries and throughout the European Union, the use of inorganic N in organic agriculture is not allowed, even if it is of mineral origin [8].
Providing N is a constant challenge for organic farming as it is the most important limiting factor for sufficient yields [9,10,11]. Overall, adequate N nutrition is difficult to achieve in organic farming and N deficiency often occurs [12]. Increasing the supply of N has a great potential to increase the yield in organic farming [13], since N inputs in organic farming are, in most cases, below the optimal level [5]. The main N sources in organic farming are the incorporation of green manure or crop residues and the application of animal manure or/and compost [6]. Nevertheless, the only true imports of N in a cultivated field are those originating from manure and compost transferred from other holdings, as well as atmospheric N2 fixed biologically by soil microorganisms [14].
Legumes, i.e., plant species belonging to the Fabaceae family, form symbiotic relationships with N2-fixing bacteria, generally termed rhizobia, and, therefore, legume-based green manure can provide significant amounts of N to the succeeding crops [15,16,17]. Several studies have shown that the use of legumes as green manure can effectively enhance the yield of the following tomato crop [17,18,19]. Different legume species are colonized by different rhizobial species. Therefore, when the appropriate rhizobial species for a particular legume crop are not present in the soil accommodating this crop, inoculation is necessary for sufficient biological N2 fixation that can effectively increases the total amount of produced biomass [19,20].
Crop rotations are the cornerstone of organic farming systems, as they contribute crucially to both maintenance of soil fertility [21], and control of pests, diseases and weeds [22]. Therefore, many scientists have proposed the reintroduction of legumes into the crop rotation systems, as they act as N fertilization sources for the next crop. Ponisio et al. [2] found that the application of crop rotation schemes based on legumes in organic farming reduces the aforementioned yield gap to 8%. However, the contribution of grain legumes to the soil N budget is limited, because roughly half of the biologically fixed N is removed by grain harvest [14], so that sometimes the final surplus may not exceed a level of 25 kg N ha−1 [22].
In this framework, the present study was carried out to test whether the growth, yield, and N nutrition of organic greenhouse tomato can be substantially improved when the preceding crop is a legume cultivated for harvesting green pods or as green manure. To attain this goal, tomato was organically cultivated in a greenhouse during spring-summer following autumn-winter cultivation of three alternative legume species. Two of the tested legume species (cowpea and common bean) were destined to produce green pods for the market, while faba bean was incorporated to the soil prior to anthesis as green manure.
2. Results
2.1. Legumes Biomass, Yield, N Accumulation and BNF
Common bean rendered significantly higher pod yield and plant residues compared to cowpea (Table 1). However, the aboveground fresh and dry biomass of cowpea or common bean were significantly lower than the corresponding fresh and dry faba bean biomass incorporated into the soil as green manure. This large difference was not due to the harvested pods in the common bean and cowpea crops, since their total biomass, including the pods, was significantly lower than that of faba bean. The pod harvest index of common bean was significantly higher than that of cowpea on fresh weight basis but similar between the two legumes when calculated on dry weight basis.

Table 1.
Aboveground fresh and dry biomass of harvested pods (BHP), biomass incorporated to the soil (BIS) after crop termination, total produced biomass (BTP), and harvest index (HI) in autumn-winter crops of common bean and cowpea used for fresh pod production, and faba bean applied as green manure.
The N concentrations in the cowpea pods were higher than in those of common bean, whereas they were similar in the vegetative residues (Table 2). In both legumes, the N concentrations were higher in the pods than in the vegetative residues. The total N content, which is the product of concentration and total dry biomass, was substantially higher in both pods and vegetative residues of common bean compared to those of cowpea. However, the total N content in the faba bean biomass incorporated to the soil was about three times higher than in the total biomass of common bean and about seven times higher than in that of cowpea. The Ndfa in the cowpea biomass (39.6%) was significantly higher than in that of common bean (18.2%), but markedly lower than in the faba bean biomass (57%). Finally, the total amount of biologically fixed nitrogen in cowpea and common bean treatments was 1.51 and 1.65 g m−2, respectively, whereas it was appreciably higher in the faba bean treatment (15 g m−2). The N harvest index did not differ significantly between cowpea and common bean (44.8% and 43.4%, respectively).

Table 2.
Total-N concentrations in plant tissues, total-N provided to the next crop by incorporation of the legume biomass to the soil, N harvest index (NHI), percentage of N derived from the atmosphere (Ndfa %) in the legume biomass incorporated to the soil, and total amount of biologically fixed N (BNF) per unit area cultivated with a legume.
2.2. Soil Measurements
The fertilization treatments applied to organic greenhouse tomato in the current study had no significant impact on the soil C, total N, P (Olsen) and exchangeable K concentrations throughout the experimental period (Table 3). After termination of all legume crops, the soil C, N, P, and K concentrations were slightly higher than those measured before commencement of their cultivation, but the differences were significant only for K and P. The subsequent cultivation of organic tomato did not reduce the soil C, total-N and P concentrations, but decreased significantly the soil K concentrations.

Table 3.
Impact of different organic fertilization treatments on concentrations of organic C, total-N, available P (Olsen), and exchangeable K in the soil. FYM: Farmyard manure; CO: composted olive-mill waste.
In the tomato crop, the soil NO3-N concentration increased considerably in all treatments 26 days after incorporation of the organic matter to the soil (DAIOM) but decreased gradually thereafter (Figure 1a). The incorporation of faba bean residues to the soil as green manure combined with farmyard manure (FYM) resulted in significantly higher soil NO3-N concentrations compared to the other three treatments at 26, 57, and 91 DAIOM. However, just before incorporation of the organic matter in the soil and at crop termination, the soil NO3-N was similar in all treatments. On the day of crop termination (at 124 DAIOM), the NO3-N concentration was below 19 mg kg−1 in all treatments.
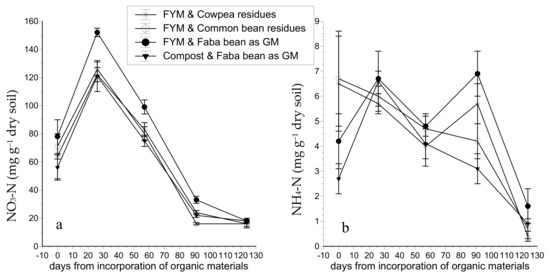
Figure 1.
Impact of different organic fertilization treatments (a) on soil NO3-N concentrations; (b) on soil NH4-N concentrations during cultivation of organic tomato. FYM: farmyard manure; Compost: composted olive-mill waste; GM: green manure.
The soil NH4-N concentration fluctuated strongly during the cropping period in the tomato crop, without significant differences between treatments (Figure 1b). Overall, the NH4-N concentration level was consistently low at all sampling dates and for all treatments ranging from 2 to 7 mg kg−1, while on the last sampling date, the NH4-N concentration dropped below 1.6 mg kg−1 in all treatments.
2.3. Tomato Yield Components
When faba bean applied as green manure was the preceding legume crop, the application of FYM as organic fertilizer increased the fruit yield in tomato, compared to application of composted olive-mill waste (Table 4). However, the fruit yield decreased significantly when the fertilization with FYM was combined with incorporation of cowpea or common bean residues to the soil originating from crops used for fresh pod production, compared to green manure with faba bean. Furthermore, the incorporation of common bean residues to the soil prior to planting further reduced the tomato yield compared to incorporation of cowpea residues. The reduction of tomato yield was exclusively due to commensurate decreases in the fruit number per plant, while the mean fruit weight was similar in all treatments.

Table 4.
Impact of different organic fertilization treatments on fruit yield and yield components in the organic greenhouse tomato crop. FYM: Farmyard manure; CO: composted olive-mill waste.
2.4. Tomato Tissue Analysis
The highest leaf N concentration was measured in tomato plants fertilized with green manure of faba bean in combination with FYM (FB + FYM). When the green manuring with faba bean was combined with application of composted olive-mill waste, the leaf N concentration in tomato was significantly lower than that obtained from FB + FYM but similar to that obtained from application of FYM together with vegetative residues of cowpea cultivated for fresh pod production. Finally, incorporating vegetative residues of common bean to the soil reduced significantly the leaf N concentration in the subsequent tomato crop compared to incorporation of cowpea residues (Table 5). On the other hand, the leaf P and K concentrations were not influenced by any fertilization treatment in the organic tomato crop.

Table 5.
Impact of different organic fertilization treatments on leaf N, P, and K concentrations in the organic greenhouse tomato crop. FYM: Farmyard manure; CO: composted olive-mill waste.
3. Discussion
3.1. Legumes Aboveground Biomass, Nitrogen Fixation and N Balance
In order to quantify the biologically fixed N, three values have to be measured, particularly total plant dry matter produced per m2, tissue N concentration (N %) and percentage of plant N derived from atmospheric N2. Of these three parameters, the dry matter production exhibits the larger variation between different crop species and cropping systems, and is, therefore the main factor that differentiates BNF in different legume crops [23]. In the present study, faba bean produced more than twice as much dry matter as common bean and more than five times that of cowpea. The major factor contributing to this large difference in dry biomass between faba bean and the other two legume crops was plant density, which was almost threefold in the faba bean plots destined for green manuring (11.7 plants m−2) compared to that applied in the crops of common bean and cowpea (4.27 plants m−2). In addition, the climatic conditions during the last two months of the legume crops were much more favorable for faba bean than for common bean and cowpea, as the greenhouse was not sufficiently heated (Table 5). Finally, the faba bean biomass was incorporated into the soil at the most appropriate stage, just before anthesis, while the residues of common bean and cowpea were incorporated after harvesting of all pods. The growth stage of legumes has also a substantial impact on the N accumulation in shoot [14,24]. Thus, at the time of incorporation into the soil, the N concentration in the faba bean tissues was significantly higher compared to those measured in common bean and cowpea residues, which were at a late reproductive stage. In addition to this, an appreciable amount of the total N contained in the plant biomass was removed through pod in the plots of cowpea and common bean, thereby further reducing the amount of N provided to the soil through incorporation of plant biomass. Thus, the amount of N provided to the soil through incorporation of plant biomass in the faba bean plots was ten times more than the corresponding amounts in the cowpea plots and five times more than in the common bean plots. The significant difference in total N provided to the soil by incorporation of common bean residues at crop termination compared to that provided by cowpea residues was solely due to commensurate differences in biomass production.
The %Ndfa ranged low in all treatments compared to values reported for the same legume species by Gatsios et al. [19], who worked in the same greenhouse soil, and by other investigators, who worked in different locations [25,26]. This is attributed to the high concentration of soil inorganic N, especially NO3, which ranged from 73 to 95 mg kg−1 when the legumes were sowed. High levels of plant available N (PAN) accumulated during soil solarization, which was applied for almost three months during the summer preceding the establishment of the legume crops. The high temperature and humidity conditions prevailing during soil solarization favored N mineralization, while the absence of plants during that time maintained the produced inorganic N available for the next crop. It is well documented [24,26,27] that high concentrations of inorganic N in the soil inhibit colonization of legume roots with rhizobia and concomitantly restrict symbiotic N2 fixation. Nevertheless, the %Ndfa of faba bean was significantly higher than that found in the other two legumes, presumably because of the high plant density of faba bean, as this crop was intended for application of green manure. Consequently, although the mineral N level in the soil was the same for all legumes at crop establishment, the amount of N relative to the total plant needs was less in faba bean and thus it was less restrictive for rhizobia colonization than in the other two legume species. An additional reason is that the BNF efficiency of faba bean is not particularly affected by the level of inorganic N in the soil, presumably because this plant has a relatively low efficiency to exploit the soil N [25,26,28]. Furthermore, the N2 fixation efficiency of common bean is generally lower than that of other legumes, according to several studies [21,23,26]. The present study confirmed the lower N2 fixation efficiency of common bean as the %Ndfa in this legume was appreciably lower than that found in cowpea and faba bean. The biologically fixed N per cultivated area unit that was provided to the next crop by incorporating faba bean to the soil as green manure amounted to 15 g m−2. This level is slightly lower than the 19 g m−2 found by Ntatsi et al. [29] in a crop cultivated for fresh pod production, but similar to that reported by Stagnari et al. [28]. Compared to the other two legumes, the amount of biologically fixed N2 accumulated by faba bean was ten times higher than that of cowpea and common bean, which was at a similar level. This is ascribed not only to the higher %Ndfa of faba bean but also to the markedly higher biomass produced by this legume.
Soil solarization increases the PAN levels in the soil due to the high temperatures that accelerate N mineralization, and thus its application after an N-demanding crop, such as tomato that depletes the PAN pool in the soil is highly beneficial. Thus, it can be applied in organically cultivated tomatoes in greenhouses. However, the release of PAN through soil solarization requires the presence of organic matter with a low C/N ratio in the soil. The present study showed that this could be achieved by the introduction of a legume crop in a yearly rotation scheme with tomato. The legume crop can be cultivated either for harvesting pods or as green manure, which is especially useful in infertile soils as has been reported by Thönnissen et al. [30]. In addition, legumes that maintain high %Ndfa even at high concentrations of PAN in the soil, such as faba bean, can be selected as shown in the current experiment and in previous studies [26,28]. Furthermore, at high PAN concentrations in the soil, the nodulation efficiency may be influenced also by the variety of a particular legume species which can interact with the rhizobial strains, as reported by Peoples et al. [26].
The dry matter harvest index of common bean and cowpea was rather low compared to that found in other studies [31,32] and this led to a low N harvest index (NHI). The final contribution of legumes to the N soil balance depends on the difference between the index of Ndfa index and the NHI. Therefore, in the case of common bean, in which the Ndfa index is appreciably lower than the NHI, the soil N balance was negative, while for cowpea it was neutral, or even slightly positive if also the root biomass is taken into account. The contribution of the below-ground legume biomass (root, exudates and nodules) to the N balance must be taken into account, as it is estimated to be about 30% of the above-ground biomass [25,26,33]. However, the N balance of the common bean remains negative even after taking into consideration the below-ground N contributed by symbiotic N fixation. Thus, the lower soil mineral N and fruit yield in tomato following common bean, compared to cowpea, is ascribed to the difference between N contributed by BNF and N removed through harvesting of pods. Indeed, this difference was strongly negative in common bean (1.65 vs. 3.68 g m−2), while in cowpea it was almost balanced (1.51 vs. 1.71 g m−2) and presumably slightly positive, if also the root biomass is taken into account. This means that the common bean crop removed part of the soil N reserves, while the cowpea crop retained the soil N reserves and presumably left some N from BNF to the following tomato crop. For this reason, the soil mineral N and the fruit yield were significantly lower in tomato following common bean, compared to tomato following cowpea, although common bean contributed more biomass to the soil than cowpea.
3.2. Soil Measurements
Ammonium concentrations were quite low in all samplings, and differences between all treatments were insignificant. This was expected because in nonacidic, well-aerated topsoils with high microbial activity, the nitrification rate of NH4 is high [34,35,36]. In contrast, NO3 concentrations were rather high before the incorporation of organic matter, indicating the partial exploitation of PAN by legumes. Indeed, as several studies have shown [26,32,37], the cultivation of legumes does not deplete the pool of inorganic N, as is the case with other plants and especially cereals. Under greenhouse conditions, nitrate leaching is unlikely because there is no rainfall and irrigation is completely controlled. The sharp increase in the soil NO3 concentration 26 DAIOM in all treatments reflects the high rates of mineralization that occur in the first weeks after incorporation of legume biomass to the soil [37,38,39]. However, the levels of NO3 were higher than the optimal range suggested for tomato cultivation in the literature [40,41]. At 57 DAΙOM, the NO3 concentration was within the optimal range, but at 91 DAIOM it dropped to lower levels than those suggested as sufficient for tomato cultivation [42,43,44]. In the latter critical period, the FYM + FB treatment maintained a significantly higher NO3 concentration than the other three treatments, while the FYM + CB treatment resulted in a significantly lower NO3 level compared to FYM + CP and CO + FB treatments. These results show that a higher amount of inorganic N was released through mineralization in the FYM + FB treatment compared to CO + FB, although the total N concentration in FYM was significantly lower than in CO. This is attributed to the much slower N mineralization rate in CO during the first year of application, which is estimated to 10% compared to 50% in FYM [45,46].
3.3. Tomato Growth and Yield
In agreement with the high level of soil PAN, tomato plants in the first stage of development showed symptoms of vigorous vegetative growth, which according to Papadopoulos [47] include curled thick leaves, thick stem with large diameter, and large clusters. Eight weeks after planting, tomato plants were balanced in terms of N nutrition, while in the third month of development they showed symptoms of N deficiency. This deficiency was mainly manifested by thin stems and light green leaves as described in relevant literature [40,47], while deficiency symptoms were mildest in the FYM + FB treatment.
The total N concentration measured in the leaves 2.5 months after planting was lower than the optimal values for tomato cultivation in all treatments, except for FYM + FB which was marginally lower [40,48]. These results were in line with the observed N deficiency symptoms that became visible at that cropping stage. The level of P and K concentrations in tomato leaves in all treatments was within the optimal range [40,48], as expected considering the concentrations of these elements in the soil.
The significant difference in the yield of tomato fruit between treatments is ascribed to PAN levels in the soil during the reproductive stage, when they became lower than those suggested for tomato, confirming that an adequate N supply is the most critical factor for organic cultivation [9,10,11]. Thus, the tomato yield in FYM + FB treatment was significantly higher than in the other three treatments, followed by FYM + CP, which is in line with commensurate differences in the soil NO3-N levels.
Several researchers [26,28,49] estimated that about 30% of the pre-crop effect of legumes on subsequent crop yield should be attributed to other factors beyond N supply, such as improvements in the physical, chemical, and biological properties of the soil. This effect could not be assessed in the present study. Thus, it is not possible to conclude if the yield differences were imposed only by differences in mineral N availability, or by other factors such as improvements in the physical, chemical, and biological properties of the soil. However, the lack of any impact of the different legume treatments on soil C and available K and P levels may indicate that physical, biological and chemical factors other than N mineralization did not have a measurable effect on the observed yield differences.
In organic farming, the most critical challenge is to synchronize the rate of N mineralization and plant N needs, as has been reported by many researchers [6,12,50]. In the present study, the data shown in Figure 1 and the visual appearance of the plants indicate that the N supply exceeded the N requirements of tomato during the first month of plant growth. This is ascribed to high N mineralization rates of legume biomass during the first 8 weeks after its incorporation into the soil, which are in agreement with previous reports [38]. However, after the first eight weeks, when the plants started to produce fruit, the soil mineral N levels decreased rapidly, suggesting that the rates of N mineralization were not sufficient to satisfy plant N uptake requirements. This lack in synchronization between N supply and N demand is crucial and needs to be mitigated in some way. Shortening the time interval between incorporation of the organic materials to the soil and planting of tomato might decrease both the peak in soil mineral N and the rate of the subsequent decrease, thereby maintaining sufficient soil N levels for longer time. In addition, the initial rate of plant biomass decomposition can be reduced by proper treatments such as adjusting the size of the shoot fragments or partially drying the biomass on the soil surface before its incorporation [12,51]. Moreover, legumes can be intercropped with other plants with a higher C/N ratio in order to reduce the initial rate of mineralization [14,37]. Finally, one should take into consideration that in drip-irrigated organic tomato crops in greenhouses, only a part of the organic biomass incorporated into the soil is utilized by the plants, as the drippers moisten constantly only an aliquot of the soil bulk. Thus, measures to moisten constantly additional parts of the soil at a later cropping stage might considerably enhance the soil N reserves that can be utilized by plants, thereby avoiding or minimizing yield restriction due to N deficiency. Nevertheless, this hypothesis has to be tested experimentally by modifying accordingly the irrigation system.
4. Materials and Methods
4.1. Plant Material, Growth Conditions, and Treatments
An experiment with legumes as preceding crop and tomato as the main crop was conducted in a greenhouse NNE–SSW oriented, which was located in Preveza, northwestern Greece (38°59′29.2″ N; 20°45′36.1″ E, 5 m a.s.l.). The exact dates for each crop establishment, commencement of harvesting, and crop termination are provided in Table 6. Prior to the establishment of the experimental treatments, soil solarization was applied to control soil-borne pathogens. The soil solarization started on 13th June and lasted up to 5th September 2018. The experiment was carried out in a commercial arch type greenhouse with vertical sidewalls, covered by low-density polyethylene film. The geometrical characteristics of the greenhouse were as follows: eaves height = 2.80 m, ridge height = 3.5 m, span width = 7.5 m, length = 44 m, ground area = 330 m2. The greenhouse was ventilated via side vents (total opening area 150 m2), which were opened whenever the greenhouse air temperature exceeded 26 °C. The plot size was 3.75 × 5.00 m2 (i.e., 18.75 m2). The soil type was sandy loam with neutral pH (7.3 measured in water extract) and an organic matter content of 4.14%. The concentrations of plant available N (NO3-N and NH4-N) in the soil before the experiment are shown in Figure 1 (concentrations on day 0). The total N, P and K in the soil before the experiment are presented in Table 3.

Table 6.
Dates of crop establishment, commencement of harvesting, and crop termination for the legume and the tomato crop.
During the experimental period, climatic data, particularly air temperature and relative humidity, were collected on an hourly basis. Monthly average temperature (mean, maximum, minimum) and relative humidity (%) values for all experiments are presented in Table 7.

Table 7.
Monthly averages for mean, maximum, and minimum daily temperatures (Tmean, Tmax and Tmin, respectively) and relative humidity (RHmean, RHmax and RHmin, respectively) inside the greenhouse during the experimental period (2018–2019) in Preveza, Greece.
In this experiment, four different treatments were established to test the impact of legumes cultivated as preceding crops on the succeeding organic tomato cultivation (Table 8). Specifically, in treatments 1 and 2, cowpea and common bean, respectively, were cultivated for harvesting fresh pods during autumn and winter of 2018. In treatments 3 and 4 faba bean was grown and incorporated into the soil before anthesis as green manure. Treatments 3 and 4 were identical at the stage of legume cultivation and differentiated afterwards by applying different sources of organic matter in each of them prior to establishment of the tomato crop.

Table 8.
Description of the treatments applied to test the impact of legumes as preceding crops to organic tomato cultivation.
In treatment 1, the seeds of cowpea were inoculated with Bradyrhizobium sp. VULI11 [52] and putative plant growth promoting rhizobacteria (PGPR). In treatment 2, the seeds of common bean were inoculated with Rhizobium sp. PVKA6 and PGPR, while in treatments 3 and 4 the seeds of faba bean were inoculated with Rhizobium sp. VFLE1 [53] and PGPR. In all treatments, the microorganisms applied as putative PGPR, which had been isolated from cowpea nodules were Enterobacter sp. strain C1.2, Enterobacter sp. strain C1.5, Enterobacter sp. strain C3.1, and Lelliottia sp. strain D2.4. Strain designations “C” and “D” represent the geographical regions of field-collected cowpea root nodules in Greece that are Epirus and Crete, respectively, and followed by a lab code number. Legume seeds were soaked for 2 min in gum arabic solution (20%) as adhesive to deliver 109 cfu/mL of the microbial cell suspensions. For combined inoculation, the liquid cultures were mixed in equal proportions. The inoculated seeds were spread in the shade, air-dried for 12 h and sown in well-prepared soil.
The crop residues of cowpea and common bean in treatments 1 and 2, respectively, and the entire biomass of faba bean in treatments 3 and 4 were incorporated into the soil at the end of January 2019. In treatments 1, 2 and 3, farm-yard manure (FYM) originating from free-range cattle farming was applied on 1st February 2019 at a rate of 50 t ha−1. The FYM contained 0.34% N, 0.15% P, and 0.48% K. This amount of FYM was equivalent to a N supply of 170 kg ha−1, which is in compliance with the European Union Regulation 889/2008. In treatment 4, olive-mill waste compost was applied, containing 1.26% N, 0.08% P and 1.03% K, at a rate of 30 t ha−1, on the same date with FYM, which provided 378 kg N ha−1. Four replicates were applied in each treatment.
The tomato crop was established 20 days after incorporation of the legume biomass and FYM or compost to the soil. The commercial tomato hybrid ‘Nissos F1’ (Hazera seeds Ltd., Berurim M.P Shikmim, Israel) grafted onto the commercial rootstock ‘Maxifort F1’ (Solanum lycopersicum × Solanum habrochaites) was transplanted on 20th February 2019. All plants were pruned to a single stem and the plant density was 2.13 plants/m2. The tomato and legume plants were drip-irrigated. During the cropping period, no additional fertilizers were provided to the plants in all treatments.
4.2. Growth, Mineral Analysis, and Nitrogen Fixation by Legumes
Immediately after termination of the autumn-winter legume crops, their plant biomass was incorporated into the soil. The aboveground biomass incorporated into the soil included only the vegetative plant residues remaining after harvesting of the pods in the cowpea and common bean plots. However, in the faba bean plots, the entire plant biomass was incorporated to the soil as green manure. Before incorporation into the soil, the aboveground biomass was quantified by harvesting the shoots from an area of 1 m2 in each plot center and measuring their total fresh weight. The samples of the aboveground fresh biomass were oven-dried at 65 °C to a constant weight and weighed to determine their dry biomass. Subsequently, each dry biomass sample was homogenized and a subsample was collected, ground using a ball mill and sieved through a 40 mesh sieve to determine total-N and carbon (C) contents in plant tissue samples by high temperature combustion using an elemental analyzer (Unicube, Elementar Analysensysteme GmbH, Hanau, Germany).
The dry biomass data from the cowpea and common bean crops were further used to determine the pod harvest index (PHI) as a percentage of harvested pod biomass to the total biomass of both pods and vegetative residues. Similarly, the N harvest index (NHI) was calculated as the percentage of N accumulated in the pods relative to the total N accumulated in pods and residues.
The N derived from the atmosphere in the aboveground biomass of legumes was determined by applying a method based on the natural abundance of 15N in plant tissues relative to the air [19,54,55]. To apply this method, the stable N isotopic composition of legume tissue samples was determined using an Isoprime 100 continuous flow isotope ratio mass spectrometer coupled to a Vario Isotope Select elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). The δ-values were calibrated relative to air by means of a three-point calibration using standard reference materials IAEA-N1, IAEA-600, and IAEA-N2. Measurement uncertainty was monitored by repeated measurements of internal laboratory standards and standard reference materials. Precision was determined to be ±0.19‰ based on repeated measurements of calibration standards and internal laboratory standards. Accuracy was determined to be ±0.19‰ on the basis of the difference between the observed and known δ values of check standards and their standard deviations. The total analytical uncertainty was estimated to be ±0.27‰ for δ15N. The δ15N values were estimated as parts per thousand (‰) deviations relative to the nominated international standard of atmospheric N2 (0.3663%), using the following equation [56]:
Subsequently, the percentage of N derived from the atmosphere (%Ndfa) was estimated by substituting the δ15N (‰) of the N2-fixing legume and a non-N2-fixing reference plant grown in the same soil, as calculated using Equation (1), into the following equation suggested by Unkovich et al. [54]:
where “B” is the δ15N in shoots of cowpea, common bean or faba bean plants grown on an inert medium and starved of N throughout their life, thereby being fully dependent on N2 fixation. The B values used in the current study were −1.61 for cowpea, –2.16 for common bean and −0.50 for faba bean, as suggested by Unkovich et al. [54]. The reference plant used in this study to determine the corresponding δ15N values was the grass weed Digitaria sanguinalis (L.).
To determine the total amounts of biologically-fixed N2 by cowpea, common bean and faba bean per cultivated area unit (BNF, kg ha−1), the following equation was used [57]:
where DB is the total dry biomass of the shoot, Nt is the total N concentration (% w/w) in the aboveground dry biomass, and %Ndfa are the values obtained from (2).
4.3. Tomato Tissue Sampling and Mineral Analysis
The N, P and K nutrition of the tomato crop was estimated by collecting samples of the youngest fully expanded leaves from all plots of the experiment 2.5 months after planting. The leaves were washed with distilled water, chopped, and oven-dried at 65 °C until they reached constant weight, powdered using a ball mill, and passed through a 40 mesh sieve. Subsequently, 0.5 g of powdered material was dry ashed in a muffle furnace at 550 °C for 5 h, and the ash was dissolved in 1 N HCl. Phosphorus (P) was measured photometrically as phosphomolybdate blue complex at 880 nm using a spectrophotometer (U-2000, Hitachi, Tokyo, Japan). Potassium (K) was determined using a flame photometer (Sherwood Model 410, Cambridge, UK). Organic C and total N in plant tissue samples were determined as described above for the legume crops.
4.4. Soil Analysis
Soil samples were collected from the central square of each plot (dimensions 2 × 2.5 m2). In each plot, 5 soil cores weighing about 400 g were collected from the root zone of 5 plants at a depth of 0–20 cm. Samples were prepared according to Miller et al. [58] and analyzed to determine the total N, NO3-N, NH4-N, and plant-available P and K concentrations. Total N in soil samples was determined by high temperature combustion using an elemental analyzer (Unicube, Elementar Analysensysteme GmbH, Hanau, Germany). To determine the concentration of mineral N (N-min, i.e., NO3-N + NH4-N) in the soil, each sample of sieved soil was extracted using a KCl solution, as described by Keeney and Nelson [59]. Subsequently, the NO3 and NH4 concentrations in the sample extracts were determined by applying the cadmium reduction to NO2 and the indophenol blue methods, respectively [59], using a Spectronic Helios spectrophotometer (Thermo Electron Corporation, Mercers Row, Cambridge CB5 8HY, UK). Plant-available P was determined using the Olsen method [60] and quantified by molybdate colorimetry [61]. Exchangeable soil K was determined using a flame photometer (Sherwood Model 420, Sherwood Scientific, Cambridge, UK) following extraction with an ammonium acetate solution.
4.5. Tomato Production and Yield Components
The impact of the experimental treatments on crop yield was assessed by harvesting all ripe tomatoes from 10 plants of the plot center twice per week and recording their number and total weight.
4.6. Statistical Analysis
The experiment was set as randomized block designs with 4 treatments and 4 replicates per treatment. The data were statistically analyzed by applying ANOVA using the STATISTICA software package, version 12.0 for Windows. The Duncan’s multiple range test was applied to separate means when the ANOVA was significant at p < 0.05. Data are presented in graphs and tables as means ± SE of four replicates.
5. Conclusions
In organic greenhouse tomato, a pre-crop of legumes can provide additional N to the crop, as the high N demands cannot be fully covered by application of animal manure due to restrictions in the maximum allowed amounts imposed by EU legislation. Common bean aiming to produce green pods does not provide substantial amounts of N, as its efficiency to fix N2 symbiotically is low. Hence, the amount of N removed by harvesting green pods is higher than that fixed symbiotically. Cowpea is more efficient in symbiotic N fixation, while its potential for green pod production is lower, and thus it may leave some of the symbiotically fixed N to the next crop. However, the best option proved to be the cultivation of faba bean as green manure, which can successfully supplement or even substitute farm-yard manure in some cultivation plans. In all treatments, the %Ndfa in the current study was lower than the potential levels, due to the relatively high mineral N concentrations in the soil before sowing legumes, originating from intensive mineralization during summer, when soil solarization was applied.
The compost of olive-mill waste did not adequately replace farm-yard manure as it provided lower amounts of soil mineral N during tomato cultivation that led to lower fruit yield.
The lack of synchronization in N supply through legume biomass mineralization and N demand by tomato was confirmed in the present study. During the vegetative and the initial reproductive stage of tomato there was an excess in soil mineral N. However, the mineral N levels dropped to lower levels than the optimal range during the latter cropping stage in all treatments, although this reduction was milder in the faba bean plots. The yield performance was commensurate with the levels of mineral N at the late cropping stage of tomato. This result indicates that the mineral N level is the main restrictive factor for yield performance in organic greenhouse tomato, which has a longer harvesting period than open-field crops. Practices increasing the exploitation of the organic matter incorporated to the soil as N fertilizer source in greenhouse organic tomato might prevent N deficiencies at the late cropping stages and enhance yield performance.
Author Contributions
D.S. and A.G. conceived and designed the experiments. A.G., G.N., L.C., D.S.-P. and A.T. performed the experiments and the analyses. A.G. and D.S. analyzed the data and wrote the paper. A.G., G.N., L.C., D.S.-P., A.T. and D.S. reviewed the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Commission within the HORIZON2020 project “TOMRES—A novel and integrated approach to increase multiple combined stress tolerance in plants using tomato as a model” (Grant Agreement 727929).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Willer, H.; Lernoud, J. The World of Organic Agriculture: Statistics and Emerging Trends 2019; Research Institute of Organic Agriculture FiBL: Frick, Switzerland; IFOAM Organics International: Bonn, Germany, 2019; pp. 1–336. [Google Scholar]
- Ponisio, L.C.; Gonigle, L.K.M.; Mace, K.C.; Palomino, J.; De Valpine, P.; Kremen, C. Diversification practices reduce organic to conventional yield gap. Proc. Royal Soc. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef]
- Birkhofer, K.; Smith, H.G.; Rundlöf, M. Environmental impacts of organic farming. eLS 2016, 1–7. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N. Many shades of gray—The context-dependent performance of organic agriculture. Sci. Adv. 2017, 3, e1602638. [Google Scholar] [CrossRef]
- Röös, E.; Mie, A.; Wivstad, M.; Salomon, E.; Johansson, B.; Gunnarsson, S.; Wallenbeck, A.; Hoffmann, R.; Nilsson, U.; Sundberg, C.; et al. Risks and opportunities of increasing yields in organic farming. A review. Agron. Sustain. Dev. 2018, 38, 14. [Google Scholar] [CrossRef]
- Watson, C.A.; Atkinson, D.; Gosling, P.; Jackson, L.R.; Rayns, F.W. Managing soil fertility in organic farming systems. Soil Use Manag. 2002, 18, 239–247. [Google Scholar] [CrossRef]
- Dumas, Y.; Quijada, J.S.; Bonafous, M. Influence of nitrogen availability on growth and development of tomato plants until fruit-setting. In Optimization of Plant Nutrition; Springer: Dordrecht, The Netherlands, 1993; pp. 235–241. [Google Scholar]
- Commission Regulation (EC) 889 Commission Regulation (EC) No 889/2008 of 5 September 2008 Laying down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Co. Available online: http://data.europa.eu/eli/reg/2008/889/oj (accessed on 7 January 2020).
- Askegaard, M.; Olesen, J.E.; Rasmussen, I.A.; Kristensen, K. Nitrate leaching from organic arable crop rotations is mostly determined by autumn field management. Agric. Ecosyst. Environ. 2011, 142, 149–160. [Google Scholar] [CrossRef]
- Bustamante, S.C.; Hartz, T.K. Nitrogen management in organic processing tomato production: Nitrogen sufficiency prediction through early-season soil and plant monitoring. HortScience 2015, 50, 1055–1063. [Google Scholar] [CrossRef]
- Clark, M.S.; Horwath, W.R.; Shennan, C.; Scow, K.M.; Lantni, W.T.; Ferris, H. Nitrogen, weeds and water as yield-limiting factors in conventional, low-input, and organic tomato systems. Agric. Ecosyst. Environ. 1999, 73, 257–270. [Google Scholar] [CrossRef]
- Dahlin, S.; Kirchmann, H.; Kätterer, T.; Gunnarsson, S.; Bergström, L. Possibilities for improving nitrogen use from organic materials in agricultural cropping systems. AMBIO A J. Hum. Environ. 2005, 34, 288–295. [Google Scholar] [CrossRef]
- Doltra, J.; Lægdsmand, M.; Olesen, J.E. Cereal yield and quality as affected by nitrogen availability in organic and conventional arable crop rotations: A combined modeling and experimental approach. Eur. J. Agron. 2011, 34, 83–95. [Google Scholar] [CrossRef]
- Briggs, S. Nitrogen Supply and Management in Organic Farming; Report; Institute of Organic Training and Advice (IOTA): Craven Arms, Shropshire, UK, 2008; pp. 1–30. [Google Scholar]
- Vasconcelos, M.W.; Grusak, M.A.; Pinto, E.; Gomes, A.; Ferreira, H.; Balázs, B.; Centofanti, T.; Ntatsi, G.; Savvas, D.; Karkanis, A.; et al. The Biology of Legumes and Their Agronomic, Economic, and Social Impact. In The Plant Family Fabaceae; Springer: Singapore, 2020; pp. 3–25. [Google Scholar]
- Fatima, T.; Teasdale, J.R.; Bunce, J.; Mattoo, A.K. Tomato response to legume cover crop and nitrogen: Differing enhancement patterns of fruit yield, photosynthesis and gene expression. Funct. Plant Biol. 2012, 39, 246. [Google Scholar] [CrossRef] [PubMed]
- Araki, H. Tomato production with cover cops in greenhouse. In Alternative Crops and Cropping Systems; Intechopen: London, UK, 2016; p. 87. [Google Scholar]
- Galieni, A.; Stagnari, F.; Speca, S.; D’Egidio, S.; Pagnani, G.; Pisante, M. Management of crop residues to improve quality traits of tomato (Solanum lycopersicum L.) fruits. Ital. J. Agron. 2017, 12, 59–62. [Google Scholar] [CrossRef]
- Gatsios, A.; Ntatsi, G.; Celi, L.; Said-Pullicino, D.; Tampakaki, A.; Giannakou, I.; Savvas, D. Nitrogen nutrition optimization in organic greenhouse tomato through the use of legume plants as green manure or intercrops. Agronomy 2019, 9, 766. [Google Scholar] [CrossRef]
- Denton, M.D.; Phillips, L.A.; Peoples, M.B.; Pearce, D.J.; Swan, A.D.; Mele, P.M.; Brockwell, J. Legume inoculant application methods: Effects on nodulation patterns, nitrogen fixation, crop growth and yield in narrow-leaf lupin and faba bean. Plant Soil 2017, 419, 25–39. [Google Scholar] [CrossRef]
- Karavidas, I.; Ntatsi, G.; Ntanasi, T.; Vlachos, I.; Tampakaki, A.; Iannetta, P.P.M.; Savvas, D. Comparative assessment of different crop rotation schemes for organic common bean production. Agronomy 2020, 10, 1269. [Google Scholar] [CrossRef]
- Berry, P.M.; Stockdale, E.A.; Sylvester-Bradley, R.; Philipps, L.; Smith, K.A.; Lord, E.I.; Watson, C.A.; Fortune, S. N, P and K budgets for crop rotations on nine organic farms in the UK. Soil Use Manag. 2003, 19, 112–118. [Google Scholar] [CrossRef]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadisch, G.; Boddey, B.; Giller, K.; Alves, B.; Chalk, P. Biological nitrogen fixation. In Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; Australian Centre for International Agricultural Research (ACIAR): Canberra, ACT, Australia, 2008; pp. 9–20. ISBN 1921531266. [Google Scholar]
- Barker, A.V. Science and Technology of Organic Farming; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1439882134. [Google Scholar]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Ntatsi, G.; Karkanis, A.; Yfantopoulos, D.; Olle, M.; Travlos, I.; Thanopoulos, R.; Bilalis, D.; Bebeli, P.; Savvas, D. Impact of variety and farming practices on growth, yield, weed flora and symbiotic nitrogen fixation in faba bean cultivated for fresh seed production. Acta Agric. Scand. Sect. B Soil Plant Sci. 2018, 68, 619–630. [Google Scholar] [CrossRef]
- Thönnissen, C.; Midmore, D.J.; Ladha, J.K.; Holmer, R.J.; Schmidhalter, U. Tomato crop response to short-duration legume green manures in tropical vegetable systems. Agron. J. 2000, 92, 245–253. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Hauggaard-Nielsen, H. Faba bean in cropping systems. Field Crop. Res. 2010, 115, 203–216. [Google Scholar] [CrossRef]
- Oliveira, M.; Castro, C.; Coutinho, J.; Trindade, H. N supply and pre-cropping benefits to triticale from three legumes in rainfed and irrigated Mediterranean crop rotations. Field Crop. Res. 2019, 237, 32–42. [Google Scholar] [CrossRef]
- Ruisi, P.; Amato, G.; Badagliacca, G.; Frenda, A.S.; Giambalvo, D.; Di Miceli, G. Agro-ecological benefits of faba bean for rainfed mediterranean cropping systems. Ital. J. Agron. 2017, 12, 233–245. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Z.; Tian, D.; Wang, J.; Fu, Z.; Zhang, F.; Zhang, R.; Chen, W.; Luo, Y.; Niu, S. Global patterns and controlling factors of soil nitrification rate. Glob. Chang. Biol. 2020, 26, 4147–4157. [Google Scholar] [CrossRef]
- Barnard, R.; Leadley, P.W.; Hungate, B.A. Global change, nitrification, and denitrification: A review. Glob. Biogeochem. Cycles 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Pandey, A.; Li, F.; Askegaard, M.; Rasmussen, I.A.; Olesen, J.E. Nitrogen balances in organic and conventional arable crop rotations and their relations to nitrogen yield and nitrate leaching losses. Agric. Ecosyst. Environ. 2018, 265, 350–362. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Magid, J.; Jensen, L.S. Catch crops and green manures as biological tools in nitrogen management in temperate zones. Adv. Agron. 2003, 79, 227–302. [Google Scholar]
- Sullivan, D.M.; Andrews, N. Estimating Plant-Available Nitrogen Release from Cover Crops; Oregon State University: Corvallis, OR, USA, 2012. [Google Scholar]
- Lenzi, A.; Antichi, D.; Bigongiali, F.; Mazzoncini, M.; Migliorini, P.; Tesi, R. Effect of different cover crops on organic tomato production. Renew. Agric. Food Syst. 2009, 24, 92–101. [Google Scholar] [CrossRef]
- Sainju, U.M.; Dris, R.; Singh, B. Mineral nutrition of tomato. Food Agric. Environ. 2003, 1, 176–184. [Google Scholar]
- Van Eysinga, J.R. Fertilization of Tomatoes with Nitrogen; Pudoc: Wageningen, The Netherlands, 1971. [Google Scholar]
- Bénard, C.; Gautier, H.; Bourgaud, F.; Grasselly, D.; Navez, B.; Caris-Veyrat, C.; Weiss, M.; Génard, M. Effects of low nitrogen supply on tomato (Solanum lycopersicum) fruit yield and quality with special emphasis on sugars, acids, ascorbate, carotenoids, and phenolic compounds. J. Agric. Food Chem. 2009, 57, 4112–4123. [Google Scholar] [CrossRef]
- Ren, T.; Christie, P.; Wang, J.; Chen, Q.; Zhang, F. Root zone soil nitrogen management to maintain high tomato yields and minimum nitrogen losses to the environment. Sci. Hortic. 2010, 125, 25–33. [Google Scholar] [CrossRef]
- Zotarelli, L.; Dukes, M.D.; Scholberg, J.M.S.; Muñoz-Carpena, R.; Icerman, J. Tomato nitrogen accumulation and fertilizer use efficiency on a sandy soil, as affected by nitrogen rate and irrigation scheduling. Agric. Water Manag. 2009, 96, 1247–1258. [Google Scholar] [CrossRef]
- Baldwin, K.R. Soil fertility on organic farms. Center for Environmental Farming Systems; North Carolina Cooperative Extension: Raleigh, NC, USA, 2006. [Google Scholar]
- Nair, A.; Delate, K. Composting, crop rotation, and cover crop practices in organic vegetable production. In Organic Farming for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 231–257. [Google Scholar]
- Papadopoulos, A.P. Growing Greenhouse Tomatoes in Soil and in Soilless Media; Communications Branch, Agriculture Canada: Ottawa, ON, Canada, 1991; ISBN 0662188594. [Google Scholar]
- Gianquinto, G.; Muñoz, P.; Pardossi, A.; Ramazzotti, S.; Savvas, D. Soil Fertility and Plant Nutrition. Good Agricultural Practices for Greenhouse Vegetable Crops. Principles for Mediterranean Climate Areas; FAO: Rome, Italy, 2013; ISBN 9789251076491. [Google Scholar]
- Reckling, M.; Bergkvist, G.; Watson, C.A.; Stoddard, F.L.; Zander, P.M.; Walker, R.L.; Pristeri, A.; Toncea, I.; Bachinger, J. Trade-offs between economic and environmental impacts of introducing legumes into cropping systems. Front. Plant Sci. 2016, 7, 669. [Google Scholar] [CrossRef]
- Berry, P.M.; Sylvester-Bradley, R.; Philipps, L.; Hatch, D.J.; Cuttle, S.P.; Rayns, F.W.; Gosling, P. Is the productivity of organic farms restricted by the supply of available nitrogen? Soil Use Manag. 2002, 18, 248–255. [Google Scholar] [CrossRef]
- Jensen, E.S.; Ambus, P. Prospects for manipulating crop residues to control nitrogen mineralisation-immobilisation in soil. Kungl. Skogs.-o Lantbr.-akad. Tidskr 2000, 139, 8–25. [Google Scholar]
- Tampakaki, A.P.; Fotiadis, C.T.; Ntatsi, G.; Savvas, D. Phylogenetic multilocus sequence analysis of indigenous slow-growing rhizobia nodulating cowpea (Vigna unguiculata L.) in Greece. Syst. Appl. Microbiol. 2017, 40, 179–189. [Google Scholar] [CrossRef]
- Efstathiadou, E.; Savvas, D.; Tampakaki, A.P. Genetic diversity and phylogeny of indigenous rhizobia nodulating faba bean (Vicia faba L.) in Greece. Syst. Appl. Microbiol. 2020, 43, 126149. [Google Scholar] [CrossRef]
- Unkovich, M.J.; Herridge, D.; Peoples, M.; Cadish, G.; Boddey, R.; Giller, K.; Alves, B.; Chalk, P. N natural abundance method. In Measuring Plant Associated Nitrogen Fixation in Agricultural Systems; Australian Centre for International Agricultural Research (ACIAR): Canberra, ACT, Australia, 2008; pp. 131–162. ISBN 978-1-921531-26-2. [Google Scholar]
- Ntatsi, G.; Karkanis, A.; Yfantopoulos, D.; Pappa, V.; Konosonoka, I.H.; Travlos, I.; Bilalis, D.; Bebeli, P.; Savvas, D. Evaluation of the field performance, nitrogen fixation efficiency and competitive ability of pea landraces grown under organic and conventional farming systems. Arch. Agron. Soil Sci. 2019, 65, 294–307. [Google Scholar] [CrossRef]
- Bedard-Haughn, A.; van Groenigen, J.W.W.; van Kessel, C. Tracing 15N landscapes: Potential uses and precautions. J. Hydrol. 2003, 272, 175–190. [Google Scholar] [CrossRef]
- Collino, D.J.; Salvagiotti, F.; Perticari, A.; Piccinetti, C.; Ovando, G.; Urquiaga, S.; Racca, R.W. Biological nitrogen fixation in soybean in Argentina: Relationships with crop, soil, and meteorological factors. Plant Soil 2015, 392, 239–252. [Google Scholar] [CrossRef]
- Miller, R.O.; Gavlak, R.; Horneck, D. Soil, Plant and Water Reference Methods for the Western Region, 4th ed.; WREP-125: Fort Collins, CO, USA, 2013. [Google Scholar]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic Forms. Methods Soil Anal. 1983, 9, 643–698. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954; p. 939. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).