The Absence of Hydrodynamic Stress Promotes Acquisition of Freezing Tolerance and Freeze-Dependent Asexual Reproduction in the Red Alga ‘Bangia’ sp. ESS1
Abstract
1. Introduction
2. Results
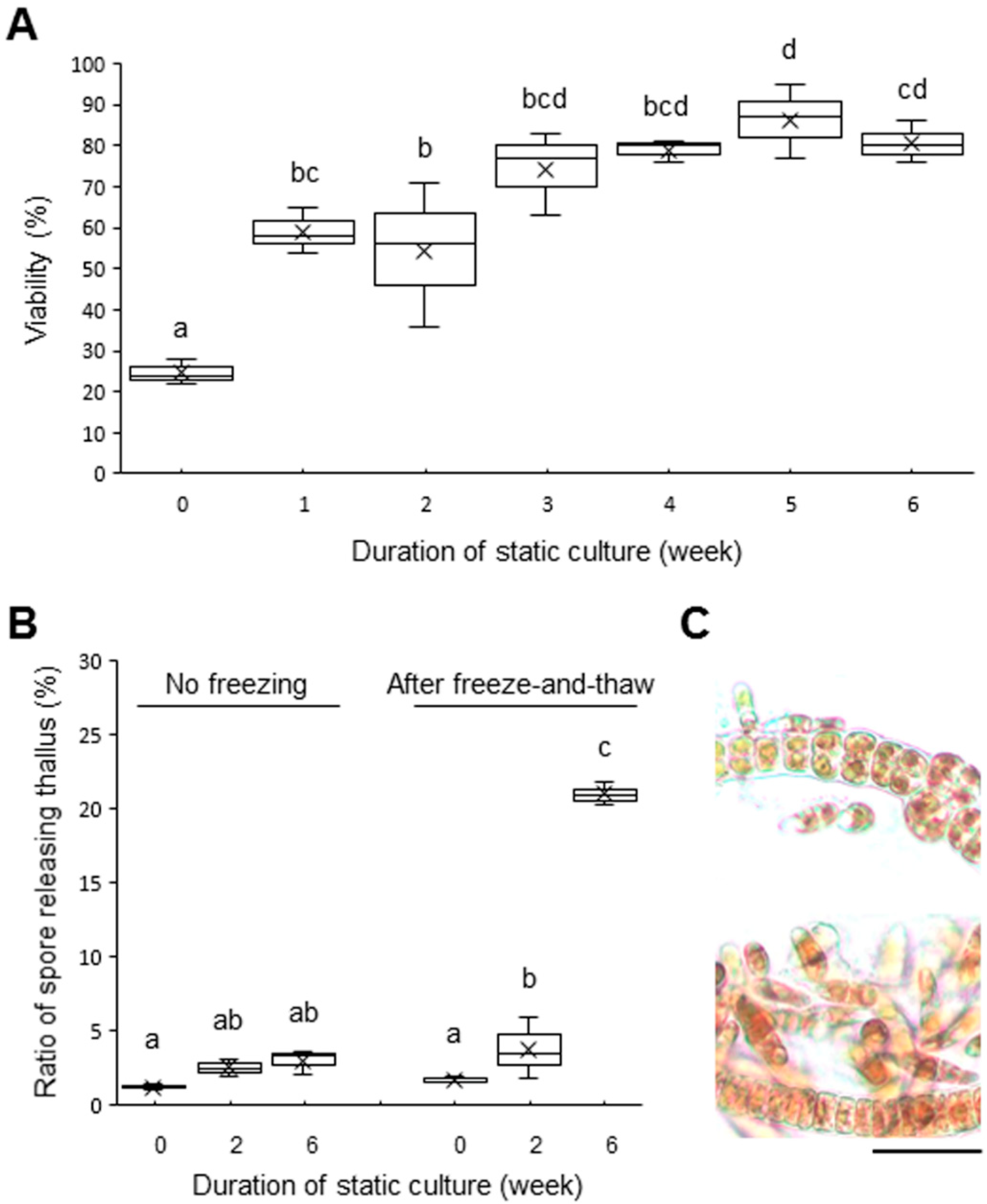
2.1. Acquisition of Freezing Tolerance by Exposure to Calm Stress
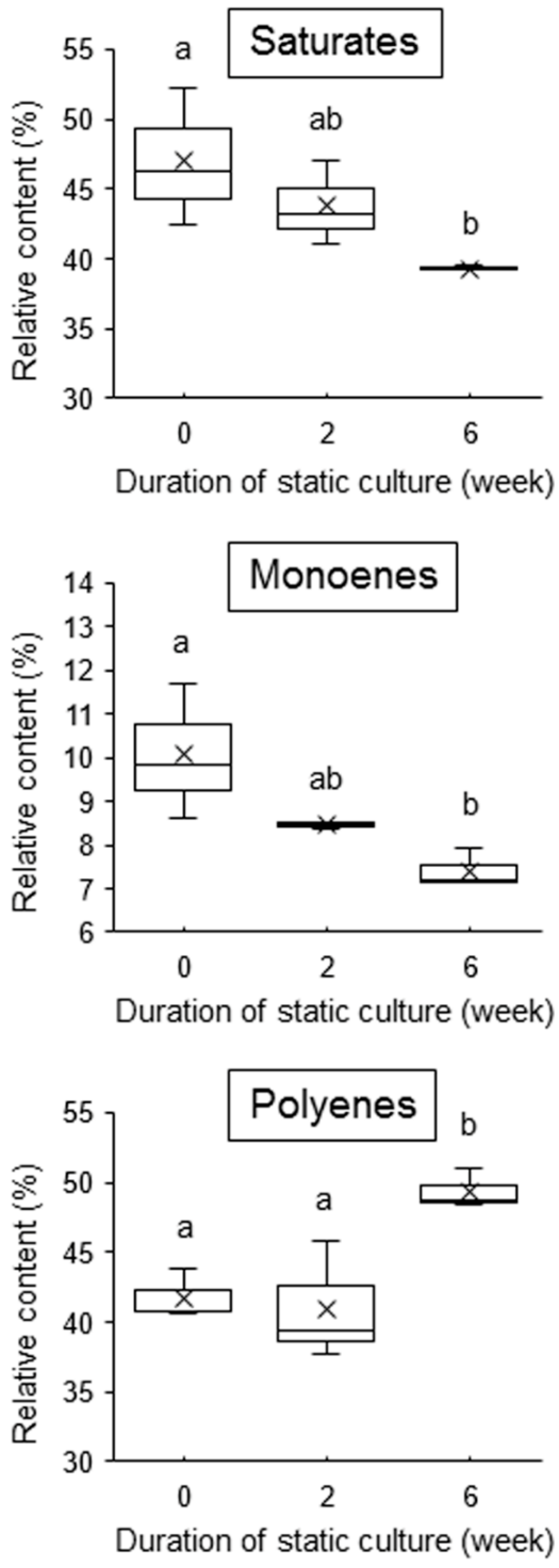
2.2. Unsaturation of Membrane Fatty Acids under Calm Stress Conditions
3. Discussion
4. Materials and Methods
4.1. Algal Material and Stress Treatment
4.2. Viability Test and Observation of Asexual Spore Release
4.3. Analysis of Membrane Fatty Acid Composition
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sutherland, J.E.; Lindstrom, S.C.; Nelson, W.A.; Brodie, J.; Lynch, M.D.J.; Hwang, M.S.; Choi, H.-G.; Miyata, M.; Kikuchi, N.; Oliveira, M.C.; et al. A new look at an ancient order: Generic revision of the Bangiales (Rhodophyta). J. Phycol. 2011, 47, 1131–1151. [Google Scholar] [CrossRef]
- Yang, L.E.; Lu, Q.Q.; Brodie, J. A review of the bladed Bangiales (Rhodophyta) in China: History, culture and taxonomy. Eur. J. Phycol. 2017, 52, 251–263. [Google Scholar] [CrossRef]
- Sun, P.; Mao, Y.; Li, G.; Cao, M.; Kong, F.; Wang, L.; Bi, G. Comparative transcriptome profiling of Pyropia yezoensis (Ueda) M.S. Hwang & H.G. Choi in response to temperature stresses. BMC Genom. 2015, 16, 463. [Google Scholar]
- Sun, P.; Tang, X.; Bi, G.; Xu, K.; Kong, F.; Mao, Y. Gene expression profiles of Pyropia yezoensis in response to dehydration and rehydration stresses. Mar. Genomics 2019, 43, 43–49. [Google Scholar] [CrossRef]
- Cao, M.; Wang, D.; Mao, Y.; Kong, F.; Bi, G.; Xing, Q.; Weng, Z. Integrating transcriptomics and metabolomics to characterize the regulation of EPA biosynthesis in response to cold stress in seaweed ‘Bangia’ fuscopurpurea. PLoS ONE 2017, 12, e0186986. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Kong, F.; Sun, P.; Bi, G.; Mao, Y. Transcriptome-wide identification of optimal reference genes for expression analysis of Pyropia yezoensis responses to abiotic stress. BMC Genom. 2018, 19, 251. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Teng, F.; Lin, Y.; Ji, D.; Xu, Y.; Chen, C.; Xie, C. Transcriptomic study to understand thermal adaptation in a high temperature-tolerant strain of Pyropia haitanensis. PLoS ONE 2018, 13, e0195842. [Google Scholar] [CrossRef]
- Wang, W.; Shen, Z.; Sun, X.; Liu, F.; Liang, Z.; Wang, F.; Zhu, J. De novo transcriptomics analysis revealed a global reprogramming towards dehydration and hyposalinity in ‘Bangia’ fuscopurpurea gametophytes (Rhodophyta). J. Appl. Phycol. 2019, 31, 637–651. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Chen, T.; Xing, L.; Xu, K.; Xu, Y.; Ji, D.; Chen, C.; Xie, C. Regulatory mechanisms underlying the maintenance of homeostasis in Pyropia haitanensis under hypersaline stress conditions. Sci. Total Environ. 2019, 662, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Wanner, L.A.; Junttila, O. Cold-induced freezing tolerance in Arabidopsis. Plant Physiol. 1999, 120, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef]
- Tan, T.; Sun, Y.; Peng, X.; Wu, G.; Bao, G.; He, F.; Zhou, H.; Lin, H. ABSCISIC ACID INSENSITIVE3 is involved in cold response and freezing tolerance regulation in Physcomitrella patens. Front. Plant Sci. 2017, 8, 1599. [Google Scholar]
- Thomashow, M.F. PLANT COLD ACCLIMATION: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Furumoto, T. Cold signaling and cold response in plants. Int. J. Mol. Sci. 2013, 14, 5312–5337. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Gilboa, A. Cryopreservation of marine unicellular algae. II. Induction of freezing tolerance. Mar. Ecol. 1980, 2, 157161. [Google Scholar] [CrossRef]
- Nagao, M.; Matsui, K.; Uemura, M. Klebsormidium flaccidum, a charophycean green alga, exhibits cold acclimation that is closely associated with compatible solute accumulation and ultrastructural changes. Plant Cell Environ. 2008, 31, 872–885. [Google Scholar] [CrossRef]
- Anandarajah, K.; Perumal, G.M.; Sommerfeld, M.; Hu, Q. Induced freezing and desiccation tolerance in the microalgae wild type Nannochloropsis sp. and Scenedesmus dimorphus. Aust. J. Basic Appl. Sci. 2011, 5, 678–686. [Google Scholar]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Foyer, C.H.; Rasool, B.; Davey, J.W.; Hancock, R.D. Cross-tolerance to biotic and abiotic stresses in plants: A focus on resistance to aphid infestation. J. Exp. Bot. 2016, 67, 2025–2037. [Google Scholar] [CrossRef]
- Yasuda, H. Cross-tolerance to thermal stresses and its application to the development of cold tolerant rice. Jpn. Agric. Res. Q. 2017, 51, 99–105. [Google Scholar] [CrossRef]
- Siminovitch, D.; Cloutier, Y. Twenty-four-hour induction of freezing and drought tolerance in plumules of winter rye seedlings by desiccation stress at room temperature in the dark. Plant Physiol. 1982, 69, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, Y.; Andrews, C.J. Efficiency of cold hardiness induction by desiccation stress in four winter cereals. Plant Physiol. 1984, 76, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Mikami, K.; Murata, N. Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog. Lipid. Res. 2003, 42, 527–543. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid. Res. 2006, 45, 160–186. [Google Scholar] [CrossRef]
- Barrero-Sicilia, C.; Silvestre, S.; Haslam, R.P.; Michaelson, L.V. Lipid remodelling: Unravelling the response to cold stress in Arabidopsis and its extremophile relative Eutrema salsugineum. Plant Sci. 2017, 263, 194–200. [Google Scholar] [CrossRef]
- Kodama, H.; Hamada, T.; Horiguchi, G.; Nishimura, M.; Iba, K. Genetic enhancement of cold tolerance by expression of a gene for chloroplast [omega]-3 fatty acid desaturase in transgenic tobacco. Plant Physiol. 1994, 105, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Barg, R.; Yin, M.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated fatty acid desaturation via overexpression of two distinct omega-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J. 2005, 44, 361–371. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; McAvoy, R.; Peters, J.; Wu, H.; Li, Y. Enhanced cold tolerance in transgenic tobacco expressing a chloroplast omega-3 fatty acid desaturase gene under the control of a cold-inducible promoter. Planta 2006, 223, 1090–1100. [Google Scholar] [CrossRef]
- Yu, C.; Wang, H.S.; Yang, S.; Tang, X.F.; Duan, M.; Meng, Q.W. Overexpression of endoplasmic reticulum omega-3 fatty acid desaturase gene improves chilling tolerance in tomato. Plant Physiol. Biochem. 2009, 47, 1102–1112. [Google Scholar] [CrossRef]
- Román, Á.; Andreu, V.; Hernández, M.L.; Lagunas, B.; Picorel, R.; Martínez-Rivas, J.M.; Alfonso, M. Contribution of the different omega-3 fatty acid desaturase genes to the cold response in soybean. J. Exp. Bot. 2012, 63, 4973–4982. [Google Scholar] [CrossRef]
- Shi, J.; Cao, Y.; Fan, X.; Li, M.; Wang, Y.; Ming, F. A rice microsomal delta-12 fatty acid desaturase can enhance resistance to cold stress in yeast and Oryza sativa. Mol. Breed. 2012, 29, 743–757. [Google Scholar] [CrossRef]
- Shi, Y.; Yue, X.; An, L. Integrated regulation triggered by a cryophyte ω-3 desaturase gene confers multiple-stress tolerance in tobacco. J. Exp. Bot. 2018, 69, 2131–2148. [Google Scholar] [CrossRef]
- Xue, M.; Guo, T.; Ren, M.; Wang, Z.; Tang, K.; Zhang, W. Constitutive expression of chloroplast glycerol-3-phosphate acyltransferase from Ammopiptanthus mongolicus enhances unsaturation of chloroplast lipids and tolerance to chilling, freezing and oxidative stress in transgenic Arabidopsis. Plant Physiol. Biochem. 2019, 143, 375–387. [Google Scholar] [CrossRef]
- Li, C.; Irie, R.; Shimada, S.; Mikami, K. Requirement of different normalization genes for quantitative gene expression analysis under abiotic stress conditions in ‘Bangia’ sp. ESS1. J. Aquat. Res. Mar. Sci. 2019, 2019, 194–205. [Google Scholar]
- Mikami, K.; Kishimoto, I. Temperature promoting the asexual life cycle program in ‘Bangia’ fuscopurpurea (Bangiales, Rhodophyta) from Esashi in the Hokkaido Island, Japan. Algal Resour. 2018, 11, 25–32. [Google Scholar]
- Kishimoto, I.; Ariga, I.; Itabashi, Y.; Mikami, K. Heat-stress memory is responsible for acquired thermotolerance in Bangia fuscopurpurea. J. Phycol. 2019, 55, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Hirata, R.; Takahashi, M.; Saga, N.; Mikami, K. Transient gene expression system established in Porphyra yezoensis is widely applicable in Bangiophycean algae. Mar. Biotechnol. 2011, 13, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.A.; Serrão, E.A.; Brawley, S.H. Control of gamete release in fucoid algae: Sensing hydrodynamic conditions via carbon acquisition. Ecology 1998, 79, 1725–1739. [Google Scholar] [CrossRef]
- Brawley, S.H.; Johnson, L.E.; Pearson, G.A.; Speransky, V.; Li, R.; Serrão, E.A. Gamete release at low tide in fucoid algae: Maladaptive or advantageous? Am. Zool. 1999, 39, 218–229. [Google Scholar] [CrossRef]
- Speransky, S.R.; Brawley, S.H.; Halteman, W.A. Gamete re-lease is increased by calm conditions in the coenocytic green alga Bryopsis (Chlorophyta). J. Phycol. 2000, 36, 730–739. [Google Scholar] [CrossRef]
- Li, L.; Saga, N.; Mikami, K. Phosphatidylinositol 3-kinase activity and asymmetrical accumulation of F-actin are necessary for establishment of cell polarity in the early development of monospores from the marine red alga Porphyra yezoensis. J. Exp. Bot. 2008, 59, 3575–3586. [Google Scholar] [CrossRef]
- Takahashi, M.; Mikami, K. Oxidative stress promotes asexual reproduction and apogamy in the red seaweed Pyropia yezoensis. Front. Plant Sci. 2017, 8, 62. [Google Scholar] [CrossRef]
- Suda, M.; Mikami, K. Reproductive responses to wounding and heat stress in gametophytic thalli of the red alga Pyropia yezoensis. Front. Mar. Sci. 2020, 7, 394. [Google Scholar] [CrossRef]
- Takahashi, M.; Mikami, K.; Mizuta, H.; Saga, N. Identification and efficient utilization of antibiotics for the development of a stable transformation system in Porphyra yezoensis (Bangia, Rhodophyta). J. Aquac. Res. Dev. 2011, 2, 118. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W.; Han, X. Preparation of derivatives of fatty acids. In Lipid Analysis, 4th ed.; Christie, W.W., Han, X., Eds.; The Oily Press: Bridgwater, UK, 2010; pp. 145–158. [Google Scholar]
- Mikami, K.; Ito, M.; Taya, K.; Kishimoto, I.; Kobayashi, T.; Itabashi, Y.; Tanaka, R. Parthenosporophytes of the brown alga Ectocarpus siliculosus exhibit sex-dependent differences in thermotolerance as well as fatty acid and sterol composition. Mar. Environ. Res. 2018, 137, 188–195. [Google Scholar] [CrossRef]
- Arisz, S.A.; Heo, J.Y.; Koevoets, I.T.; Zhao, T.; van Egmond, P.; Meyer, A.J.; Zeng, W.; Niu, X.; Wang, B.; Mitchell-Olds, T.; et al. DIACYLGLYCEROL ACYLTRANSFERASE1 contributes to freezing tolerance. Plant Physiol. 2018, 177, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.-J.; Yang, Y.-C.; Zhou, Y.; Huang, L.-P.; Xu, L.; Chen, Q.-F.; Yu, L.-J.; Xiao, S. DIACYLGLYCEROL ACYLTRANSFERASE and DIACYLGLYCEROL KINASE modulate triacylglycerol and phosphatidic acid production in the plant response to freezing stress. Plant Physiol. 2018, 177, 1303–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dong, J.; Zhao, X.; Zhang, Y.; Ren, J.; Xing, L.; Jiang, C.; Wang, X.; Wang, J.; Zhao, S.; et al. Research progress in membrane lipid metabolism and molecular mechanism in peanut cold tolerance. Front. Plant Sci. 2019, 10, 838. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omuro, Y.; Khoa, H.V.; Mikami, K. The Absence of Hydrodynamic Stress Promotes Acquisition of Freezing Tolerance and Freeze-Dependent Asexual Reproduction in the Red Alga ‘Bangia’ sp. ESS1. Plants 2021, 10, 465. https://doi.org/10.3390/plants10030465
Omuro Y, Khoa HV, Mikami K. The Absence of Hydrodynamic Stress Promotes Acquisition of Freezing Tolerance and Freeze-Dependent Asexual Reproduction in the Red Alga ‘Bangia’ sp. ESS1. Plants. 2021; 10(3):465. https://doi.org/10.3390/plants10030465
Chicago/Turabian StyleOmuro, Yoshiki, Ho Viet Khoa, and Koji Mikami. 2021. "The Absence of Hydrodynamic Stress Promotes Acquisition of Freezing Tolerance and Freeze-Dependent Asexual Reproduction in the Red Alga ‘Bangia’ sp. ESS1" Plants 10, no. 3: 465. https://doi.org/10.3390/plants10030465
APA StyleOmuro, Y., Khoa, H. V., & Mikami, K. (2021). The Absence of Hydrodynamic Stress Promotes Acquisition of Freezing Tolerance and Freeze-Dependent Asexual Reproduction in the Red Alga ‘Bangia’ sp. ESS1. Plants, 10(3), 465. https://doi.org/10.3390/plants10030465








