Removal of Methylene Blue and Congo Red Using Adsorptive Membrane Impregnated with Dried Ulva fasciata and Sargassum dentifolium
Abstract
1. Introduction
2. Results and Discussion
2.1. One Factor at a Time (OFAT) Method (First Optimization Step)
2.1.1. Effect of Contact Time
2.1.2. Effect of pH
2.1.3. Effect of Algal Dose in the Membrane
2.1.4. Effect of Dyes Concentration
2.2. Full Factorial Design Experiment (Second Optimization Step)
2.2.1. Main Effects
2.2.2. Interaction Effect
2.2.3. Pareto Chart
2.2.4. Normal Probability Plot
2.2.5. Response Optimizer
2.3. Langmuir and Freundlich Isotherms
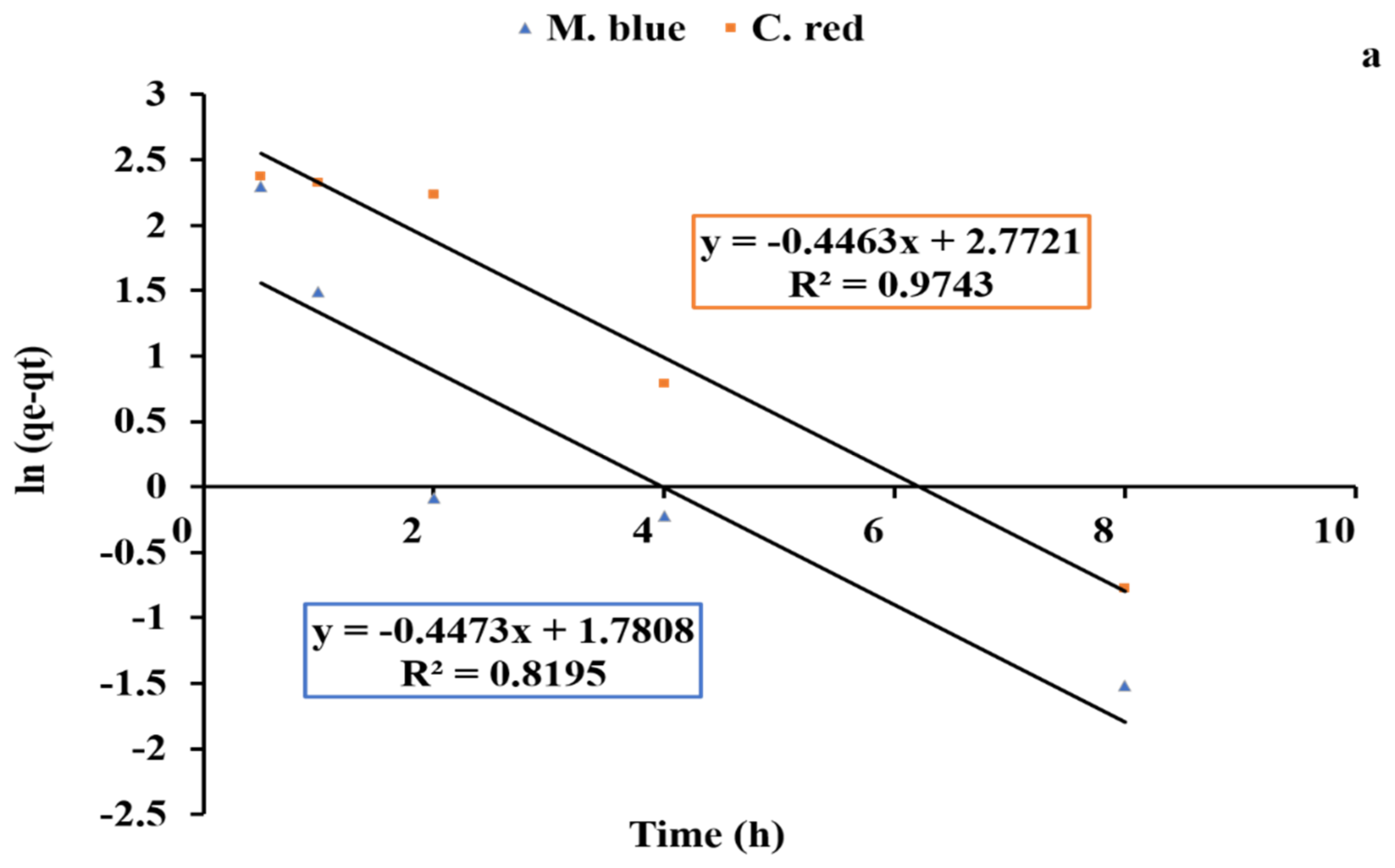
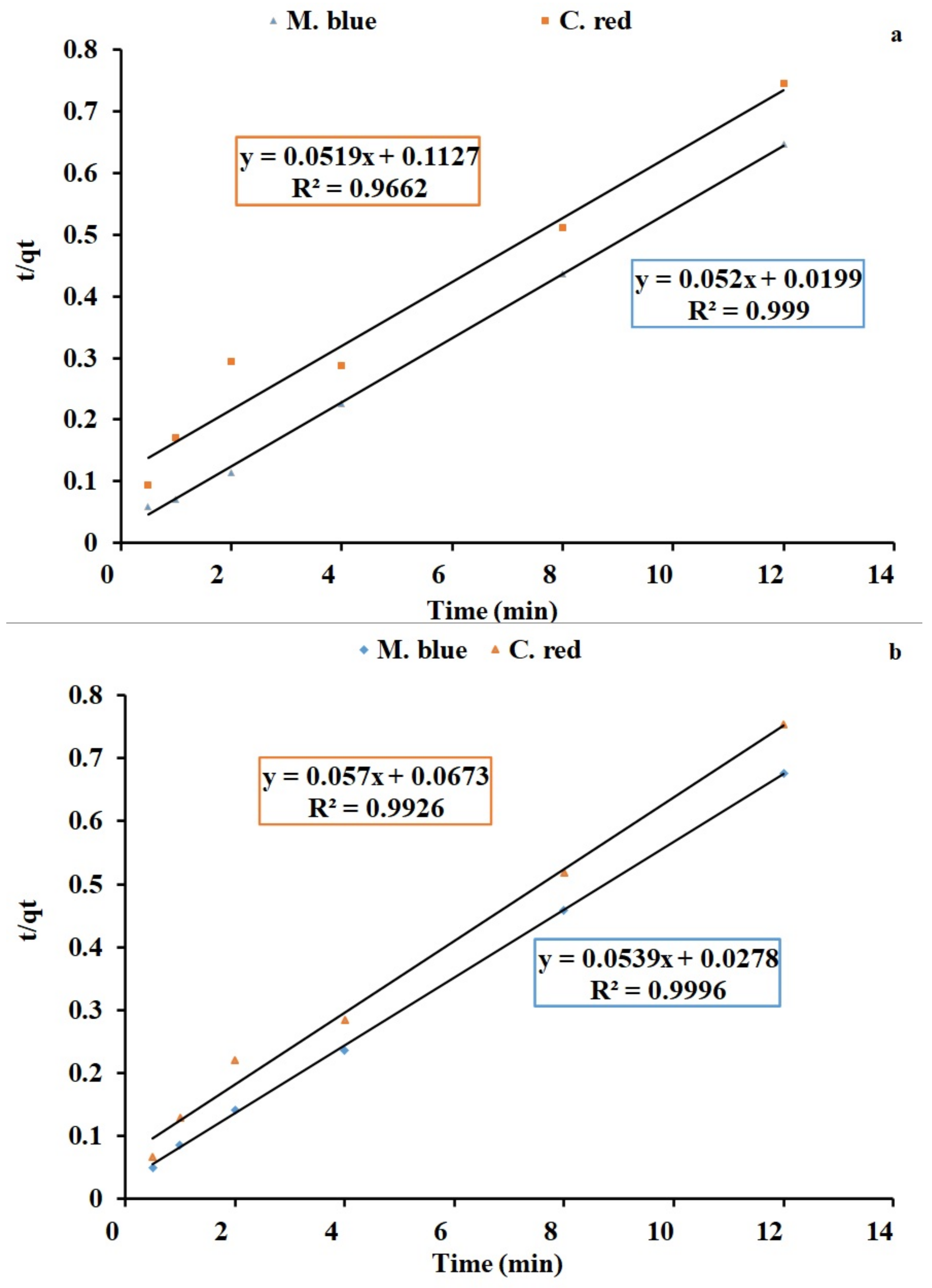
2.4. Kinetics Studies
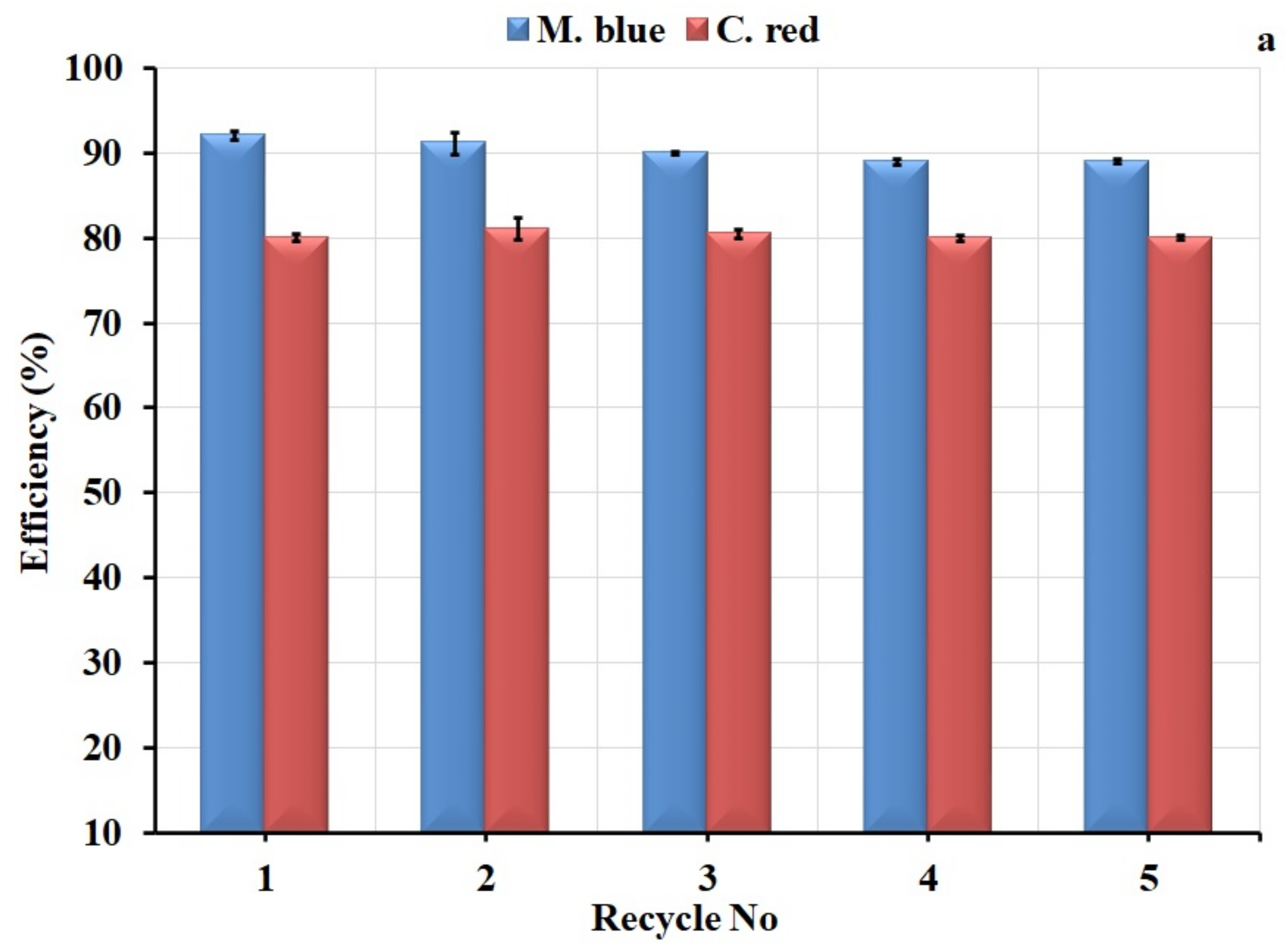
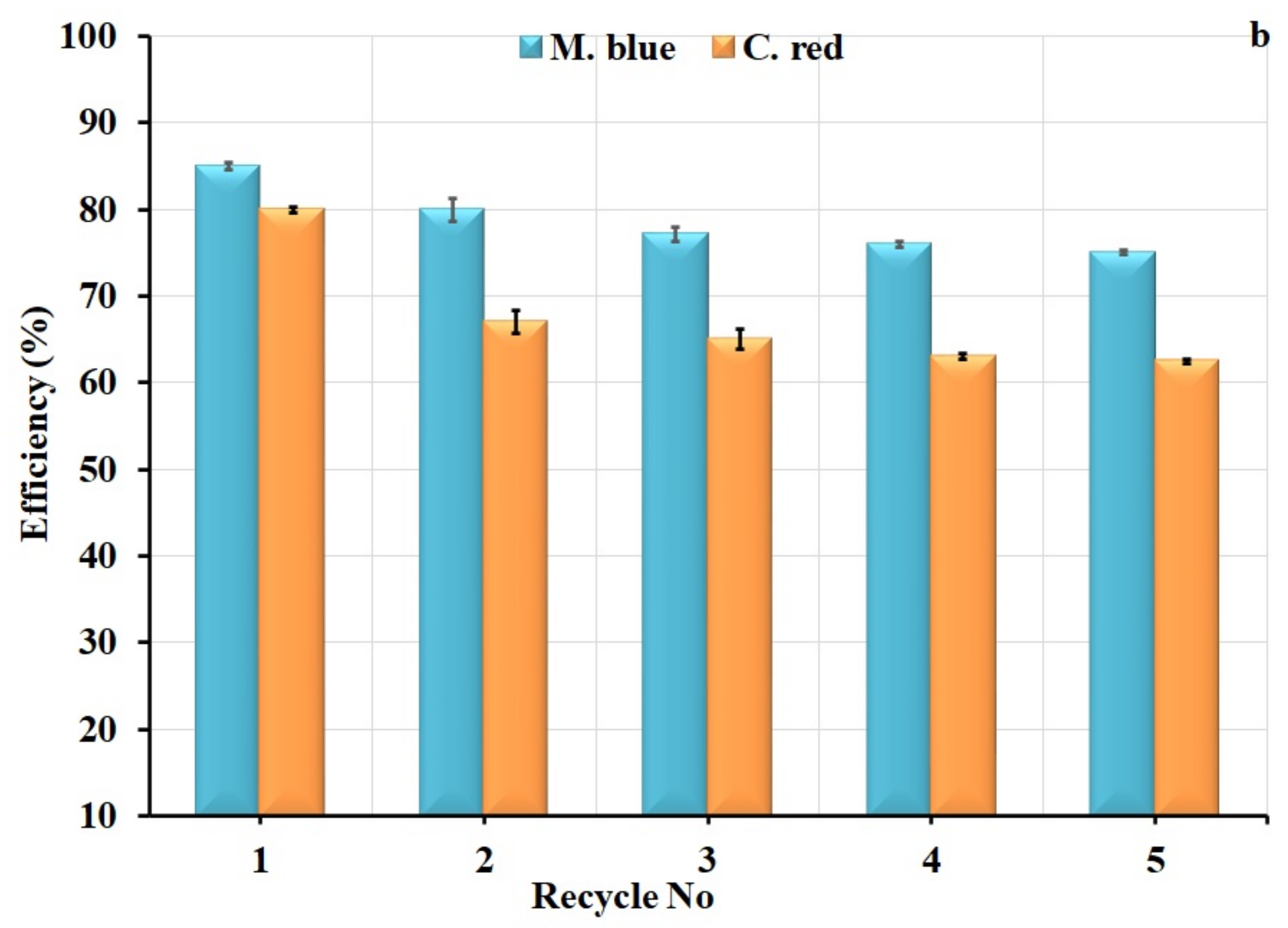
2.5. Regeneration Studies
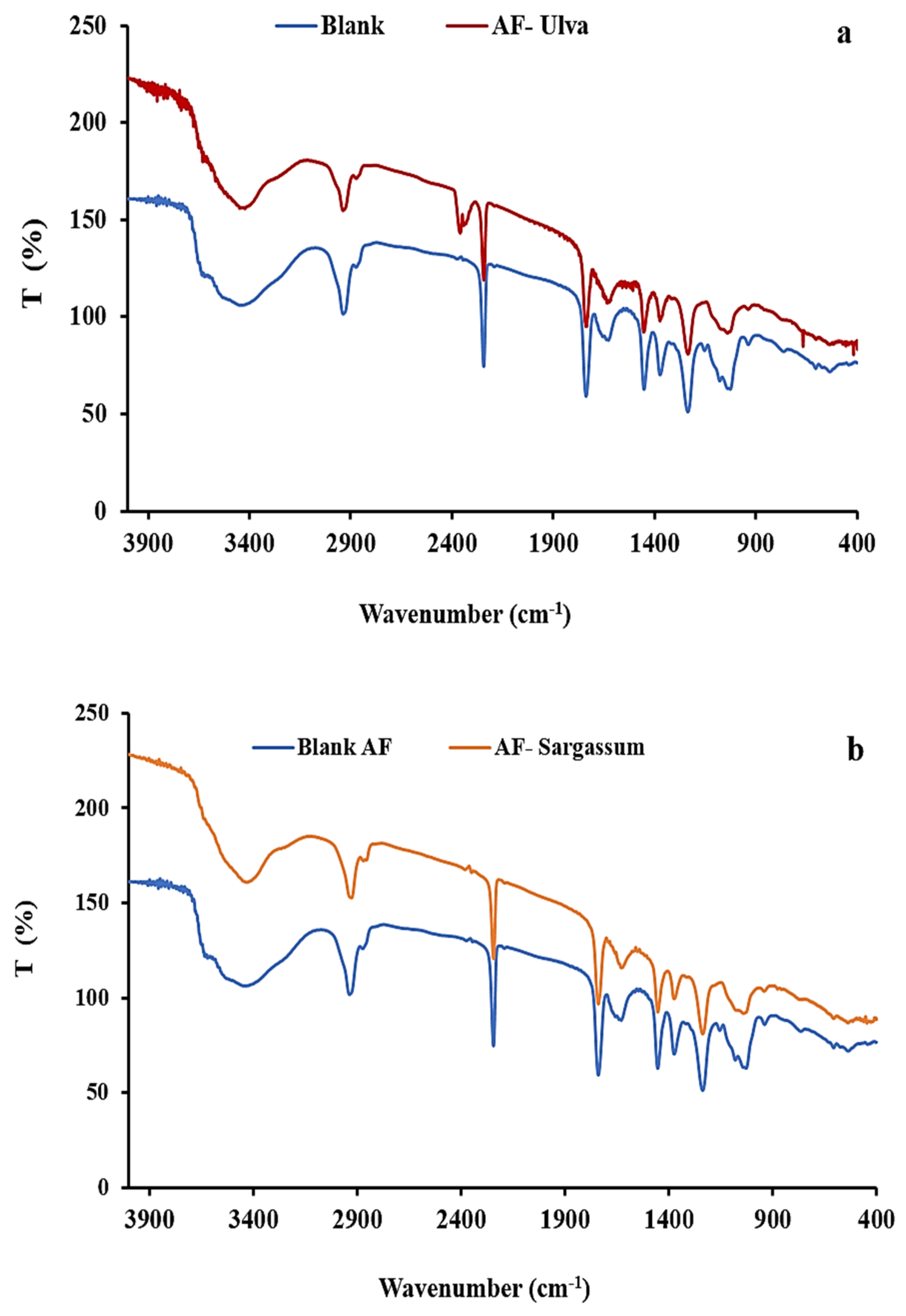
2.6. Membrane Characterization
2.6.1. Swelling and Porosity Characteristics of the Membranes
2.6.2. Fourier Transform Infrared (FTIR) Spectrophotometer
2.6.3. SEM Analysis
3. Materials and Methods
3.1. Algal Biomass Preparation
3.2. Membrane Preparation
Pretreatment Method
3.3. Adsorbate Preparation
3.4. Batch Sorption Experiments
3.5. Full Factorial Design Experiments (Second Optimization Step)
3.6. Langmuir and Freundlich Isotherm
3.6.1. Langmuir Isotherm
3.6.2. Freundlich Isotherm
3.7. Kinetics Studies
3.7.1. Pseudo First-Order Model
3.7.2. Pseudo Second-Order Model
3.8. Characterization of the Composite Membranes
3.8.1. Swelling Properties
3.8.2. Porosity Properties
3.8.3. Fourier Transform Infrared Spectroscopy (FT-IR)
3.8.4. Scanning Electron Microscopy (SEM)
3.9. Desorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunger, K. (Ed.) Industrial Dyes: Chemistry, Properties, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Soliman, M.; Alhaithloul, H.A.; Hakeem, K.R.; Alharbi, B.M.; El-Esawi, M.; Elkelish, A. Exogenous Nitric Oxide Mitigates Nickel-Induced Oxidative Damage in Eggplant by Upregulating Antioxidants, Osmolyte Metabolism, and Glyoxalase Systems. Plants 2019, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- McKay, G.; Otterburn, M.S.; Aga, J.A. Fuller’s earth and fired clay as adsorbents for dyestuffs. Water Air Soil Pollut. 1985, 24, 307–322. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.; Nada, A.A.; O’Donovan, A.; Kumar Thakur, V.; Elkelish, A. Mycogenic Silver Nanoparticles from Endophytic Trichoderma Atroviride with Antimicrobial Activity. J. Ren. Mat. 2019, 7, 171–185. [Google Scholar] [CrossRef]
- Szlachta, M.; Wójtowicz, P. Adsorption of methylene blue and Congo red from aqueous solution by activated carbon and carbon nanotubes. Water Sci. Technol. 2013, 68, 2240–2248. [Google Scholar] [CrossRef]
- Xu, G.; Zhu, Y.; Wang, X.; Wang, S.; Cheng, T.; Ping, R.; Cao, J.; Lv, K. Novel chitosan and Laponite based nanocomposite for fast removal of Cd (II), methylene blue and Congo red from aqueous solution. e-Polymers 2019, 19, 244–256. [Google Scholar] [CrossRef]
- Abdelhamid, A.E.; Khalil, A.M. Polymeric membranes based on cellulose acetate loaded with candle soot nanoparticles for water desalination. J. Macromol. Sci. Part A 2019, 56, 153–161. [Google Scholar] [CrossRef]
- Abdelhamid, A.E.; Elawady, M.M.; El-Ghaffar, M.A.A.; Rabie, A.M.; Larsen, P.; Christensen, M.L. Surface modification of reverse osmosis membranes with zwitterionic polymer to reduce biofouling. Water Sci. Technol. Water Supply 2015, 15, 999–1010. [Google Scholar] [CrossRef]
- Zidan, T.; Abdelhamid, A.E.; Zaki, E. N-Aminorhodanine modified chitosan hydrogel for antibacterial and copper ions removal from aqueous solutions. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sarioglu, O.F.; San Keskin, N.O.; Celebioglu, A.; Tekinay, T.; Uyar, T. Bacteria encapsulated electrospun nanofibrous webs for remediation of methylene blue dye in water. Colloids Surf. B Biointerfaces 2017, 152, 245–251. [Google Scholar] [CrossRef]
- Sen, S.K.; Raut, S.; Bandyopadhyay, P.; Raut, S. Fungal decolouration and degradation of azo dyes: A review. Fungal Biol. Rev. 2016, 30, 112–133. [Google Scholar] [CrossRef]
- Singh, H.; Chauhan, G.; Jain, A.K.; Sharma, S. Adsorptive potential of agricultural wastes for removal of dyes from aqueous solutions. J. Environ. Chem. Eng. 2017, 5, 122–135. [Google Scholar] [CrossRef]
- Moghazy, R.M. Activated biomass of the green microalga Chlamydomonas variabilis as an efficient biosorbent to remove methylene blue dye from aqueous solutions. Water Sa 2019, 45, 20–28. [Google Scholar] [CrossRef]
- El-Shahat, M.; Abdelhamid, A.E.; Abdelhameed, R.M. Capture of iodide from wastewater by effective adsorptive membrane synthesized from MIL-125-NH2 and cross-linked chitosan. Carbohydr. Polym. 2020, 231, 115742. [Google Scholar] [CrossRef] [PubMed]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018. [Google Scholar] [CrossRef] [PubMed]
- El-Wakeel, S.T.; Moghazy, R.M.; Labena, A.; Husien, S. Algal biosorbent as a basic tool for heavy metals removal; the first step for further applications. J. Mater. Environ. Sci. 2019, 10, 75–87. [Google Scholar]
- Husien, S.; Labena, A.; El-Belely, E.; Mahmoud, H.; Hamouda, A. Application of Nostoc sp. for hexavalent chromium Cr(VI) removal: Planktonic and biofilm. Int. J. Environ. Anal. Chem. 2020, 1–22. [Google Scholar] [CrossRef]
- Husien, S.; Labena, A.; El-Belely, E.; Mahmoud, H.M.; Hamouda, A.S. Adsorption studies of hexavalent chromium Cr (VI) on micro-scale biomass of Sargassum dentifolium, Seaweed. J. Environ. Chem. Eng. 2019, 7, 103444. [Google Scholar] [CrossRef]
- Husien, S.; Labena, A.; El-Belely, E.; Mahmoud, H.M.; Hamouda, A.S. Absorption of hexavalent chromium by green micro algae Chlorella sorokiniana: Live planktonic cells. Water Pract. Technol. 2019, 14, 515–529. [Google Scholar] [CrossRef]
- Moghazy, R.M.; Labena, A.; Husien, S. Eco-friendly complementary biosorption process of methylene blue using micro-sized dried biosorbents of two macro-algal species (Ulva fasciata and Sargassum dentifolium): Full factorial design, equilibrium, and kinetic studies. Int. J. Biol. Macromol. 2019, 134, 330–343. [Google Scholar] [CrossRef]
- Mirzababaei, M.; Miraftab, M.; Mohamed, M.; McMahon, P. Impact of carpet waste fibre addition on swelling properties of compacted clays. Geotech. Geol. Eng. 2013, 31, 173–182. [Google Scholar] [CrossRef]
- Mansor, E.S.; Labena, A.; Moghazy, R.M.; Abdelhamid, A.E. Advanced eco-friendly and adsorptive membranes based on Sargassum dentifolium for heavy metals removal, recovery and reuse. J. Water Process Eng. 2020, 37, 101424. [Google Scholar] [CrossRef]
- Bajaj, P.; Paliwal, D.; Gupta, A. Modification of Acrylic Fibres for Specific End Uses; NISCAIR-CSIR: New Delhi, India, 1996. [Google Scholar]
- Zhang, L.; Zhang, X.; Li, P.; Zhang, W. Effective Cd2+ chelating fiber based on polyacrylonitrile. React. Funct. Polym. 2009, 69, 48–54. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Su, Y.; Su, H.; Wang, X.; Zhu, X. Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J. Hazard. Mater. 2007, 143, 220–225. [Google Scholar] [CrossRef]
- Deokar, R.; Sabale, A. Biosorption of methylene blue and malachite green from binary solution onto Ulva Lactuca. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 295–304. [Google Scholar]
- Omar, H.; El-Gendy, A.; Al-Ahmary, K. Bioremoval of toxic dye by using different marine macroalgae. Turk. J. Bot. 2018, 42, 15–27. [Google Scholar] [CrossRef]
- Pigorsch, E.; Elhaddaoui, A.; Turrell, S. Spectroscopic study of pH and solvent effects on the structure of Congo red and its binding mechanism to amyloid-like proteins. Spectrochim. Acta Part A Mol. Spectrosc. 1994, 50, 2145–2152. [Google Scholar] [CrossRef]
- Fu, Y.; Viraraghavan, T. Removal of Congo Red from an aqueous solution by fungus Aspergillus niger. Adv. Environ. Res. 2002, 7, 239–247. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A. Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J. Hazard. Mater. 2008, 153, 759–766. [Google Scholar] [CrossRef]
- Singh, S.K.; Dixit, K.; Sundaram, S. Effect of acidic and basic pretreatment of wild algal biomass on Cr (VI) biosorption. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 38–41. [Google Scholar] [CrossRef]
- Moghazy, R.M.; Labena, A.; Husien, S.; Mansor, E.S.; Abdelhamid, A.E. Neoteric approach for efficient eco-friendly dye removal and recovery using algal-polymer biosorbent sheets: Characterization, factorial design, equilibrium and kinetics. Int. J. Biol. Macromol. 2020, 157, 494–509. [Google Scholar] [CrossRef]
- Saadat, S.; Karimi-Jashni, A. Optimization of Pb (II) adsorption onto modified walnut shells using factorial design and simplex methodologies. Chem. Eng. J. 2011, 173, 743–749. [Google Scholar] [CrossRef]
- Bingol, D.; Tekin, N.; Alkan, M. Brilliant Yellow dye adsorption onto sepiolite using a full factorial design. Appl. Clay Sci. 2010, 50, 315–321. [Google Scholar] [CrossRef]
- Boubakri, A.; Helali, N.; Tlili, M.; Amor, M.B. Fluoride removal from diluted solutions by Donnan dialysis using full factorial design. Korean J. Chem. Eng. 2014, 31, 461–466. [Google Scholar] [CrossRef]
- Hasan, S.; Srivastava, P.; Talat, M. Biosorption of lead using immobilized Aeromonas hydrophila biomass in up flow column system: Factorial design for process optimization. J. Hazard. Mater. 2010, 177, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.K.; Mondal, N.K. Biosorption of Congo Red from aqueous solution onto burned root of Eichhornia crassipes biomass. Appl. Water Sci. 2017, 7, 1841–1854. [Google Scholar] [CrossRef]
- Rouf, S.; Nagapadma, M.; Rao, R.R. Removal of harmful textile dye congo red from aqueous solution using chitosan and chitosan beads modified with CTAB. Int. J. Eng. Res. Appl. 2015, 5, 75–82. [Google Scholar]
- Iqbal, M.J.; Ashiq, M.N. Adsorption of dyes from aqueous solutions on activated charcoal. J. Hazard. Mater. 2007, 139, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Woolard, C.; Strong, J.; Erasmus, C. Evaluation of the use of modified coal ash as a potential sorbent for organic waste streams. Appl. Geochem. 2002, 17, 1159–1164. [Google Scholar] [CrossRef]
- Cengiz, S.; Cavas, L. Removal of methylene blue by invasive marine seaweed: Caulerpa racemosa var. cylindracea. Bioresour. Technol. 2008, 99, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Moeinian, K.; Rastgoo, T.; Mehdinia, S. Effects of pH, particle size and porosity of raw rice husk and its silica on removing lead and hexavalent chromium from aqueous solution. Casp. J. Environ. Sci. 2017, 15, 263–270. [Google Scholar]
- Tuzen, M.; Sarı, A.; Saleh, T.A. Response surface optimization, kinetic and thermodynamic studies for effective removal of rhodamine B by magnetic AC/CeO2 nanocomposite. J. Environ. Manag. 2018, 206, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.E.; El-Sayed, A.A.; Khalil, A.M. Polysulfone nanofiltration membranes enriched with functionalized graphene oxide for dye removal from wastewater. J. Polym. Eng. 2020, 40, 833–841. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc oxide based photocatalytic degradation of persistent pesticides: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar]
- Mathias, P.M.; Kumar, R.; Moyer, J.D.; Schork, J.M.; Srinivasan, S.R.; Auvil, S.R.; Talu, O. Correlation of multicomponent gas adsorption by the dual-site Langmuir model. Application to nitrogen/oxygen adsorption on 5A-zeolite. Ind. Eng. Chem. Res. 1996, 35, 2477–2483. [Google Scholar] [CrossRef]
- Herbert, F. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar]
- Ho, Y.; Ng, J.; McKay, G. Kinetics of pollutant sorption by biosorbents. Sep. Purif. Methods 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Marrez, D.A.; Abdelhamid, A.E.; Darwesh, O.M. Eco-friendly cellulose acetate green synthesized silver nano-composite as antibacterial packaging system for food safety. Food Packag. Shelf Life 2019, 20, 100302. [Google Scholar] [CrossRef]
- Su, T.; Han, X.; Lu, X. Palladium(II)-catalyzed oxidative annulation of alkenylindoles with alkynes initiated by C–H activation. Tetrahedron Lett. 2014, 55, 27–30. [Google Scholar] [CrossRef]
- Rabiee, H.; Vatanpour, V.; Farahani, M.H.D.A.; Zarrabi, H. Improvement in flux and antifouling properties of PVC ultrafiltration membranes by incorporation of zinc oxide (ZnO) nanoparticles. Sep. Purif. Technol. 2015, 156, 299–310. [Google Scholar] [CrossRef]
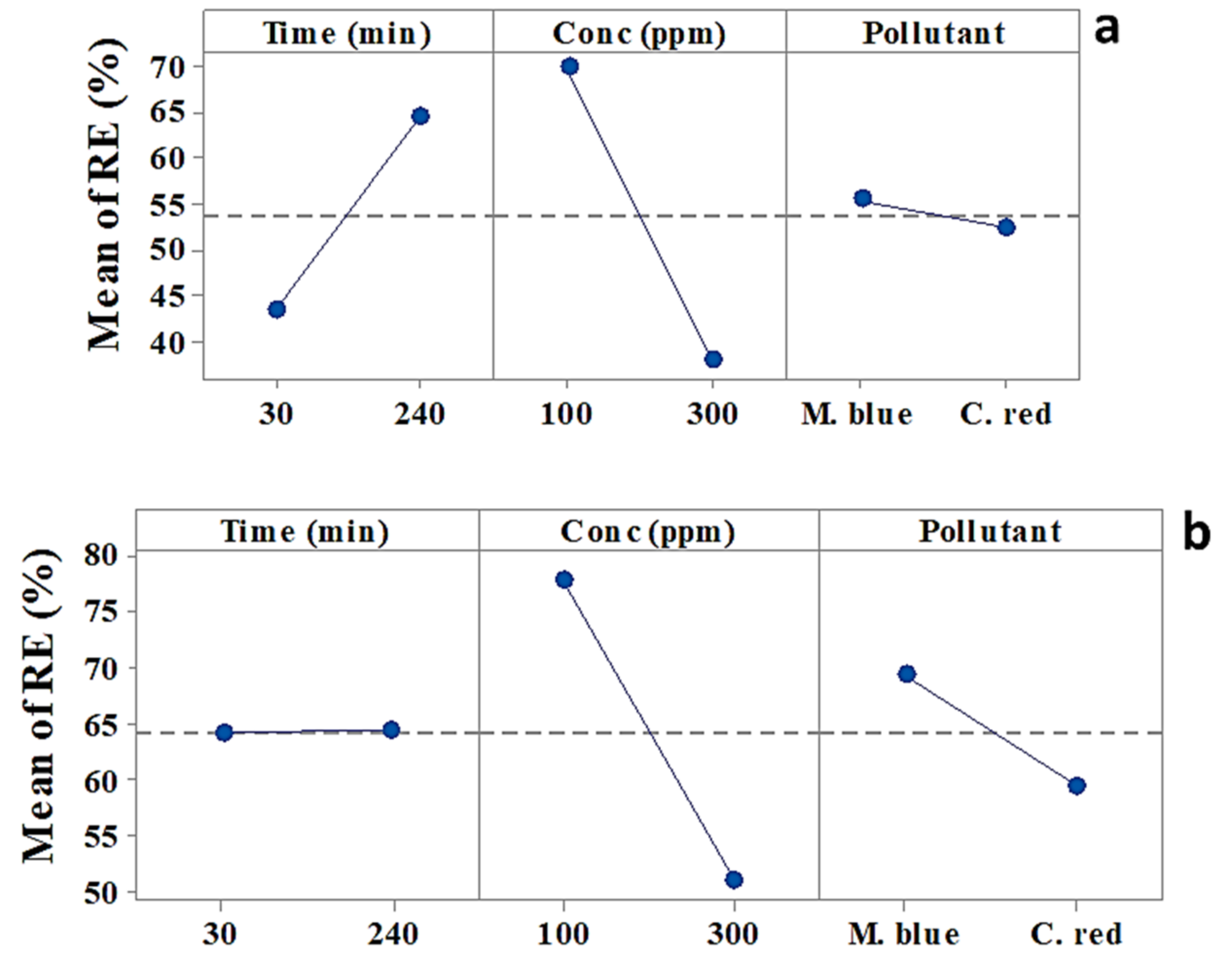
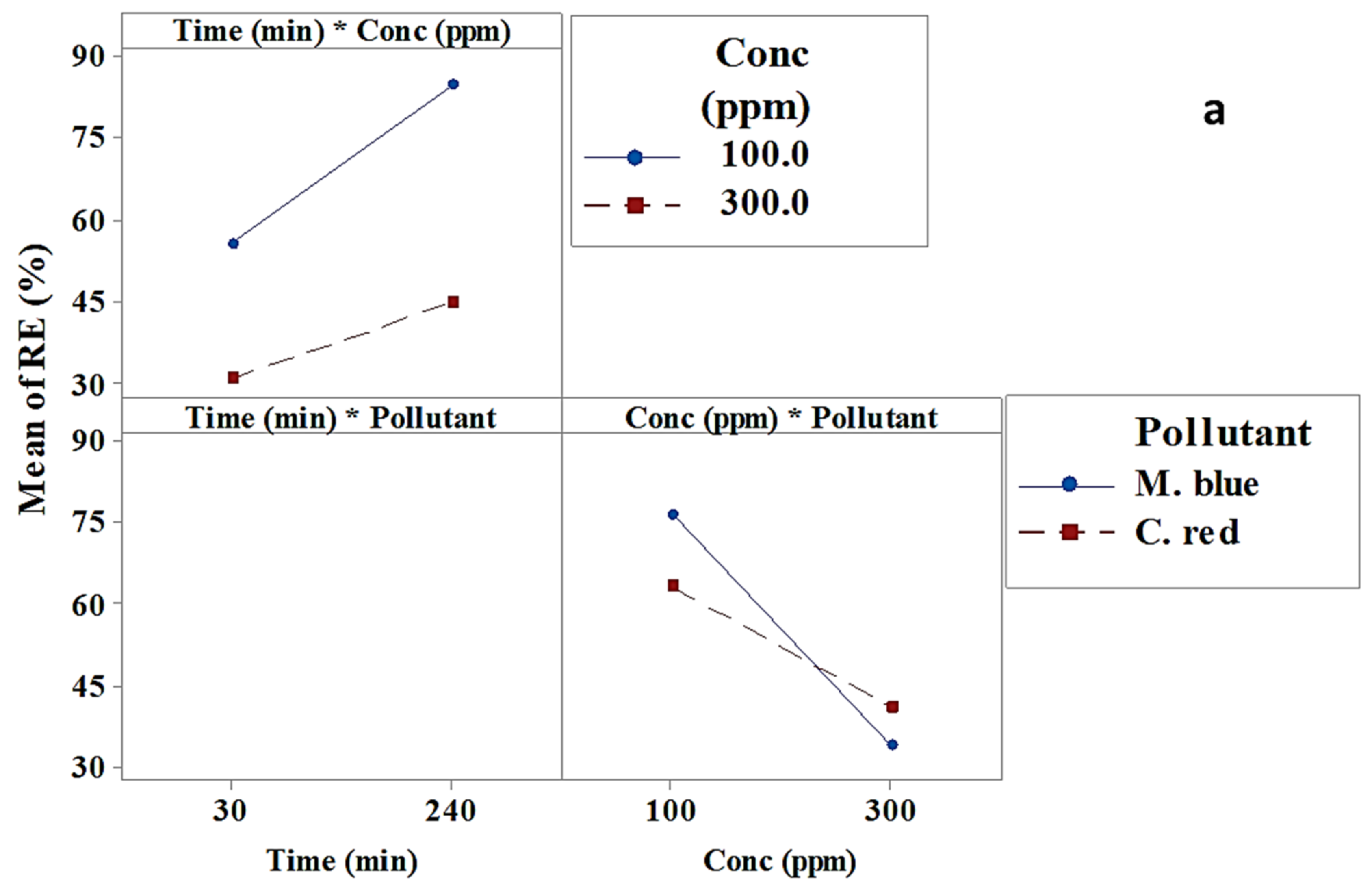
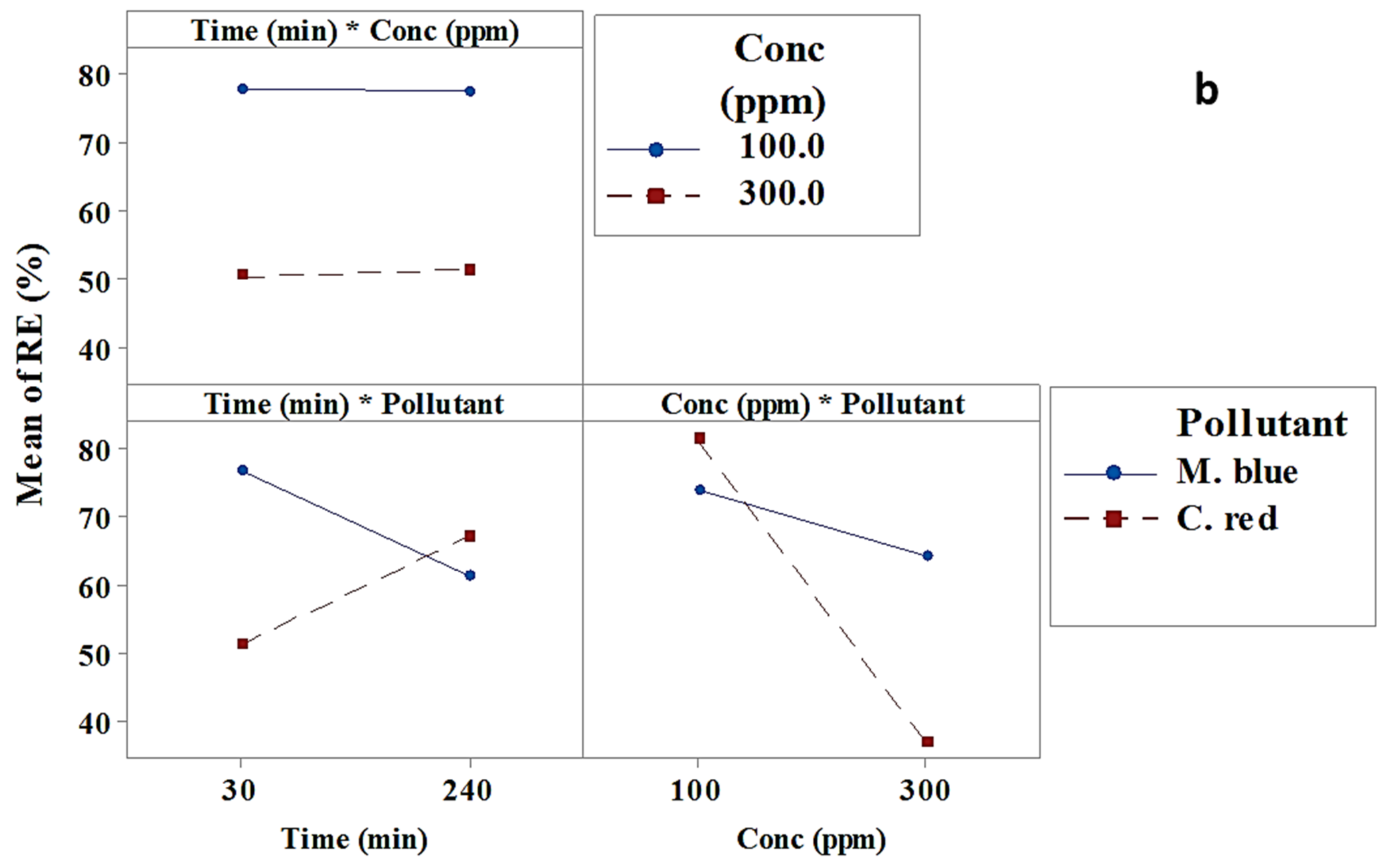


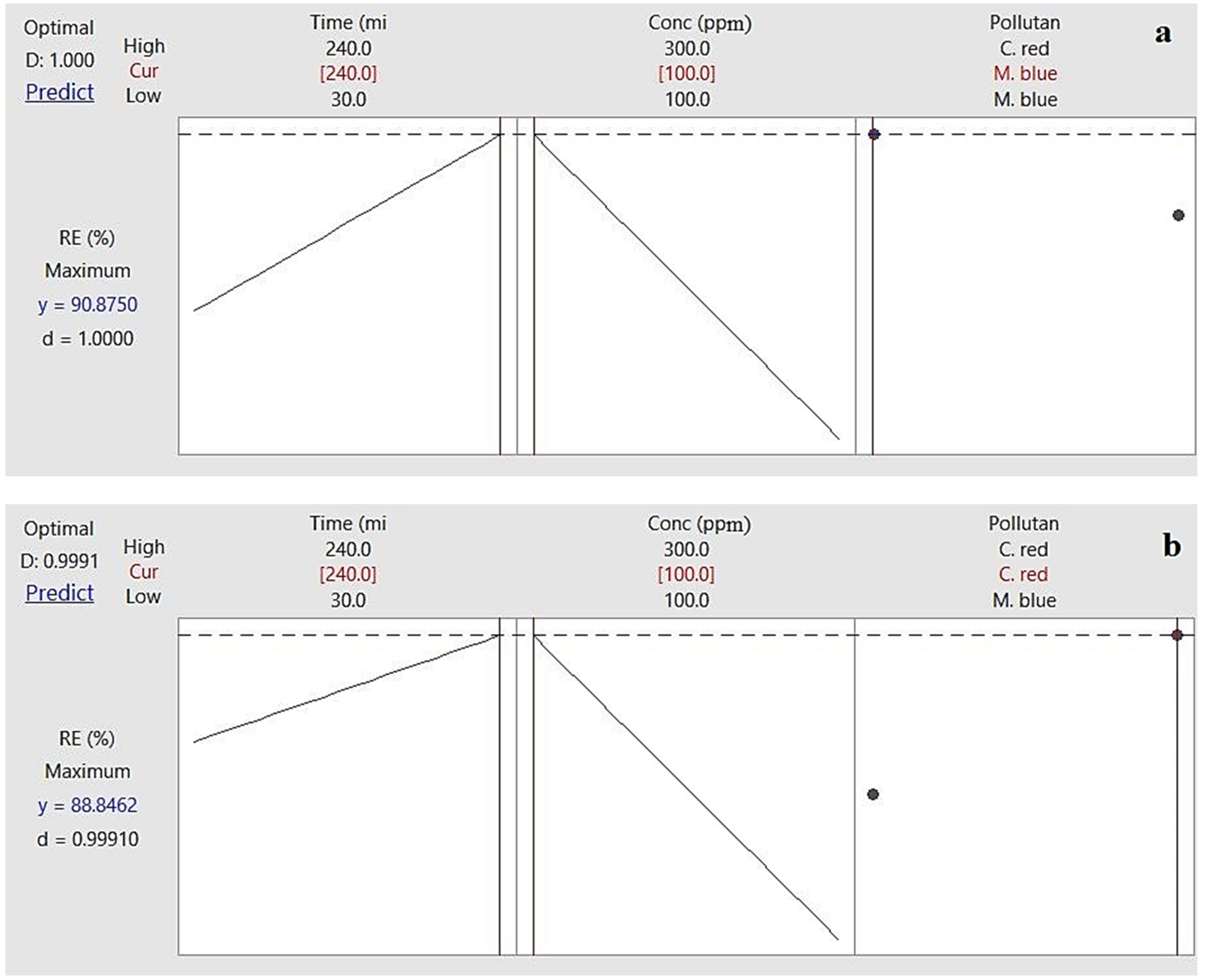




| Term | Effect | Coef | SE Coef | T-Value | p-Value |
|---|---|---|---|---|---|
| Constant | 53.80 | 2.58 | 20.89 | 0.002 | |
| Time (min) | 21.42 | 10.71 | 2.58 | 4.16 | 0.053 |
| Conc (ppm) | −32.05 | −16.03 | 2.58 | −6.22 | 0.025 |
| Pollutant | −3.10 | −1.55 | 2.58 | −0.60 | 0.608 |
| Time (min) × Conc (ppm) | −7.43 | −3.72 | 2.58 | −1.44 | 0.286 |
| Conc (ppm) × Pollutant | 10.15 | 5.08 | 2.58 | 1.97 | 0.188 |
| Term | Effect | Coef | SE Coef | T-Value | p-Value |
|---|---|---|---|---|---|
| Constant | 64.192 | 0.133 | 483.69 | 0.001 | |
| Time (min) | 0.303 | 0.152 | 0.173 | 0.88 | 0.542 |
| Conc (ppm) | −26.851 | −13.425 | 0.103 | −130.34 | 0.005 |
| Pollutant | −9.933 | −4.967 | 0.133 | −37.42 | 0.017 |
| Time (min) × Conc (ppm) | 0.698 | 0.349 | 0.126 | 2.78 | 0.220 |
| Time (min) × Pollutant | 15.587 | 7.793 | 0.173 | 44.96 | 0.014 |
| Conc (ppm) × Pollutant | −17.199 | −8.600 | 0.103 | −83.49 | 0.008 |
| Model | Parameters | |||||
|---|---|---|---|---|---|---|
| Langmuir | Qmax (mg/g) | B | R2 | |||
| M. blue | C. red | M. blue | C. red | M. blue | C. red | |
| 45.871 | 30.959 | 0.2485 | 0.0408 | 0.9341 | 0.9891 | |
| Freundlich | n | K | R2 | |||
| M. blue | C. red | M. blue | C. red | M. blue | C. red | |
| 1.0922 | 0.5924 | 3.9682 | 4.9306 | 0.9387 | 0.9384 | |
| Model | Parameters | |||||
|---|---|---|---|---|---|---|
| Langmuir | Qmax b | B | R2 | |||
| M. blue | C. red | M. blue | C. red | M. blue | C. red | |
| 65.789 | 28.24 | 0.0553 | 0.073 | 0.9875 | 0.9937 | |
| Freundlich | n | K | R2 | |||
| M. blue | C. red | M. blue | C. red | M. blue | C. red | |
| 1.244 | 1.969 | 3.4689 | 3.14412 | 0.9466 | 0.9295 | |
| Model | Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| 1st order Kinetics | qe calc. | 1st order Kinetics | qe calc. | 1st order Kinetics | ||||
| M. blue | C. red | M. blue | C. red | M. blue | C. red | M. blue | C. red | |
| 60.3 | 778.7 | 60.3 | 16.1 | 60.3 | 1.07 | 60.3 | 0.96 | |
| 2nd order kinetics | qe calc. | 2nd order kinetics | qe calc. | 2nd order kinetics | ||||
| M. blue | C. red | M. blue | C. red | M. blue | C. red | M. blue | C. red | |
| 19.23 | 19.26 | 19.23 | 16.1 | 19.23 | 0.023 | 19.23 | 0.9926 | |
| Model | Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| 1st order Kinetics | qe calc. | 1st order Kinetics | qe calc. | 1st order Kinetics | ||||
| M. blue | C. red | M. blue | C. red | M. blue | C. red | M. blue | C. red | |
| 301.23 | 3.16 | 301.23 | 16.1 | 301.23 | −2.09 | 301.23 | 0.9602 | |
| 2nd order kinetics | qe calc. | 2nd order kinetics | qe calc. | 2nd order kinetics | ||||
| M. blue | C. red | M. blue | C. red | M. blue | C. red | M. blue | C. red | |
| 18.5529 | 17.5439 | 18.5529 | 16.1 | 18.5529 | 0.04828 | 18.5529 | 0.9926 | |
| Factor | Unit | Symbol | Statistical Code | Values of Coded Levels | |
|---|---|---|---|---|---|
| (Low) − 1 | (High) + 1 | ||||
| Time | min | Time | A | 30 | 240 |
| Dyes Concentration | ppm | Conc | B | 100 | 300 |
| Pollutant | Pollutant | C | M. blue | C. red | |
| Factor | Unit | Symbol | Statistical Code | Values of Coded Levels | |
|---|---|---|---|---|---|
| (Low) − 1 | (High) + 1 | ||||
| Time | min | Time | A | 30 | 240 |
| Dyes Concentration | ppm | Conc | B | 100 | 300 |
| Pollutant | Pollutant | C | M. blue | C. red | |
| StdOrder | RunOrder | PtType | Blocks | Time (min) | Conc (ppm) | Pollutant | RE (%) | FITS1 | RESI1 |
|---|---|---|---|---|---|---|---|---|---|
| 4 | 1 | 1 | 1 | 240 | 300 | M. blue | 46 | 41.24167 | 4.758333 |
| 2 | 2 | 1 | 1 | 30 | 300 | M. blue | 22.5 | 27.25833 | −4.75833 |
| 1 | 3 | 1 | 1 | 30 | 100 | M. blue | 64 | 62.025 | 1.975 |
| 3 | 4 | 1 | 1 | 240 | 100 | M. blue | 88.9 | 90.875 | −1.975 |
| 4 | 5 | 1 | 1 | 240 | 300 | C. red | 43.53333 | 48.29167 | −4.75833 |
| 1 | 6 | 1 | 1 | 30 | 100 | C. red | 46.8 | 48.775 | −1.975 |
| 2 | 7 | 1 | 1 | 30 | 300 | C. red | 39.06667 | 34.30833 | 4.758333 |
| 3 | 8 | 1 | 1 | 240 | 100 | C. red | 79.6 | 77.625 | 1.975 |
| StdOrder | RunOrder | PtType | Blocks | Time (min) | Conc (ppm) | Pollutant | RE (%) | FITS1 | RESI1 |
|---|---|---|---|---|---|---|---|---|---|
| 3 | 1 | 1 | 1 | 240 | 100 | M. blue | 75 | 75.12552 | −0.12552 |
| 4 | 2 | 1 | 1 | 240 | 300 | M. blue | 65.5 | 65.37448 | 0.125517 |
| 1 | 3 | 1 | 1 | 30 | 100 | M. blue | 82.1 | 81.97448 | 0.125517 |
| 2 | 4 | 1 | 1 | 30 | 300 | M. blue | 71.5 | 71.62552 | −0.12552 |
| 4 | 5 | 1 | 1 | 240 | 300 | C. red | 45.44 | 45.49379 | −0.05379 |
| 3 | 6 | 1 | 1 | 240 | 100 | C. red | 88.9 | 88.84621 | 0.053793 |
| 2 | 7 | 1 | 1 | 30 | 300 | C. red | 28.96 | 28.90621 | 0.053793 |
| 1 | 8 | 1 | 1 | 30 | 100 | C. red | 73.6 | 73.65379 | −0.05379 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labena, A.; Abdelhamid, A.E.; Amin, A.S.; Husien, S.; Hamid, L.; Safwat, G.; Diab, A.; Gobouri, A.A.; Azab, E. Removal of Methylene Blue and Congo Red Using Adsorptive Membrane Impregnated with Dried Ulva fasciata and Sargassum dentifolium. Plants 2021, 10, 384. https://doi.org/10.3390/plants10020384
Labena A, Abdelhamid AE, Amin AS, Husien S, Hamid L, Safwat G, Diab A, Gobouri AA, Azab E. Removal of Methylene Blue and Congo Red Using Adsorptive Membrane Impregnated with Dried Ulva fasciata and Sargassum dentifolium. Plants. 2021; 10(2):384. https://doi.org/10.3390/plants10020384
Chicago/Turabian StyleLabena, Ahmed, Ahmed E. Abdelhamid, Abeer S. Amin, Shimaa Husien, Liqaa Hamid, Gehan Safwat, Ayman Diab, Adil A. Gobouri, and Ehab Azab. 2021. "Removal of Methylene Blue and Congo Red Using Adsorptive Membrane Impregnated with Dried Ulva fasciata and Sargassum dentifolium" Plants 10, no. 2: 384. https://doi.org/10.3390/plants10020384
APA StyleLabena, A., Abdelhamid, A. E., Amin, A. S., Husien, S., Hamid, L., Safwat, G., Diab, A., Gobouri, A. A., & Azab, E. (2021). Removal of Methylene Blue and Congo Red Using Adsorptive Membrane Impregnated with Dried Ulva fasciata and Sargassum dentifolium. Plants, 10(2), 384. https://doi.org/10.3390/plants10020384







