Underutilized Mexican Plants: Screening of Antioxidant and Antiproliferative Properties of Mexican Cactus Fruit Juices
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Soluble Solids, Betacyanin, Betaxanthin Content, and Antioxidant Activity
2.2. Phenolic Composition Analysis
2.3. Cell Viability Assay
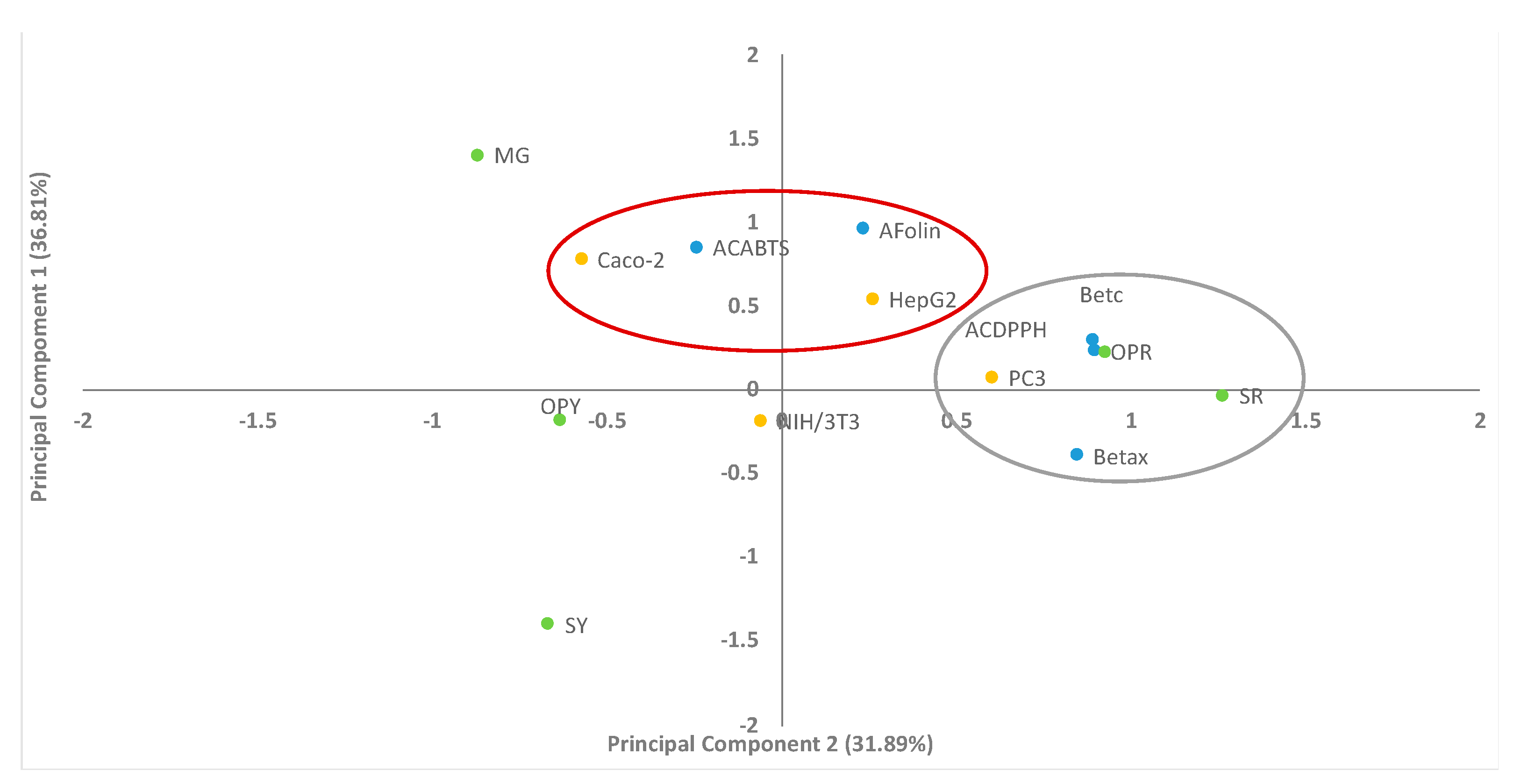
2.4. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Production of Clarified Juice
3.2.1. Preparation of Pitaya, Garambullo, and Tuna Pulps
3.2.2. Production of Clarified Juice
3.3. Total Soluble Solids
3.4. Betacyanin and Betaxanthin Content and Antioxidant Activity Assay
3.4.1. Quantification of Betacyanin and Betaxanthin
3.4.2. Antioxidant Activity by 2,2’-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) Diammonium Salt Capacity (ABTS)
3.4.3. Antioxidant Activity by α-α-Diphenyl-β-picrylhydrazyl (DPPH)
3.4.4. Ferric Reducing Antioxidant Power (FRAP)
3.5. Total Phenolic Composition
3.6. HPLC-DAD Analysis
3.7. Cell Viability Assay
3.7.1. Cell Culture
3.7.2. Cell Proliferation Assay
3.8. Principal Component Analysis (PCA)
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Cancer. 2020. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 22 January 2021).
- Govea-Salas, M.; Morlett-Chávez, J.; Rodriguez-Herrera, R.; Ascacio-Valdés, J. Some Mexican Plants Used in Traditional Medicine. Aromat. Med. Plants Back Nat. Intech. 2017, 1, 191–200. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, Y.; Martínez-Huélamo, M.; Pedraza-Chaverri, J.; Ramírez, V.; Martínez-Tagüeña, N.; Trujillo, J. Ethnobotanical, Nutritional and Medicinal Properties of Mexican Drylands Cactaceae Fruits: Recent Findings and Research Opportunities. Food Chem. 2020, 312, 126073. [Google Scholar] [CrossRef]
- Chuck-Hernández, C.; Parra-Saldívar, R.; Sandate-Flores, L. Pitaya (Stenocereus Spp.). Encycl. Food Health 2015, 385–391. [Google Scholar] [CrossRef]
- Quiroz-González, B.; Rodriguez-Martinez, V.; Welti-Chanes, J.; García-Mateos, M.d.R.; Corrales-García, J.; Ybarra-Moncada, M.C.; Leyva-Ruelas, G.; Torres, J.A. Refrigerated storage of high hydristatic pressure (HHP) treated pitaya (Stenocereus pruinosus) juice. Rev. Mex. Ing. Quim. 2020, 19, 387–399. [Google Scholar] [CrossRef]
- García-Cruz, L.; Dueñas, M.; Santos-Buelgas, C.; Valle-Guadarrama, S.; Salinas-Moreno, Y. Betalains and Phenolic Compounds Profiling and Antioxidant Capacity of Pitaya (Stenocereus Spp.) Fruit from Two Species (S. Pruinosus and S. Stellatus). Food Chem. 2017, 234, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Rios, L.J.; Bautista-Ramírez, M.E.; Gómez y Gómez, Y.D.L.M.; Torres-Bustillos, L.G. Frutas de cactáceas: Compuestos bioactivos y sus propiedades nutracéuticas. In Propiedades Funcionales de Hoy; OmniaScience: Barcelona, España, 2016; pp. 35–65. [Google Scholar] [CrossRef]
- Cota-Sanchez, J.H. Nutritional composition of the prickly pear (Opuntia ficus-indica) fruit. In Nutritional Composition of Fruit Cultiva, 1st ed.; Saskatoon, Ed.; Academic Press: London, UK, 2016; pp. 691–712. [Google Scholar] [CrossRef]
- Albano, C.; Negro, C.; Tommasi, N.; Gerardi, C.; Mita, G.; Miceli, A.; de Bellis, L.; Blando, F. Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia Ficus-Indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes. Antioxidants 2015, 4, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Moaty, H.I. Structural Elucidation of Phenolic Compounds Isolated from Opuntia Littoralis and Their Antidiabetic, Antimicrobial and Cytotoxic Activity. S. Afr. J. Bot. 2020, 131, 320–327. [Google Scholar] [CrossRef]
- Allegra, M.; D’anneo, A.; Frazzitta, A.; Restivo, I.; Livrea, M.A.; Attanzio, A.; Tesoriere, L. The Phytochemical Indicaxanthin Synergistically Enhances Cisplatin-Induced Apoptosis in Hela Cells via Oxidative Stress-Dependent P53/P21waf1 Axis. Biomolecules 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Santoscoy, R.A.; Gutierrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic Composition, Antioxidant Capacity and In Vitro Cancer Cell Cytotoxicity of Nine Prickly Pear (Opuntia Spp.) Juices. Plant Foods Hum. Nutr. 2009, 64, 146–152. [Google Scholar] [CrossRef]
- Serra, A.T.; Poejo, J.; Matias, A.A.; Bronze, M.R.; Duarte, C.M.M. Evaluation of Opuntia Spp. Derived Products as Antiproliferative Agents in Human Colon Cancer Cell Line (HT29). Food Res. Int. 2013, 54, 892–901. [Google Scholar] [CrossRef]
- Reynoso-Camacho, R.; Martinez-Samayoa, P.; Ramos-Gomez, M.; Guzmán, H.; Salgado, L.M. Antidiabetic and Renal Protective Properties of Berrycactus Fruit (Myrtillocactus geometrizans). J. Med. Food 2015, 18, 565–571. [Google Scholar] [CrossRef] [PubMed]
- García-Cruz, L.; Valle-Guadarrama, S.; Salinas-Moreno, Y.; Joaquín-Cruz, E. Physical, Chemical, and Antioxidant Activity Characterization of Pitaya (Stenocereus pruinosus) Fruits. Plant Foods Hum. Nutr. 2013, 68, 403–410. [Google Scholar] [CrossRef]
- Tatar, B.C.; Sumnu, G.; Oztop, M.; Ayaz, E. Effects of centrifugation, encapsulation method and different coating materials on the total antioxidant activity of the microcapsules of powdered cherry laurels. Int. J. Nut. Food Engin. 2017, 10, 901–904. [Google Scholar]
- Guzmán-Maldonado, S.H.; Herrera-Hernández, G.; Hernández-López, D.; Reynoso-Camacho, R.; Guzmán-Tovar, A.; Vaillant, F.; Brat, P. Physicochemical, nutritional and functional characteristics of two underutilised fruit cactus species (Myrtillocactus) produced in central Mexico. Food Chem. 2010, 121, 381–386. [Google Scholar] [CrossRef]
- Herrera-Hernández, M.G.; Guevara-Lara, F.; Reynoso-Camacho, R.; Guzmán-Maldonado, S.H. Effects of Maturity Stage and Storage on Cactus Berry (Myrtillocactus geometrizans) Phenolics, Vitamin C, Betalains and Their Antioxidant Properties. Food Chem. 2011, 129, 1744–1750. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Chambers, E.; Adhikari, K.; Carbonell-Barrachina, Á.A. Sensory and physicochemical characterization of juices made with pomegranate and blueberries, blackberries, or raspberries. J. Food Sci. 2010, 75, 398–404. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Boston, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Zeghad, N.; Ahmed, E.; Belkhiri, A.; Heyden, Y.V.; Demeyer, K. Antioxidant Activity of Vitis Vinifera, Punica Granatum, Citrus Aurantium and Opuntia Ficus Indica Fruits Cultivated in Algeria. Heliyon 2019, 5, e01575. [Google Scholar] [CrossRef] [PubMed]
- Konan, A.; Konan, Y.; Kone, M. Polyphenols Content and Antioxidant Capacity of Traditional Juices Consumed in Côte d’Ivoire. J. Appl. Biosci. 2015, 87, 8015. [Google Scholar] [CrossRef]
- Gupta, A.K.; Rather, M.A.; Jha, A.K.; Shashank, A.; Singhal, S.; Sharma, M.; Pathak, U.; Sharma, D.; Mastinu, A. Artocarpus lakoocha roxb. And artocarpus heterophyllus lam. flowers: New sources of bioactive compounds. Plants 2020, 9, 1329. [Google Scholar] [CrossRef]
- Osorio-Esquivel, O.; Álvarez, V.B.; Dorantes-Álvarez, L.; Giusti, M.M. Phenolics, Betacyanins and Antioxidant Activity in Opuntia Joconostle Fruits. Food Res. Int. 2011, 44, 2160–2168. [Google Scholar] [CrossRef]
- Farag, M.A.; Sallam, I.E.; Fekry, M.I.; Zaghloul, S.S.; El-Dine, R.S. Metabolite Profiling of Three Opuntia Ficus-Indica Fruit Cultivars Using UPLC-QTOF-MS in Relation to Their Antioxidant Potential. Food Biosci. 2020, 36, 100673. [Google Scholar] [CrossRef]
- Vorster, C.; Stander, A.; Joubert, A. Differential signaling involved in Sutherlandia frutescens-induced cell death in MCF-7 and MCF-12A cells. J. ethnopharmacol. 2012, 140, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Migowska, N.; Caban, M.; Plenis, A.; Stepnowski, P. Chemometric Analysis for Optimizing Derivatization in Gas Chromatography-Based Procedures. J. Chemom. 2011, 25, 636–643. [Google Scholar] [CrossRef]
- Rockett, F.C.; de Oliveira Schmidt, H.; Schmidt, L.; Rodrigues, E.; Tischer, B.; de Oliveira, V.R.; Rios, A. Phenolic compounds and antioxidant activity in vitro and in vivo of Butia and Opuntia fruits. Food. Res. Int. 2020, 137, 109740. [Google Scholar] [CrossRef] [PubMed]
- Al Juhaimi, F.; Ghafoor, K.; Uslu, N.; Ahmed, I.A.M.; Babiker, E.E.; Özcan, M.M.; Fadimu, G.J. The effect of harvest times on bioactive properties and fatty acid compositions of prickly pear (Opuntia ficus-barbarica A. Berger) fruits. Food Chem. 2020, 303, 125387. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemistry (AOAC). Official Method of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemistry (AOAC): Arlington, VA, USA, 1990; pp. 141–144. [Google Scholar]
- Sandate-Flores, L.; Rodríguez-Rodríguez, J.; Calvo-Segura, S.; Mayorga-Martínez, A.; Parra-Saldivar, R.; Chuck-Hernández, C. Evaluation of different methods for betanin quantification in pitaya (Stenocereus spp). Agro. Food. Ind. Hi Tech. 2016, 27, 20–25. [Google Scholar]
- Sandate-Flores, L.; Romero-Esquivel, E.; Rodríguez-Rodríguez, J.; Rostro-Alanis, M.; Melchor-Martínez, E.M.; Castillo-Zacarías, C.; Parra-Saldívar, R. Functional Attributes and Anticancer Potentialities of Chico (Pachycereus Weberi) and Jiotilla (Escontria Chiotilla) Fruits Extract. Plants 2020, 9, 1623. [Google Scholar] [CrossRef]
- Nilsson, T. Studies into the pigments in beetroot (Beta vulgaris L. ssp. Vulgaris var. rubra L.). Lantbrukshögskolans Ann. 1970, 36, 179–219. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggenete, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol.Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rostro-Alanis, M.D.J.; Báez-González, J.; Torres-Alvarez, C.; Parra-Saldívar, R.; Rodriguez-Rodriguez, J.; Castillo, S. Chemical composition and biological activities of oregano essential oil and its fractions obtained by vacuum distillation. Molecules 2019, 24, 1904. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]


| Parameter | SY | SR | OPY | OPR | MG |
|---|---|---|---|---|---|
| Betacyanins (µg/g FS) | 47.99 ± 0.18 d | 334.06 ± 2.08 b | 13.29 ± 0.06 e | 403.56 ± 1.41 a | 103.50 ± 0.01 c |
| Betaxanthins (µg/g FS) | 240.52 ± 0.88 b | 404.59 ± 2.33 a | 59.28 ± 0.41 c | 263.24 ± 36.36 b | 45.76 ± 0.42 c |
| Total phenolic compounds (mg GA/100 mL FS) | 57.16 ± 1.52 e | 108.75 ± 0.35 c | 79.73 ± 1.04 d | 111.72 ± 0.35 b | 138.38 ± 0.14 a |
| ABTS (µmol TE/100 g FS) | 542.62 ± 7.20 d | 994.40 ± 32.28 c | 370.41 ± 10.69 e | 1097.35 ± 20.20 b | 3123.77 ± 26.15 a |
| DPPH (µmol TE/100 mL FS) | 80.71 ± 6.65 | 854.60 ± 17.60 b | 379.87 ± 70.86 c | 1115.25 ± 86.46 a | 329.24 ± 9.48 c |
| FRAP (µmol TE/100 mL FS) | 865.20 ± 10.24 c | 2744.48 ± 42.16 a | 480.20 ± 7.80 d | 2532.05 ± 48.63 b | ND |
| Parameter | MG Fruit mg/L FS | OPY Fruit mg/L FS |
|---|---|---|
| p-Coumaric acid | 60.60 ± 0.25 a | 16.85 ± 1.02 b |
| Gallic acid | 14.95 ± 0.01 a | 21.75 ± 0.75 a |
| Caffeic acid | 1.90 ± 0.01 b | 4.60 ± 0.01 a |
| Vanillic acid | 10.05 ± 0.04 a | 13.00 ± 0.45 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, E.M.M.; Sandate-Flores, L.; Rodríguez-Rodríguez, J.; Rostro-Alanis, M.; Parra-Arroyo, L.; Antunes-Ricardo, M.; Serna-Saldívar, S.O.; Iqbal, H.M.N.; Parra-Saldívar, R. Underutilized Mexican Plants: Screening of Antioxidant and Antiproliferative Properties of Mexican Cactus Fruit Juices. Plants 2021, 10, 368. https://doi.org/10.3390/plants10020368
Martínez EMM, Sandate-Flores L, Rodríguez-Rodríguez J, Rostro-Alanis M, Parra-Arroyo L, Antunes-Ricardo M, Serna-Saldívar SO, Iqbal HMN, Parra-Saldívar R. Underutilized Mexican Plants: Screening of Antioxidant and Antiproliferative Properties of Mexican Cactus Fruit Juices. Plants. 2021; 10(2):368. https://doi.org/10.3390/plants10020368
Chicago/Turabian StyleMartínez, Elda M. Melchor, Luisaldo Sandate-Flores, José Rodríguez-Rodríguez, Magdalena Rostro-Alanis, Lizeth Parra-Arroyo, Marilena Antunes-Ricardo, Sergio O. Serna-Saldívar, Hafiz M. N. Iqbal, and Roberto Parra-Saldívar. 2021. "Underutilized Mexican Plants: Screening of Antioxidant and Antiproliferative Properties of Mexican Cactus Fruit Juices" Plants 10, no. 2: 368. https://doi.org/10.3390/plants10020368
APA StyleMartínez, E. M. M., Sandate-Flores, L., Rodríguez-Rodríguez, J., Rostro-Alanis, M., Parra-Arroyo, L., Antunes-Ricardo, M., Serna-Saldívar, S. O., Iqbal, H. M. N., & Parra-Saldívar, R. (2021). Underutilized Mexican Plants: Screening of Antioxidant and Antiproliferative Properties of Mexican Cactus Fruit Juices. Plants, 10(2), 368. https://doi.org/10.3390/plants10020368









