Piceatannol Is Superior to Resveratrol at Suppressing Adipogenesis in Human Visceral Adipose-Derived Stem Cells
Abstract
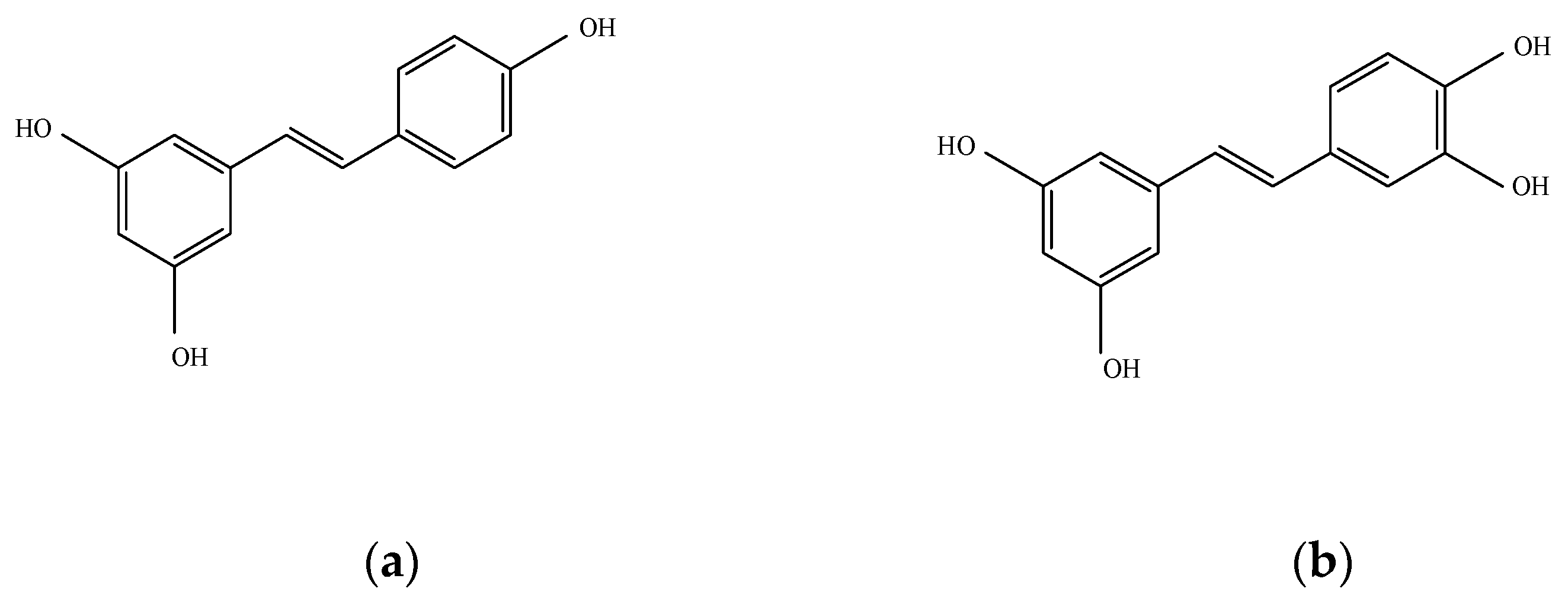
1. Introduction
2. Results
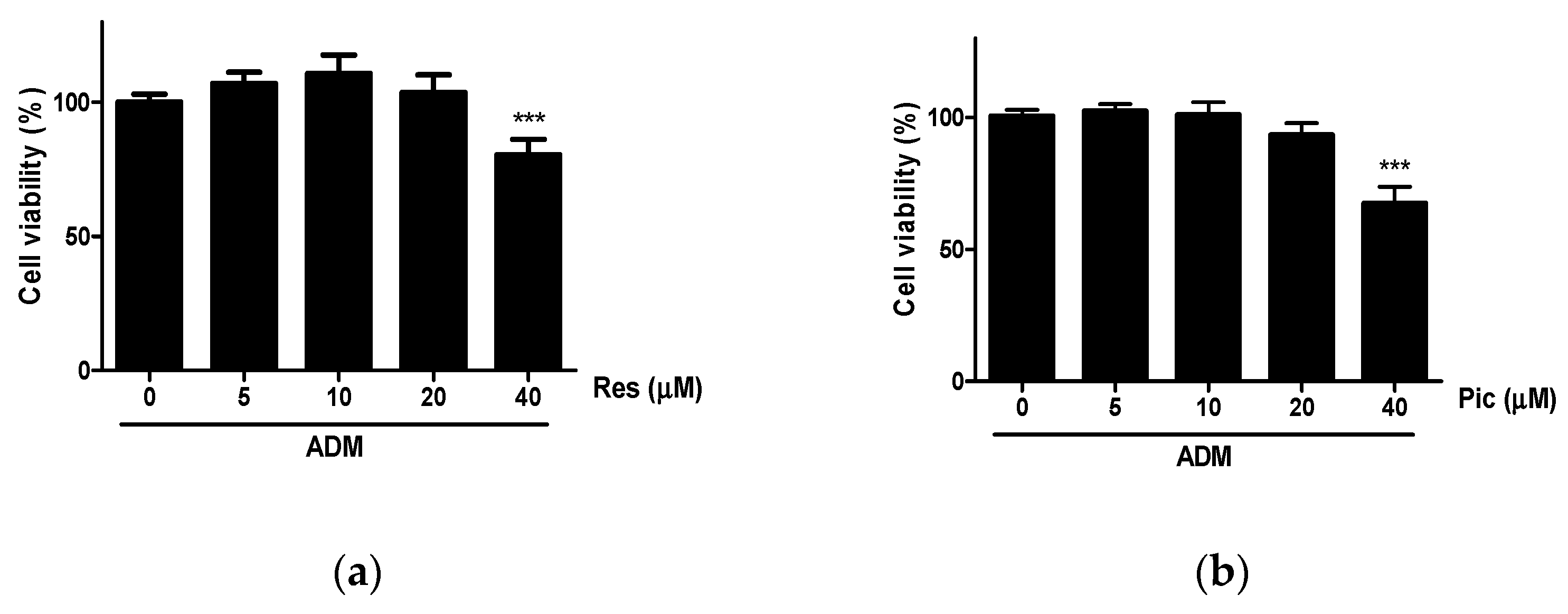
2.1. The Effect of RES and Pic on Cell Viability of vASCs
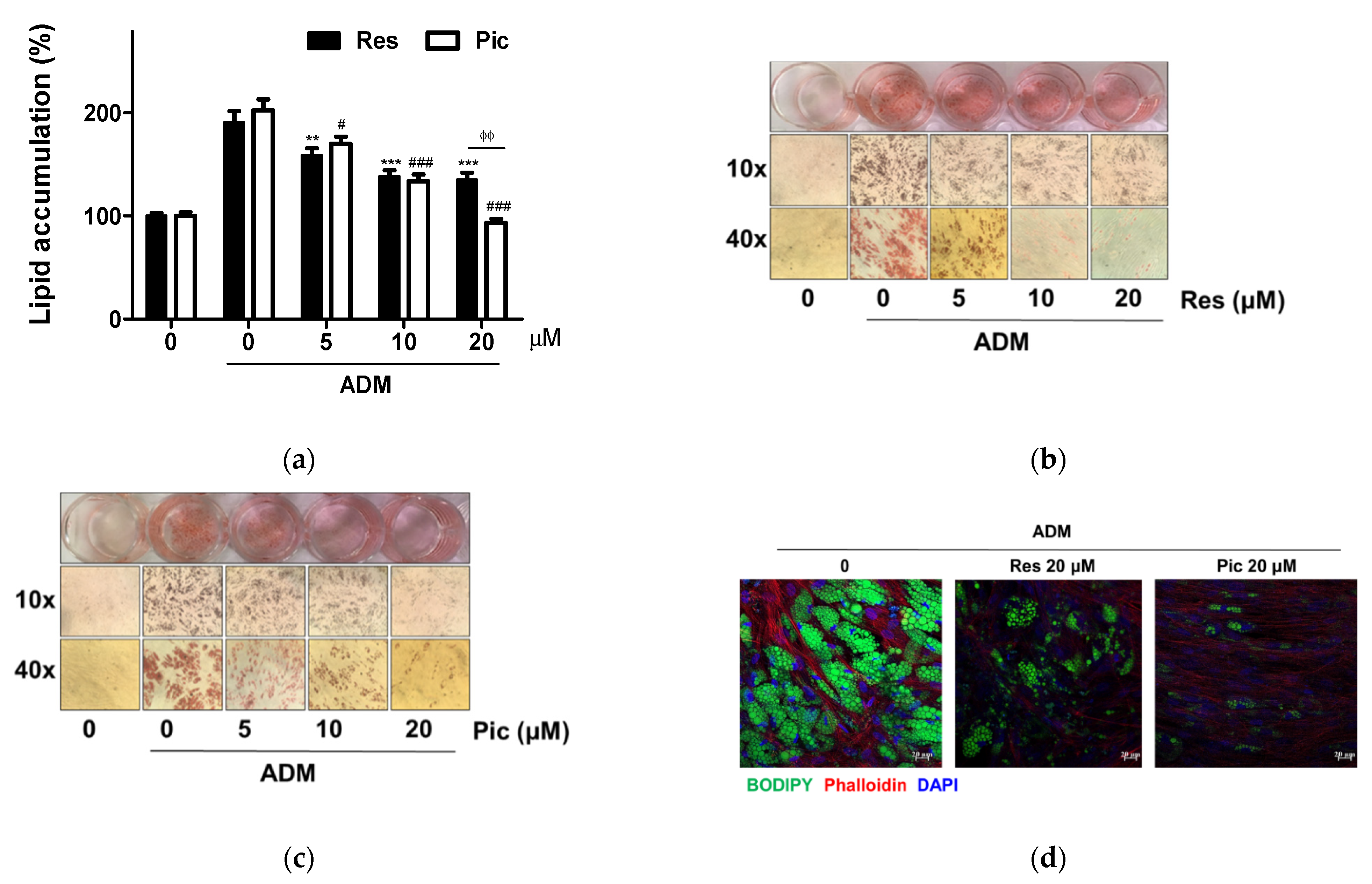
2.2. The Effect of Res and Pic on the Lipid Accumulation of vASCs
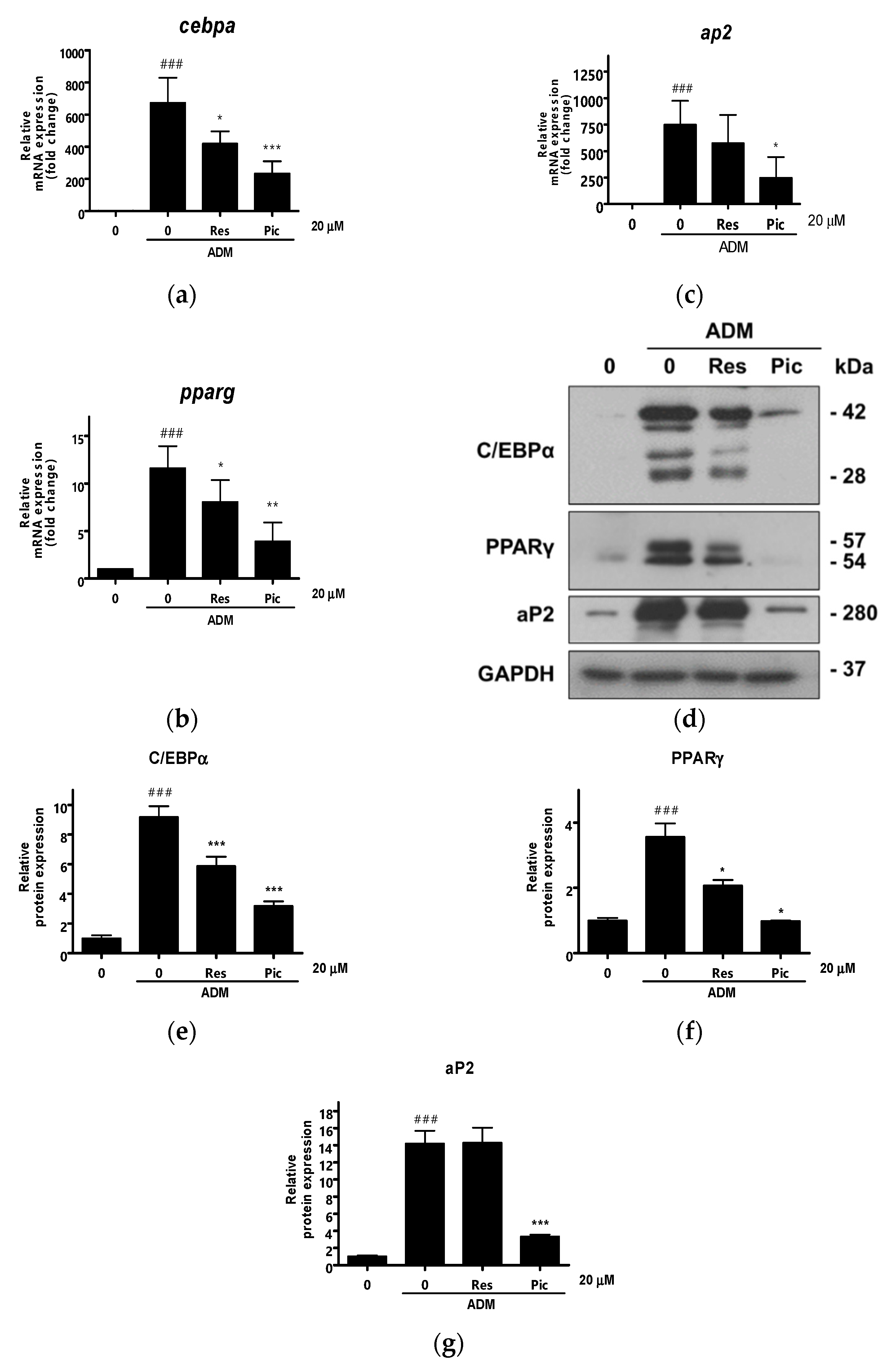
2.3. Comparison of Res and Pic on the Modulation of Adipogenic Marker Expression in vASCs
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Isolation of Human Visceral vASCs
4.3. Differentiation of Adipocytes from vASCs
4.4. ORO Staining
4.5. BODIPY Staining
4.6. qRT-PCR
4.7. Western Blot
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.W.; Lee, S.J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Resveratrol and cardiovascular diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Gal, R.; Deres, L.; Horvath, O.; Eros, K.; Sandor, B.; Urban, P.; Soos, S.; Marton, Z.; Sumegi, B.; Toth, K.; et al. Resveratrol Improves Heart Function by Moderating Inflammatory Processes in Patients with Systolic Heart Failure. Antioxidants 2020, 9, 1108. [Google Scholar] [CrossRef]
- Gwak, H.; Kim, S.; Dhanasekaran, D.N.; Song, Y.S. Resveratrol triggers ER stress-mediated apoptosis by disrupting N-linked glycosylation of proteins in ovarian cancer cells. Cancer Lett. 2016, 371, 347–353. [Google Scholar] [CrossRef]
- Han, Y.; Jo, H.; Cho, J.H.; Dhanasekaran, D.N.; Song, Y.S. Resveratrol as a Tumor-Suppressive Nutraceutical Modulating Tumor Microenvironment and Malignant Behaviors of Cancer. Int. J. Mol. Sci. 2019, 20, 925. [Google Scholar] [CrossRef]
- Ferrigni, N.R.; McLaughlin, J.L.; Powell, R.G.; Smith, C.R., Jr. Use of potato disc and brine shrimp bioassays to detect activity and isolate piceatannol as the antileukemic principle from the seeds of Euphorbia lagascae. J. Nat. Prod. 1984, 47, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Kim, J.E.; Song, N.R.; Son, J.E.; Hwang, M.K.; Byun, S.; Kim, J.H.; Lee, K.W.; Lee, H.J. Phosphoinositide 3-kinase is a novel target of piceatannol for inhibiting PDGF-BB-induced proliferation and migration in human aortic smooth muscle cells. Cardiovasc. Res. 2010, 85, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Jung, J.I.; Cho, H.J.; Her, S.; Kwon, S.H.; Yu, R.; Kang, Y.H.; Lee, K.W.; Park, J.H. Inhibition of tumor progression by oral piceatannol in mouse 4T1 mammary cancer is associated with decreased angiogenesis and macrophage infiltration. J. Nutr. Biochem. 2015, 26, 1368–1378. [Google Scholar] [CrossRef]
- Song, N.R.; Hwang, M.K.; Heo, Y.S.; Lee, K.W.; Lee, H.J. Piceatannol suppresses the metastatic potential of MCF10A human breast epithelial cells harboring mutated H-ras by inhibiting MMP-2 expression. Int. J. Mol. Med. 2013, 32, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Lin, H.; Zhao, M.; Tao, L.; Yang, Y.; Xu, X.; Jia, A.; Zhang, J.; Weng, D. Piceatannol attenuates D-GalN/LPS-induced hepatoxicity in mice: Involvement of ER stress, inflammation and oxidative stress. Int. Immunopharmacol. 2018, 64, 131–139. [Google Scholar] [CrossRef]
- Lai, T.N.; André, C.M.; Chirinos, R.; Nguyen, T.B.; Larondelle, Y.; Rogez, H. Optimisation of extraction of piceatannol from Rhodomyrtus tomentosa seeds using response surface methodology. Sep. Purif. Technol. 2014, 134, 139–146. [Google Scholar] [CrossRef]
- Krambeck, K.; Oliveira, A.; Santos, D.; Pintado, M.M.; Baptista Silva, J.; Sousa Lobo, J.M.; Amaral, M.H. Identification and Quantification of Stilbenes (Piceatannol and Resveratrol) in Passiflora edulis By-Products. Pharmaceuticals 2020, 13, 73. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Moreno, J.J. Resveratrol analogs with antioxidant activity inhibit intestinal epithelial cancer Caco-2 cell growth by modulating arachidonic acid cascade. J. Agric. Food Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.; Hsieh, T.C.; Halicka, H.D.; Darzynkiewicz, Z.; Wu, J.M. Upregulation of PDL1 expression by resveratrol and piceatannol in breast and colorectal cancer cells occurs via HDAC3/p300mediated NFkappaB signaling. Int. J. Oncol. 2018, 53, 1469–1480. [Google Scholar] [CrossRef]
- Wen, H.; Fu, Z.; Wei, Y.; Zhang, X.; Ma, L.; Gu, L.; Li, J. Antioxidant Activity and Neuroprotective Activity of Stilbenoids in Rat Primary Cortex Neurons via the PI3K/Akt Signalling Pathway. Molecules 2018, 23, 2328. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization Obesity and Overweight Fact Sheet; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Heyn, G.S.; Corrêa, L.H.; Magalhães, K.G. The Impact of Adipose Tissue-Derived miRNAs in Metabolic Syndrome, Obesity, and Cancer. Front. Endocrinol. 2020, 11, 563816. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metab. Clin. Exp. 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Liu, W.; Li, D.; Cao, H.; Li, H.; Wang, Y. Expansion and inflammation of white adipose tissue—Focusing on adipocyte progenitors. Biol. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Qu, R.; Fan, T.; Ouyang, J.; Dai, J. A glance on the role of actin in osteogenic and adipogenic differentiation of mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xiao, X.; Feng, X.; Li, W.; Zhou, N.; Zheng, L.; Sun, Y.; Zhang, Z.; Zhu, W. Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J. Nutr. Biochem. 2012, 23, 1100–1112. [Google Scholar] [CrossRef]
- Mitterberger, M.C.; Zwerschke, W. Mechanisms of resveratrol-induced inhibition of clonal expansion and terminal adipogenic differentiation in 3T3-L1 preadipocytes. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1356–1376. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Seo, S.G.; Heo, Y.S.; Yue, S.; Cheng, J.X.; Lee, K.W.; Kim, K.H. Piceatannol, natural polyphenolic stilbene, inhibits adipogenesis via modulation of mitotic clonal expansion and insulin receptor-dependent insulin signaling in early phase of differentiation. J. Biol. Chem. 2012, 287, 11566–11578. [Google Scholar] [CrossRef]
- Carpene, C.; Pejenaute, H.; Del Moral, R.; Boulet, N.; Hijona, E.; Andrade, F.; Villanueva-Millan, M.J.; Aguirre, L.; Arbones-Mainar, J.M. The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes. Int. J. Mol. Sci. 2018, 19, 2081. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, B.; Kim, M.K.; Gong, S.P.; Park, N.H.; Chung, H.H.; Kim, H.S.; No, J.H.; Park, W.Y.; Park, A.K.; et al. Gene expression profiles of human subcutaneous and visceral adipose-derived stem cells. Cell Biochem. Funct. 2016, 34, 563–571. [Google Scholar] [CrossRef]
- Shin, S.; El-Sabbagh, A.S.; Lukas, B.E.; Tanneberger, S.J.; Jiang, Y. Adipose stem cells in obesity: Challenges and opportunities. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Arai, D.; Kataoka, R.; Otsuka, S.; Kawamura, M.; Maruki-Uchida, H.; Sai, M.; Ito, T.; Nakao, Y. Piceatannol is superior to resveratrol in promoting neural stem cell differentiation into astrocytes. Food Funct. 2016, 7, 4432–4441. [Google Scholar] [CrossRef]
- Setoguchi, Y.; Oritani, Y.; Ito, R.; Inagaki, H.; Maruki-Uchida, H.; Ichiyanagi, T.; Ito, T. Absorption and metabolism of piceatannol in rats. J. Agric. Food Chem. 2014, 62, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Kinoshita, Y.; Maruki-Uchida, H.; Yanae, K.; Sai, M.; Ito, T. Piceatannol and its metabolite, isorhapontigenin, induce SIRT1 expression in THP-1 human monocytic cell line. Nutrients 2014, 6, 4794–4804. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Irwin-Houston, J.M.; Weiss, C. Combination of resveratrol and 5-azacytydine improves osteogenesis of metabolic syndrome mesenchymal stem cells. J. Cell. Mol. Med. 2018, 22, 4771–4793. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Shayan, P.; Busch, F.; Aldinger, C.; Buhrmann, C.; Lueders, C.; Mobasheri, A. Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: Potential role of Runx2 deacetylation. PLoS ONE 2012, 7, e35712. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Murias, M.; Jager, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure-activity relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef]
- Wagner, G.; Lindross-Christensen, J.; Einwallner, E.; Husa, J.; Zap, T.C.; Lipp, K.; Rauscher, S.; Groger, M.; Spittler, A.; Loewe, R.; et al. HO-1 inhibits preadipocyte proliferation and differentiation at the onset of obesity via ROS dependent activation of Akt. Sci. Rep. 2017, 7, 40881. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Zhang, C.; Gao, L.; Li, J. Piceatannol pretreatment alleviates acute cardiac injury via regulating PI3K-Akt-eNOS signaling in H9c2 cells. Biomed. Pharmacother. 2019, 109, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Kim, B.; Han, Y.; Yang, H.; Cho, U.; Kim, S.I.; Kim, J.H.; Yoon Park, J.H.; Lee, K.W.; Song, Y.S. Decursin and Decursinol Angelate Suppress Adipogenesis through Activation of β-catenin Signaling Pathway in Human Visceral Adipose-Derived Stem Cells. Nutrients 2019, 12, 13. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Xu, X.; Wang, S.; Yang, Y.; Zhou, J.; Zhang, L.; Zheng, F.; Li, X.; Wang, B. Embelin attenuates adipogenesis and lipogenesis through activating canonical Wnt signaling and inhibits high-fat diet-induced obesity. Int. J. Obes. 2017, 41, 729–738. [Google Scholar] [CrossRef]
- Tabrizi, R.; Tamtaji, O.R.; Lankarani, K.B.; Akbari, M.; Dadgostar, E.; Dabbaghmanesh, M.H.; Kolahdooz, F.; Shamshirian, A.; Herabi, M.M.; Asemi, Z. The effects of resveratrol intake on weight loss: A systematic review and meta-analysis of randomized controlled trial. Crit. Rev. Food Sci. Nutr. 2020, 60, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stødkilde-Jørgensen, H.; Møller, N.; Jessen, N.; Pedersen, S.B.; Jørgensen, J.O. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef]
- Dai, Y.; Lim, J.X.; Yeo, S.C.M.; Xiang, X.; Tan, K.S.; Fu, J.H.; Huang, L.; Lin, H.S. Biotransformation of piceatannol, a dietary resveratrol derivative: Promises to human health. Mol. Nutr. Food Res. 2020, 64, 19000905. [Google Scholar] [CrossRef]
- Yeo, S.C.M.; Fenwick, P.; Barnes, P.J.; Lin, H.S.; Donnelly, L.E. Isorhapontigenin, a bioavailable dietary polyphenol, suppresses airway epithelial cell inflammation through a corticosteroid-independent mechanism. Br. J. Pharmacol. 2017, 174, 2043–2059. [Google Scholar] [CrossRef]
- Tung, Y.C.; Lin, Y.H.; Chen, H.J.; Chou, S.C.; Cheng, A.C.; Kalyanam, N.; Ho, C.T.; Pan, M.H. Piceatannol exerts anti-obesity effects in C57BL/6 mice through modulating adipogenic proteins and gut microbiota. Molecules 2016, 21, 1419. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, A.M.; Zlotnik, A. Translating translational research: Mouse models of human disease. Cell. Mol. Immunol. 2013, 10, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Aichler, M.; Kunzke, T.; Buck, A.; Sun, N.; Ackermann, M.; Jonigk, D.; Gaumann, A.; Walch, A. Molecular similarities and differences from human pulmonary fibrosis and corresponding mouse model: MALDI imaging mass spectrometry in comparative medicine. Lab. Investig. 2018, 98, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Safahani, M.; Aligoholi, H.; Noorbakhsh, F.; Djalali, M.; Pishva, H.; Mousavi, S.M.M.; Alipour, F.; Gorji, A.; Koohdani, F. Resveratrol promotes the arcuate nucleus architecture remodeling to produce more anorexigenic neurons in high-fat-diet-fed mice. Nutrition 2018, 50, 49. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Hedjazifar, S.; Gogg, S.; Hammarstedt, A.; Smith, U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 2015, 26, 193–200. [Google Scholar] [CrossRef]
- Vishvanath, L.; Gupta, R.L. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Investig. 2019, 129, 4022–4031. [Google Scholar] [CrossRef]
- De Koning, L.; Merchant, A.T.; Pogue, J.; Anand, S.S. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: Meta-regression analysis of prospective studies. Eur. Heart J. 2007, 28, 850–856. [Google Scholar] [CrossRef]
| Donor No. | Age | WHR | BMI |
|---|---|---|---|
| #31 | 66 | 0.98 | 23.94 |
| #34 | 66 | 0.97 | 25.56 |
| #47 | 71 | 0.98 | 24.36 |
| #50 | 54 | 0.94 | 22.99 |
| #54 | 68 | 1.04 | 27.37 |
| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| C/EBPα | GCAAACTCACCGCTCCAATG | CTTCTCTCATGGGGGTCTGC |
| PPARγ | AGGTCAGCGGACTCTGGATTC | AGTGGGGATGTCTCATAATG |
| aP2 | ATGGGGGTGTCCTGGTACAT | ACGTCCCTTGGCTTATGCTC |
| GAPDH | GAGTCAACGGATTTGGTCGT | TTGATTTTGGAGGGATCTCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, I.S.; Han, Y.; Jo, H.; Lee, K.W.; Song, Y.S. Piceatannol Is Superior to Resveratrol at Suppressing Adipogenesis in Human Visceral Adipose-Derived Stem Cells. Plants 2021, 10, 366. https://doi.org/10.3390/plants10020366
Park IS, Han Y, Jo H, Lee KW, Song YS. Piceatannol Is Superior to Resveratrol at Suppressing Adipogenesis in Human Visceral Adipose-Derived Stem Cells. Plants. 2021; 10(2):366. https://doi.org/10.3390/plants10020366
Chicago/Turabian StylePark, In Sil, Youngjin Han, HyunA Jo, Ki Won Lee, and Yong Sang Song. 2021. "Piceatannol Is Superior to Resveratrol at Suppressing Adipogenesis in Human Visceral Adipose-Derived Stem Cells" Plants 10, no. 2: 366. https://doi.org/10.3390/plants10020366
APA StylePark, I. S., Han, Y., Jo, H., Lee, K. W., & Song, Y. S. (2021). Piceatannol Is Superior to Resveratrol at Suppressing Adipogenesis in Human Visceral Adipose-Derived Stem Cells. Plants, 10(2), 366. https://doi.org/10.3390/plants10020366







