Influence of Bagging on the Development and Quality of Fruits
Abstract
1. Introduction
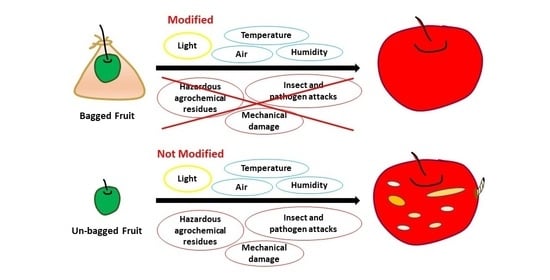
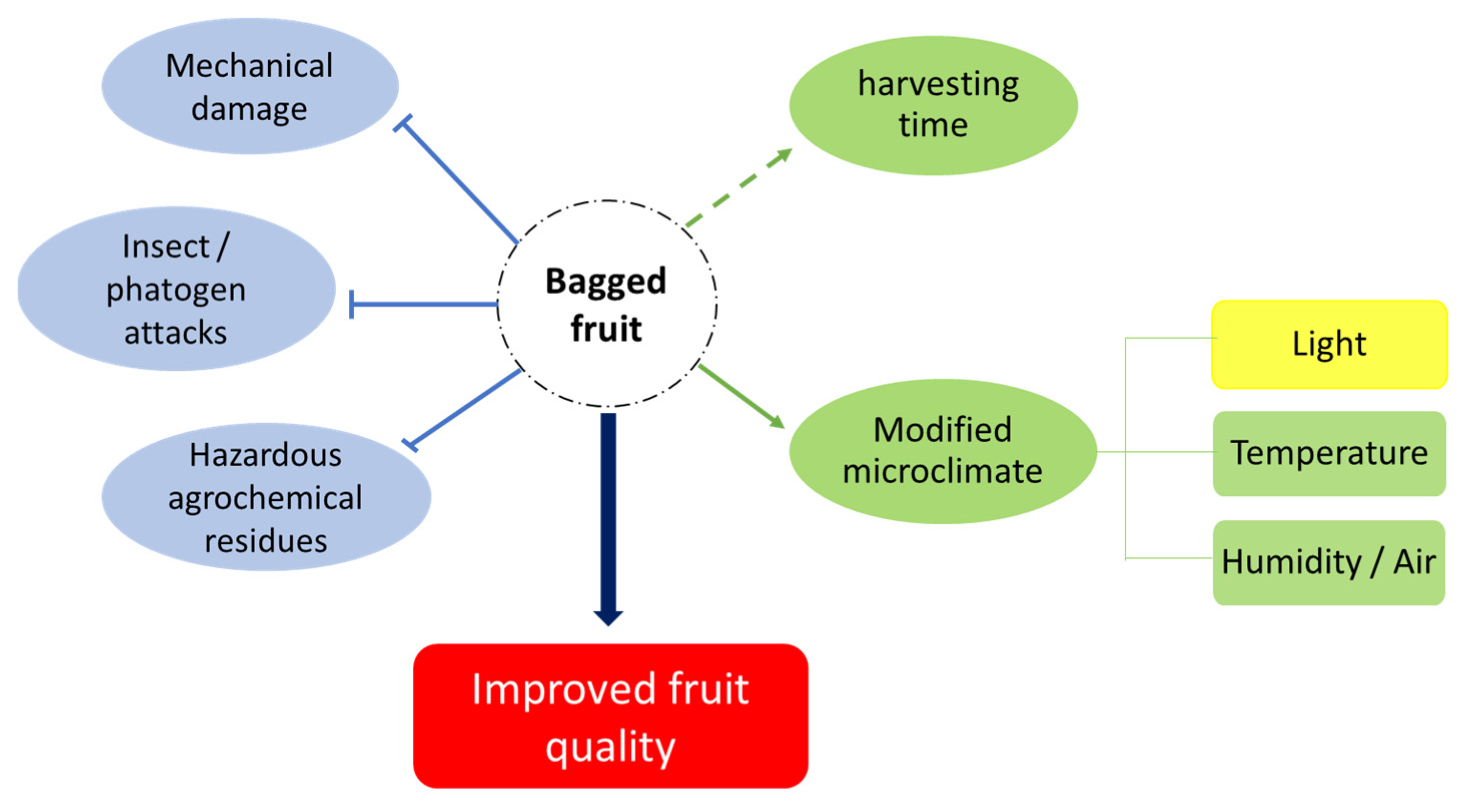
2. The Role of Bagging on Fruit Quality
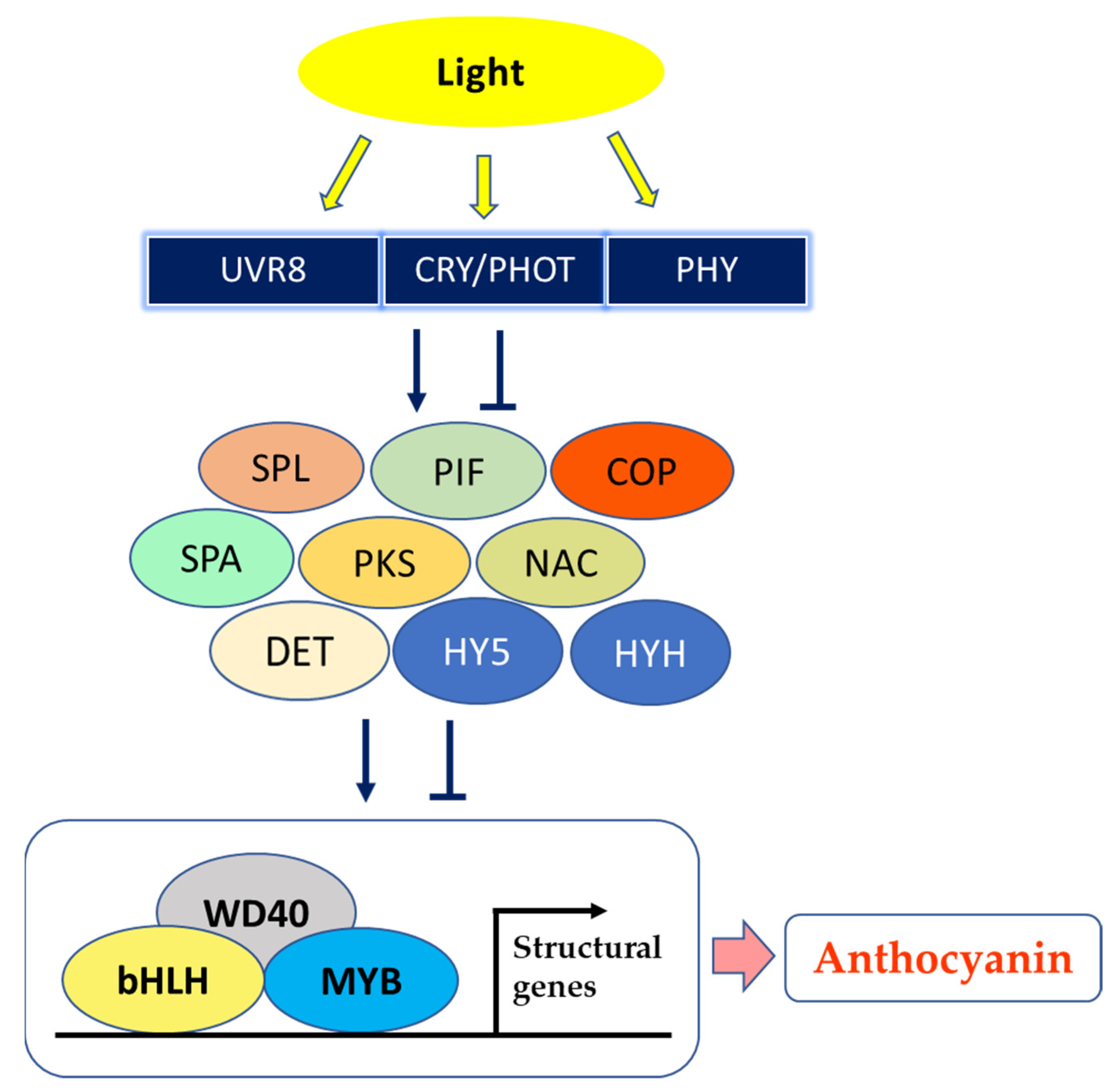
3. Light and Fruit Flavonoids
4. Bagging and the Color of Fruits
5. Bagging and Fruit Microclimate
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Sommer, A.; Vyas, K.S. A global clinical view on vitamin A and carotenoids. Am. J. Clin. Nutr. 2012, 96, 1204S–1206S. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables-the millennium’s health. Int. J. Food Sci. Technol. 2008, 36, 703–725. [Google Scholar] [CrossRef]
- Pem, D.; Jeewon, R. Fruit and vegetable intake: Benefits and progress of nutrition education interventions-narrative review article. Iran. J. Public Health 2015, 44, 1309–1321. [Google Scholar]
- Food and Agriculture Organization of the United Nations; World Health Organization. Sustainable Healthy Diets–Guiding Principles. Available online: http://www.fao.org/3/ca6640en/ca6640en.pdf (accessed on 20 December 2020).
- Nunes, M.C.N. Impact of environmental conditions on fruit and vegetable quality. Stewart Postharvest Rev. 2008, 4, 1–14. [Google Scholar] [CrossRef]
- Sharma, R.R.; Reddy, S.V.R.; Jhalegar, M.J. Pre-harvest fruit bagging: A useful approach for plant protection and improved post-harvest fruit quality—A review. J. Hortic. Sci. Biotechnol. 2014, 89, 101–113. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, H.H.; Youn, C.K.; Kweon, S.J.; Jung, H.J.; Lee, C.H. Effects of bagging material on fruit coloration and quality of “janghowon hwangdo” peach. Acta Hortic. 2008, 772, 81–86. [Google Scholar] [CrossRef]
- Frank, D. Evaluation of Fruit Bagging as a Pest Management Option for Direct Pests of Apple. Insects 2018, 9, 178. [Google Scholar] [CrossRef]
- Jing, L.; Youhong, C.; Zhimei, Y.; Xiaogang, L. Effects of bagging on the quality of pear fruit and pesticide residues. Acta Hortic. 2008, 772, 315–318. [Google Scholar] [CrossRef]
- Xu, G.; Nie, J.; Wu, Y.; Yan, Z.; Ye, M. The effects of fruit bagging on residue behavior and dietary risk for four pesticides in apple. Sci. Rep. 2018, 8, 14348. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.R.; Nagaraja, A.; Goswami, A.K.; Thakre, M.; Kumar, R.; Varghese, E. Influence of on-the-tree fruit bagging on biotic stresses and postharvest quality of rainy-season crop of ‘Allahabad Safeda’ guava (Psidium guajava L.). Crop Prot. 2020, 135, 105216. [Google Scholar] [CrossRef]
- Allran, J.; Schnabel, G.; Melgar, J.C. Peach bagging in the Southeastern US. J. Am. Pomol. Soc. 2019, 73, 38–46. [Google Scholar]
- Campbell, D.; Sarkhosh, A.; Brecht, J.K.; Gillett-Kaufman, J.L.; Liburd, O.; Melgar, J.; Treadwell, D. Bagging Organic Peaches Reduces Physical Injuries and Storage Decay with Minimal Effects on Fruit Quality. HortScience 2021, 56, 52–58. [Google Scholar] [CrossRef]
- Araújo Neto, S.E.; Rocha, C.; de Farias, J.F.; Minosso, S.C.C.; Ferreira, R.L.F. Quality of guava fruits bagged with different materials in an organic system. Comun. Sci. 2020, 11, e3206. [Google Scholar] [CrossRef]
- Shimizu, K.; Kawana, T.; Ohtani, T.; Mihira, T.; Furukawa, S. Meta-analysis of the damage suppression effect of a double-layer fruit protection bag on fruit bug injuries of loquat fruits and evaluation by explicit solution of odds ratio. Appl. Entomol. Zool. 2019, 54, 247–254. [Google Scholar] [CrossRef]
- Lima, J.D.; Engelking, E.W.; Rozane, D.E.; Gomes, E.N.; da Silva, S.H.M.G.; Kluge, R.A. Effect of bunch protection material and bagging time on the yield of ‘Nanica’ banana and chilling control. Aust. J. Crop Sci. 2020, 14, 574–580. [Google Scholar] [CrossRef]
- Issarakraisila, M. Effect of types of bagging materials on growth, quality and disease-insect damages in pummelo fruit in tropical humid conditions. Acta Hortic. 2018, 1208, 319–324. [Google Scholar] [CrossRef]
- Sarker, D.; Rahman, M.; Barman, J. Efficacy of different bagging materials for the control of mango fruit fly. Bangladesh J. Agric. Res. 2009, 34, 165–168. [Google Scholar] [CrossRef]
- Watanawan, A.; Watanawan, C.; Jarunate, J. Bagging “Nam Dok Mai #4” mango during development affects color and fruit quality. Acta Hortic. 2008, 787, 325–328. [Google Scholar] [CrossRef]
- Ding, P.; Syakirah, M.N. Influence of fruit bagging on postharvest quality of “Harumanis” mango (Mangifera indica L.). Acta Hortic. 2010, 877, 169–174. [Google Scholar] [CrossRef]
- Chonhenchob, V.; Kamhangwong, D.; Kruenate, J.; Khongrat, K.; Tangchantra, N.; Wichai, U.; Singh, S.P. Preharvest bagging with wavelength-selective materials enhances development and quality of mango (Mangifera indica L.) cv. Nam Dok Mai #4. J. Sci. Food Agric. 2011, 91, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Mathooko, F.M.; Kahangi, E.M.; Runkuab, J.M.; Onyangob, C.A.; Owinob, W.O. Pre-harvest mango (Mangifera indica L. ‘Apple’) fruit bagging controls lenticel discolouration and improves postharvest quality. Acta Hortic. 2011, 906, 55–62. [Google Scholar] [CrossRef]
- Rahman, M.A.; Alam, S.M.K.; Reza, M.H.; Uddin, M.S.; Amin, M.N.; Nasrin, T.A.A. Impact of pre-harvest fruit bagging and improved postharvest practices in reducing losses and managing quality of mango in the value chain system. Int. J. Postharvest Technol. Innov. 2019, 6, 117–136. [Google Scholar] [CrossRef]
- Xu, C.X.; Chen, H.B.; Huang, R.Y.; He, Y.J. Effects of bagging on fruit growth and quality of carambola. Acta Hortic. 2008, 773, 195–200. [Google Scholar] [CrossRef]
- Morera-Montoya, R.; Blanco-Metzler, H.; Luis-Loria, C. Evaluation of different bagging materials for the control of the fruit fly Anastrepha sp. (Diptera:Tephritidae) and fruit pathogens in Taiwanese guava fruits (Psidium guajava L.). Acta Hortic. 2010, 849, 283–292. [Google Scholar] [CrossRef]
- Hu, G.B.; Chen, D.C.; Li, P.; Ouyang, R.; Gao, F.F.; Wang, H.C.; Dong, J. Effects of bagging on fruit coloration and phenylalanine ammonia lyase and polyphenol oxidase in “Feizixiao” Litchi. Acta Hortic. 2001, 558, 273–278. [Google Scholar] [CrossRef]
- Pal, M.; Lal, R.L.; Nautiyal, P.; Joshi, P. Effect of chemicals and physical means on harvesting span, yield and quality of litchi (Litchi chinensis Sonn.) cv. Rose Scented. J. Appl. Hortic. 2016, 18, 71–75. [Google Scholar] [CrossRef]
- Xu, H.X.; Chen, J.W.; Xie, M. Effect of different light transmittance paper bags on fruit quality and antioxidant capacity in loquat. J. Sci. Food Agric. 2010, 90, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Zhang, Z.; Gao, Z.; Gu, L.; Huang, L. Effects of bagging on sugar metabolism and the activity of sugar metabolism related enzymes during fruit development of Qingzhong loquat. Afr. J. Biotechnol. 2011, 10, 4212–4216. [Google Scholar] [CrossRef]
- Yang, W.H.; Zhu, X.C.; Bu, J.H.; Hu, G.B.; Wang, H.C.; Huang, X.M. Effects of bagging on fruit development and quality in cross-winter off-season longan. Sci. Hortic. 2009, 120, 194–200. [Google Scholar] [CrossRef]
- Fumuro, M.; Gamo, H. Effects of bagging on the occurrence of black stain on the skin of ‘Shinsyu’ persimmon (Diospyros kaki L.) grown under film. J. Jpn. Soc. Hortic. Sci. 2001, 70, 261–263. [Google Scholar] [CrossRef][Green Version]
- Katagiri, T.; Satoh, Y.; Fukuda, T.; Kataoka, I. Improving marketability of ’Fuyu’ persimmon fruit by bagging culture. Acta Hortic. 2003, 601, 213–217. [Google Scholar] [CrossRef]
- Kwan, K.D.; Soo, C.D.; Sik, K.E.; Hyuan, H.K.; Ho, L.K.; Sik, K.K.; Cheol, L.K. Fruit quality of Yuzu (Citrus junos L.) as influenced by bagging time and materials of bagging treatment. J. Korean Soc. Hortic. Sci. 2000, 41, 190–193. [Google Scholar]
- Kassem, H.A.; Omar, A.K.H.; Ahmed, M.A. Response of ‘Zaghloul’ date palm productivity, ripening and quality to different polyethylene bagging treatments. Am. Eurasian J. Agric. Environ. Sci. 2011, 11, 616–621. [Google Scholar]
- Al-Obeed, R.S.; Harhash, M.M. Effect of bunch bagging color on “succary” and “khalas” date palm cultivars: Fruit chemical characteristics. Acta Hortic. 2010, 882, 1213–1217. [Google Scholar] [CrossRef]
- Awad, M.A. Increasing the rate of ripening of date palm fruit (Phoenix dactylifera L.) cv. Helali by preharvest and postharvest treatments. Postharvest Biol. Technol. 2007, 43, 121–127. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, Q.; Liu, Q.; Li, W. Effects of the micro-environment inside fruit bags on the structure of fruit peel in ‘Fuji’ apple. Acta Ecol. Sin. 2011, 31, 2831–2836. [Google Scholar]
- Hamedi Sarkomi, F.; Moradinezhad, F.; Khayat, M. Pre-harvest bagging influences sunburn, cracking and quality of pomegranate fruits. J. Hortic. Postharvest Res. 2019, 2, 131–142. [Google Scholar] [CrossRef]
- Chaiwong, S.; Saengrayap, R.; Prahsarn, C. Effects of different materials for banana bunch covers during winter in Thailand. Acta Hortic. 2019, 1245, 21–28. [Google Scholar] [CrossRef]
- Zhang, B.B.; Guo, J.Y.; Ma, R.J.; Cai, Z.X.; Yan, J.; Zhang, C.H. Relationship between the bagging microenvironment and fruit quality in ‘guibao’ peach [Prunus persica (L.) Batsch]. J. Hortic. Sci. Biotech. 2015, 90, 303–310. [Google Scholar] [CrossRef]
- Zhou, J.; Zhong, G.; Lin, Z.; Xu, H. The effects of bagging on fresh fruit quality of Canarium album. J. Food Agric. Environ. 2012, 10, 505–508. [Google Scholar]
- Jing, C.; Feng, D.; Zhao, Z.; Wu, X.; Chen, X. Effect of environmental factors on skin pigmentation and taste in three apple cultivars. Acta Physiol. Plant. 2020, 42, 69. [Google Scholar] [CrossRef]
- Islam, M.T.; Shamsuzzoha, M.; Rahman, M.S.; Bari, M.A.; Akter, M.M.; Khatun, A.; Huque, R.; Uddin, M.S. Effect of bagging time on fruit quality and shelf life of mango (Mangifera indica L.) Cv. Langra in Bangladesh. Int. J. Agric. Environ. Biores. 2019, 4, 279–289. [Google Scholar] [CrossRef]
- Bentley, W.J.; Viveros, M. Brown-bagging Granny Smith apples on trees stops codling moth damage. Calif. Agric. 1992, 46, 30–32. [Google Scholar] [CrossRef]
- Huang, C.; Yu, B.; Teng, Y.; Su, J.; Shu, Q.; Cheng, Z.; Zeng, L. Effects of fruit bagging on coloring and related physiology, and qualities of red Chinese sand pears during fruit maturation. Sci. Hortic. 2009, 121, 149–158. [Google Scholar] [CrossRef]
- Sharma, R.R.; Pal, R.K.; Asrey, R.; Sagar, V.R.; Dhiman, M.R.; Rana, M.R. Pre-harvest fruit bagging influences fruit color and quality of apple cv. Delicious. Agric. Sci. 2013, 4, 443–448. [Google Scholar] [CrossRef]
- Asrey, R.; Kumar, K.; Sharma, R.R.; Meena, N.K. Fruit bagging and bag color affects physico-chemical, nutraceutical quality and consumer acceptability of pomegranate (Punica granatum L.) arils. J. Food Sci. Technol. 2020, 57, 1469–1476. [Google Scholar] [CrossRef]
- Griñán, I.; Morales, D.; Galindo, A.; Torrecillas, A.; Pérez-López, D.; Moriana, A.; Collado-González, J.; Carbonell-Barrachina, Á.A.; Hernández, F. Effect of preharvest fruit bagging on fruit quality characteristics and incidence of fruit physiopathies in fully irrigated and water stressed pomegranate trees. J. Sci. Food Agric. 2019, 99, 1425–1433. [Google Scholar] [CrossRef]
- Dong, Z.F.; Wang, Y.Z.; Wang, L.; Liu, C.L.; Dong, X.Y.; Liu, G.S.; Yuan, Y.B. Effects of different bag treatments on the absorption of calcium in ‘Red Fuji’ apple fruit. Acta Hortic. Sin. 2007, 34, 835–840. [Google Scholar]
- Kim, D.H.; Byun, J.K.; Choi, C.; Choi, D.G.; Kang, I.K. The effect of calcium chloride, prohexadione-Ca, and Ca-coated paper bagging on reduction of bitter pit in ‘Gamhong’ apple. Korean J. Hortic. Sci. Technol. 2008, 26, 367–371. [Google Scholar]
- Teixeira, R.; Boff, M.I.C.; Amarante, C.V.T.; Steffens, C.A.; Boff, P. Effects of fruit bagging on pests and diseases control and on quality and maturity of ‘Fuji Suprema’ apples. Bragantia 2011, 70, 688–695. [Google Scholar] [CrossRef]
- Teixeira, R.; Amarante, C.V.T.; Boff, M.I.C.; Ribeiro, L.G. Control of insect pests and diseases, maturity and quality of ‘Imperial Gala’ apples submitted to bagging. Braz. Mag. Fruit Cult. 2011, 33, 394–401. [Google Scholar] [CrossRef]
- Rajametov, S.; Nurbekov, A. Effects of different types of paper bags on ‘Golden Delicious’ apple fruits. Acta Hortic. 2020, 1275, 415–418. [Google Scholar] [CrossRef]
- Matsumoto, K.; Fujita, T.; Sato, S. Effects of 1-MCP and Pre-harvest Fruit Bagging Treatments on Cold Storability of the Red-fleshed Apple ‘Kurenainoyume’. Hortic. J. 2018, 87, 443–451. [Google Scholar] [CrossRef]
- Faoro, I.D.; Mondardo, M. Ensacamento de frutos de pereira cv. Housui. Rev. Bras. Frutic. 2004, 26, 86–88. [Google Scholar] [CrossRef]
- Rajametov, S.; Nurbekov, A. Effects of different types of paper bags on ‘Carmen’ pear fruits. Acta Hortic. 2020, 1275, 337–340. [Google Scholar] [CrossRef]
- Amarante, C.; Banks, N.H.; Max, S. Pre-harvest bagging improves packout and fruit quality of pears (Pyrus communis). N. Z. J. Crop Hortic. Sci. 2002, 30, 93–98. [Google Scholar] [CrossRef]
- Lin, J.; Wang, J.H.; Li, X.J.; Chang, Y.H. Effects of bagging twice and room temperature storage on quality of ‘Cuiguan’ pear fruit. Acta Hortic. 2012, 934, 837–840. [Google Scholar] [CrossRef]
- Jia, H.; Araki, A.; Okamoto, G. Influence of fruit bagging on aroma volatiles and skin coloration of ‘Hakuho’ peach (Prunus persica Batsch). Postharvest Biol. Technol. 2005, 35, 61–68. [Google Scholar] [CrossRef]
- Das, K.; Roy, M.; Roy, D.; Dutta, P. Influence of different sources of potassium on fruit quality and shelf-life of mango cv Himsagar. Environ. Ecol. 2017, 35, 1318–1322. [Google Scholar]
- Zhang, H.P.; Qin, A.; Qi, K.J.; Tao, S.T.; Wu, J.Y.; Huang, X.S.; Zhang, S.L. The effect of bagging on ascorbate in Pyrus fruit. N. Z. J. Crop Hortic. Sci. 2019, 47, 19–31. [Google Scholar] [CrossRef]
- Zhu, M.; Fang, W.; Chen, C.; Wang, L.; Cao, K. Effects of Shading by Bagging on Carotenoid Accumulation in Peach Fruit Flesh. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- De Wit, M.; Galvão, V.C.; Fankhauser, C. Light-Mediated Hormonal Regulation of Plant Growth and Development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, Y.; Yanagisawa, S. Light signalling-induced regulation of nutrient acquisition and utilisation in plants. Semin. Cell Dev. Biol. 2018, 83, 123–132. [Google Scholar] [CrossRef]
- Sanchez, S.E.; Rugnone, M.L.; Kay, S.A. Light Perception: A Matter of Time. Mol. Plant 2020, 13, 363–385. [Google Scholar] [CrossRef]
- Teixeira, R.T. Distinct Responses to Light in Plants. Plants 2020, 9, 894. [Google Scholar] [CrossRef]
- Bakhshi, D.; Arakawa, O. Induction of phenolic compounds biosynthesis with light irradiation in the flesh of red and yellow apples. J. Appl. Hortic. 2006, 8, 101–104. [Google Scholar] [CrossRef]
- Awad, M.A.; Wagenmakers, P.S.; de Jager, A. Effects of light on flavonoid and chlorogenic acid levels in the skin of ‘Jonagold’ apples. Sci. Hortic. 2001, 88, 289–298. [Google Scholar] [CrossRef]
- Kolb, C.A.; Käser, M.A.; Kopecký, J.; Zotz, G.; Riederer, M.; Pfündel, E.E. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol. 2001, 127, 863–875. [Google Scholar] [CrossRef]
- Chaves-Silva, S.; Santos, A.L.D.; Chalfun-Júnior, A.; Zhao, J.; Peres, L.E.P.; Benedito, V.A. Understanding the genetic regulation of anthocyanin biosynthesis in plants–Tools for breeding purple varieties of fruits and vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, W.; Wang, K.; Zhang, B.; Allan, A.C.; Lin-Wang, K.; Chen, K.; Xu, C. Differential Sensitivity of Fruit Pigmentation to Ultraviolet Light between Two Peach Cultivars. Front. Plant Sci. 2017, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.S. Nature’s Swiss army knife: The diverse protective roles of anthocyanins in leaves. J. Biomed. Biotechnol. 2004, 5, 314–320. [Google Scholar] [CrossRef]
- Xie, D.; Sharma, S.; Paiva, N.; Ferreira, D.; Dixon, R. Role of Anthocyanidin Reductase, Encoded by BANYULS in Plant Flavonoid Biosynthesis. Science 2003, 299, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Maatta, K.; Pirttila, A.M.; Torronen, R.; Karenlampi, S.; Hohtola, A. Expression of Genes Involved in Anthocyanin Biosynthesis in Relation to Anthocyanin, Proanthocyanidin, and Flavonol Levels during Bilberry Fruit Development. Plant Physiol. 2002, 130, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Hohtola, A.; Jaakola, L.; Maatta-Riihinen, K.; Karenlampi, S. Activation of flavonoid biosynthesis by solar radiation in bilberry (Vaccinium myrtillus L.) leaves. Planta 2004, 218, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Gravano, E.; Pinelli, P.; Mulinacci, N.; Romani, A. Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytol. 2000, 148, 69–77. [Google Scholar] [CrossRef]
- Ravaglia, D.; Espley, R.V.; Henry-Kirk, R.A.; Andreotti, C.; Ziosi, V.; Hellens, R.P.; Costa, G.; Allan, A.C. Transcriptional regulation of flavonoid biosynthesis in nectarine (Prunus persica) by a set of R2R3 MYB transcription factors. BMC Plant Biol. 2013, 13, 68. [Google Scholar] [CrossRef]
- Ma, C.; Liang, B.; Chang, B.; Yan, J.; Liu, L.; Wang, Y.; Yang, Y.; Zhao, Z. Transcriptome profiling of anthocyanin biosynthesis in the peel of ‘Granny Smith’ apples (Malus domestica) after bag removal. BMC Genom. 2019, 20, 353. [Google Scholar] [CrossRef]
- Volz, R.K.; Oraguzie, N.C.; Whitworth, C.J.; How, N.; Chagné, D.; Carlisle, C.M.; Gardiner, S.E.; Rikkerink, E.H.A.; Lawrence, T. Breeding for Red Flesh Colour in Apple: Progress and Challenges. Acta Hortic. 2009, 814, 337–342. [Google Scholar] [CrossRef]
- Zhou, H.; Lin-Wang, K.; Wang, H.; Gu, C.; Dare, A.P.; Espley, R.V.; He, H.; Allan, A.C.; Han, Y. Molecular genetics of blood-fleshed peach reveals activation of anthocyanin biosynthesis by NAC transcription factors. Plant J. 2015, 82, 105–121. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Liu, Y.; Shi, X.; Wang, Y.; Zhang, C.; Zhao, Z. The effect of fruit bagging on the color, phenolic compounds and expression of the anthocyanin biosynthetic and regulatory genes on the ‘Granny Smith’ apples. Eur. Food Res. Technol. 2013, 237, 875–885. [Google Scholar] [CrossRef]
- Ma, C.; Jing, C.; Chang, B.; Yan, J.; Liang, B.; Liu, L.; Yang, Y.; Zhao, Z. The effect of promoter methylation on MdMYB1 expression determines the level of anthocyanin accumulation in skins of two non-red apple cultivars. BMC Plant Biol. 2018, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Khanal, B.P.; Mekonnen, S.B.; Knoche, M. Shading affects fracture force and fracture strain of apple fruit skins. Sci. Hortic. 2020, 274, 109651. [Google Scholar] [CrossRef]
- Yuri, J.A.; Neira, A.; Fuentes, M.; Razmilic, I.; Lepe, V.; González, M.F. Bagging cv. Fuji, Raku Raku Apple Fruit Affects Their Phenolic Profile and Antioxidant Capacity. Erwerbs Obstbau 2020, 62, 221–229. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, D.; Yue, X.; Wang, S.; Li, X.; Teng, Y. Analysis of different pigmentation patterns in ‘Mantianhong’ (Pyrus pyrifolia Nakai) and ‘Cascade’ (Pyrus communis L.) under bagging treatment and postharvest UV-B/visible irradiation conditions. Sci. Hortic. 2013, 151, 75–82. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kang, S.-S.; Choi, J.-J.; Park, K.-S.; Won, K.-H.; Lee, H.-C.; Han, T.-H. The effect of several paper bags on fruit skin coloration of red skin European pear ‘Kalle’. Korean J. Hortic. Sci. Technol. 2014, 32, 10–17. [Google Scholar] [CrossRef][Green Version]
- Qian, M.; Ni, J.; Niu, Q.; Bai, S.; Bao, L.; Li, J.; Sun, Y.; Zhang, D.; Teng, Y. Response of miR156-SPL module during the red peel coloration of bagging-treated Chinese sand pear (Pyrus pyrifolia Nakai). Front. Physiol. 2017, 8, 550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-F.; Su, J.; Yao, G.-F.; Liu, H.-N.; Gu, C.; Qin, M.-F.; Bai, B.; Cai, S.-S.; Wang, G.-M.; Wang, R.-Z.; et al. Different light-response patterns of coloration and related gene expression in red pears (Pyrus L.). Sci. Hortic. 2018, 229, 240–251. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, C.; Zhang, X.; Wang, C.; Yang, S. Preharvest bagging and postharvest calcium treatment affects superficial scald incidence and calcium nutrition during storage of ‘Chili’ pear (Pyrus bretschneideri) fruit. Postharvest Biol. Technol. 2020, 163, 111149. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Wang, S.; Li, W.; Liu, D.; Guo, X.; Qu, B. Transcriptomic analysis of bagging-treated ‘Pingguo’ pear shows that MYB4-like1, MYB4-like2, MYB1R1 and WDR involved in anthocyanin biosynthesis are up-regulated in fruit peels in response to light. Sci. Hortic. 2019, 244, 428–434. [Google Scholar] [CrossRef]
- Liu, T.; Song, S.; Yuan, Y.; Wu, D.; Chen, M.; Sun, Q.; Zhang, B.; Xu, C.; Chen, K. Improved peach peel color development by fruit bagging. Enhanced expression of anthocyanin biosynthetic and regulatory genes using white non-woven polypropylene as replacement for yellow paper. Sci. Hortic. 2015, 184, 142–148. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, Z.; Ye, Z. Effect of bagging duration on peach fruit peel color and key protein changes based on iTRAQ quantitation. Sci. Hortic. 2019, 246, 217–226. [Google Scholar] [CrossRef]
- Sun, R.-Z.; Cheng, G.; Li, Q.; Zhu, Y.-R.; Zhang, X.; Wang, Y.; He, Y.-N.; Li, S.-Y.; He, L.; Chen, W.; et al. Comparative physiological, metabolomic, and transcriptomic analyses reveal developmental stage-dependent effects of cluster bagging on phenolic metabolism in Cabernet Sauvignon grape berries. BMC Plant Biol. 2019, 19, 583. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.J.; He, Y.; Jiang, A.L. Bagging Affecting Sugar and Anthocyanin Metabolism in the Ripening Period of Grape Berries. Not. Bot. Hortic. Agrobot. 2019, 47, 1194–1205. [Google Scholar] [CrossRef]
- Pisciotta, A.; Planeta, D.; Giacosa, S.; Paissoni, M.A.; Di Lorenzo, R.; Rolle, L. Quality of Grapes Grown Inside Paper Bags in Mediterranean Area. Agronomy 2020, 10, 792. [Google Scholar] [CrossRef]
- Kiran, A.S.; Kavitha, C.; Soorianathasundaram, K.; Sritharan, N. Impact of Fruit Bagging with Different Coloured Non-woven Polypropylene Bags on Yield Attributes in Grapes. Asian J. Dairy Food Res. 2020, 39, 359–362. [Google Scholar] [CrossRef]
- Ji, X.-H.; Wang, B.-L.; Wang, X.-D.; Shi, X.-B.; Liu, P.-P.; Liu, F.-Z.; Wang, H.-B. Effects of different color paper bags on aroma development of Kyoho grape berries. J. Integr. Agric. 2019, 18, 70–82. [Google Scholar] [CrossRef]
- Guo, S.-H.; Xu, T.-F.; Shi, T.-C.; Jin, X.-Q.; Feng, M.-X.; Zhao, X.-H.; Zhang, Z.-W.; Meng, J.-F. Cluster bagging promotes melatonin biosynthesis in the berry skins of Vitis vinifera cv. Cabernet Sauvignon and Carignan during development and ripening. Food Chem. 2020, 305, 125502. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Feng, J.; Liu, Y.; Zhang, S.; Zhao, G. The R2R3-MYB transcription factor PaMYB10 is involved in anthocyanin biosynthesis in apricots and determines red blushed skin. BMC Plant Biol. 2019, 19, 287. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, X.; Cui, W.; Lin, M.; Qiao, C.; Zhong, Y.; Fang, J.; Hu, C. Restraint of Bagging on Fruit Skin Coloration in on-Tree Kiwifruit (Actinidia arguta). J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- He, Y.; Chen, H.; Zhou, L.; Liu, Y.; Chen, H. Comparative transcription analysis of photosensitive and non-photosensitive eggplants to identify genes involved in dark regulated anthocyanin synthesis. BMC Genom. 2019, 20, 678. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Znang, F.; Shi, Y. Ecological effects of bagging on actinidia fruits. Chin. J. Appl. Ecol. 2003, 14, 1829–1832. [Google Scholar]
- Zhang, J.-G.; Wang, H.-Y.; Wang, M.; Sun, J.-S.; Liu, Y.-F.; Schrader, L. Effect of bagging on microenvironments of apple fruits. Acta Ecol. Sin. 2005, 25, 1082–1087. [Google Scholar]
- Cheng, Z.-H.; Zhao, Y.; Meng, H.-W.; Guan, Z.-H. Effects of fruit bagging with different types of bags on growth and quality of cucumber fruit. Acta Ecol. Sin. 2007, 27, 732–739. [Google Scholar]
- Morandi, B.; Manfrini, L.; Losciale, P.; Zibordi, M.; Corelli-Grappadelli, L. The positive effect of skin transpiration in peach fruit growth. J. Plant Physiol. 2010, 167, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Liang, X.; Wang, Y.; Gleason, M.L.; Zhang, R.; Sun, G.Y. High humidity and age-dependent fruit susceptibility promote development of Trichothecium black spot on apple. Plant Dis. 2019, 103, 259–267. [Google Scholar] [CrossRef]
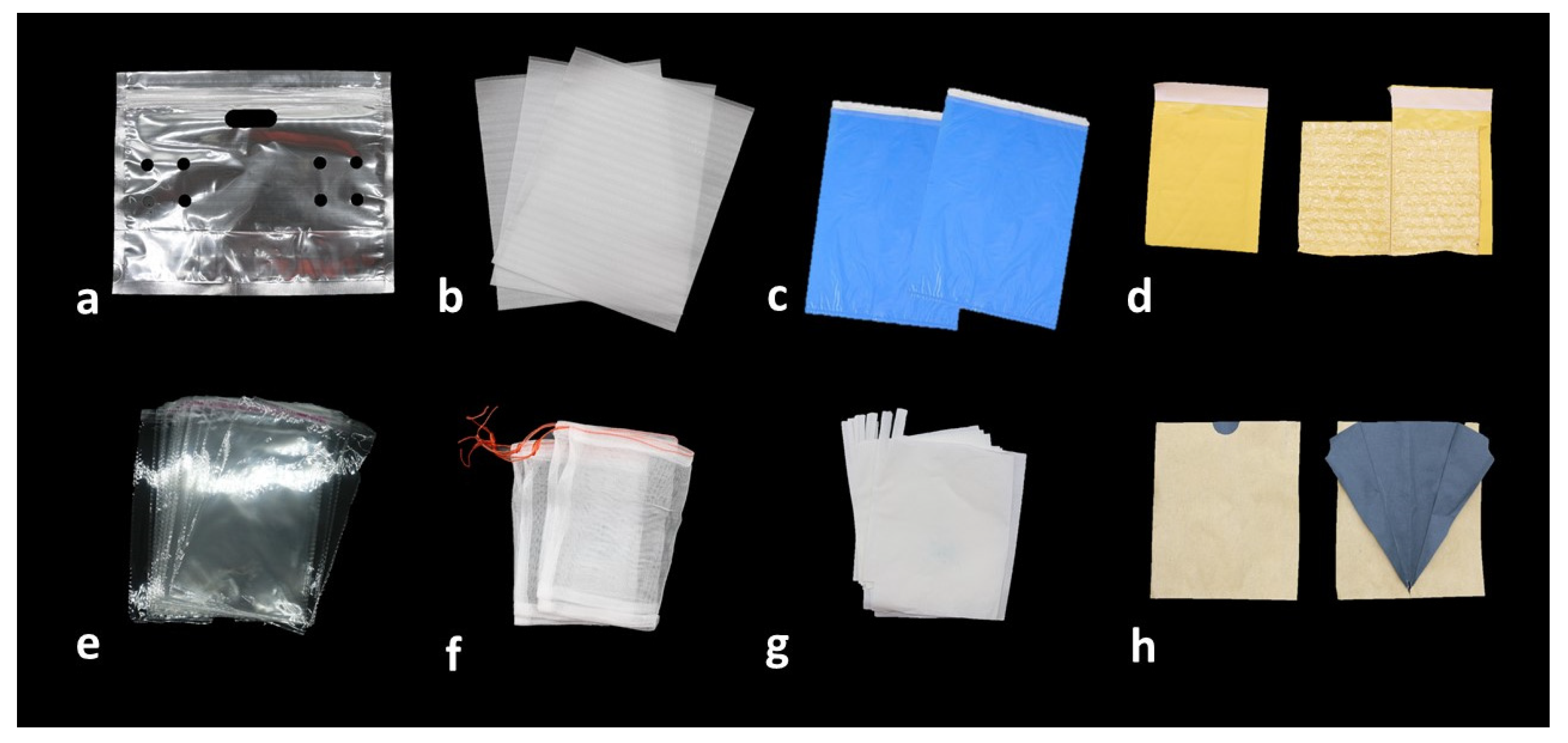
| Crop/Cultivar | Bagging Start | Bagging Material | Effect | Ref. |
|---|---|---|---|---|
| Mango “Langra” and “Khirshapat” | 30 d before harvest | black polybag, transparent polybag, brown paper | higher total soluble sugars and better physical quality of fruit | [21] |
| Mango “Nam Dok Mai #4” | 48 d after full bloom | two-layered paper (brown outside and black inside) | improvement in fruit weight and skin appearance | [22] |
| Mango “Harumanis” | 56 d before harvest | brown and black paper | improvement in skin color | [23] |
| Mango “Nam Dok Mai #4” | 45 d after full bloom | low-density polyethylene | improvement in fruit weight and skin glossiness | [24] |
| Mango “Apple” | 40–50 d before harvest | standard Kraft paper | reduction in lenticel discoloration | [25] |
| Mango “Khirsapat” | 42 d before harvest | brown paper | reduced significantly post-harvest losses | [26] |
| Carambola “Malaysia” | 10–31 d after full bloom | plastic, newspaper, and non-woven cloth | increase in fruit size, fruit weight and soluble solid content | [27] |
| Guava “Allahabad Safeda” | 30 days after pollination | nylon nets, non-woven polypropylene, butter paper and brown paper | advanced fruit maturity, improved fruit weight, texture, visual appeal, quality and functional attributes | [14] |
| Guava “Tai-Kuo” | for 146 and 175 d during fruit development | waxed paper, nylon, Taiwan bag, telephone book paper | protection against pests and mechanical damage | [28] |
| Litchi “Feizixiao” | 15 and 30 d after full bloom | cellophane or fabric | better fruit coloration/appearance | [29] |
| Litchi “Rose Scented” | 14 d before harvest | perforated transparent polyethylene | reduction in fruit drop. increase in fruit size, higher soluble solids content | [30] |
| Loquat “Baiyu” and “Ninghaibai” | after fruit thinning (early April) | white paper (50% light transmittance) and two–layered paper (out grey, inside black—0% light transmittance) | improvement in appearance decrease in fruit weight | [31] |
| Loquat “Qingzhong” | after fruit thinning | paper | promotion in appearance, increased sucrose, glucose and soluble solids content, decreased fructose, sorbitol and titratable acidity content | [32] |
| Longan “Chuliang” | 34 d after full bloom | perforated plastic, white or black adhesive-bonded | increased fruit size and fruit retention rate, reduced fruit cracking incidence | [33] |
| Persimmon “Shinsyu” | 35–50 d before harvest | paper | no black stain | [34] |
| Persimmon “Fuyu” | 1–4 months before harvest | white paper (40% shade) | reduced fruit blemishing (increase of blemishing with early removal) | [35] |
| Yuzu (Citrus junos) | early September | recycled Japanese phone book paper, grey colored paper and black polyester | significant reduction in fruit spot injury | [36] |
| Date Palm “Zaghloul” | at pollination time | transparent and blue polyethylene | reduction in tip cracked fruit | [37] |
| Date Palm “Succary” and “Khalas” | 28 d after pollination | black, white blue, yellow plastic | acceleration fruit ripening | [38] |
| Date Palm “Helali” | 30 d after pollination | black and blue polyethylene, paper | increased rate of fruit ripening | [39] |
| Apple Cultivar | Bagging Start | Bagging Material | Effect | Ref. |
|---|---|---|---|---|
| “Granny Smith” | 40 d after full bloom (removed at 160 d after full bloom) | two-layer paper (outer brown, inner red) | increase in anthocyanin content after bag removal, increased expression of genes involved in light signal perception and transduction | [81] |
| “Qinguan” (deep-red cultivar), “Cripps Pink” (pale-red cultivar), and “Golden Delicious” (non-red cultivar) | 45 d after full bloom | double layer paper (outer yellow, inner red paper coated with wax) | reduced anthocyanin accumulation in red cultivars, reduced sugar and organic acid contents | [45] |
| “Granny Smith” | 114–118 d before harvest | brown paper | improvement of sweetness, sunburn reduction, 30 to 40% additional yield | [47] |
| “Delicious” | 30 d before harvest | light yellow fabric | improvement in fruit color, firmness, and reduction in postharvest disorders | [49] |
| “Red Fuji” | 40 d after full bloom | paper | better absorption of calcium in fruit | [52] |
| “Gamhong” | 28–35 d after full bloom | Ca-coated paper | reduction in bitter pit | [53] |
| “Fuji Suprema” | 40 d after full bloom | transparent micro-holed plastic and non-textured fabric | lower incidence of bitter pit, higher incidence of russeting, improvement in Ca content | [54] |
| “Imperial Gala” | 40 d after full bloom | transparent micro-perforated plastic or non-textured fabric bags | reduction in bitter pit incidence | [55] |
| “Golden Delicious” | 113 d before harvesting | two double layer paper: (a) outside grey–inside yellow; (b) outside newspaper–inside yellow | improved fruit skin, slightly decrease in size and weight | [56] |
| “Kurenainoyume” | 39–54 days after full bloom (removed 29–48 d before harvesting) | light impermeable double-layered paper | incidence of cork spot in non-bagged fruits, no decrease in flesh firmness during storage | [57] |
| “Golden Delicious” and “Granny Smith” | 40 d after full bloom (removed at 120 d or 160 d after full bloom) | two-layer paper (outer brown, inner red) | red/pink pigmentation after bag removal, more intense in "Granny Smith” | [85] |
| “Idared” | 40 d after full bloom | 1–3 layers of black hail net | small increase in mechanical properties Increase in russet susceptibility | [86] |
| “Fuji Raku Raku” | 60–75 d after full bloom | double layer paper (outer grey, inner red) | lower internal browning with more rotting, lower phenolic content | [87] |
| Pear Cultivar/Species | Bagging Start | Bagging Material | Effect | Ref. |
|---|---|---|---|---|
| “Meirensu” and “Yunhongli No. 1" (P. pyrifolia) | 20 d after full bloom (removed 1–3 weeks before harvest) | single- or two-layer paper with different levels of light reduction | improvement of anthocyanins accumulation removing bags 2–3 weeks before harvest | [48] |
| “Housui” (P. pyrifolia) | 34 d and/or 83 d after full bloom | several colored paper combinations or transparent paraffin | improved fruit appearance (uniform, shine and smooth skin color with small lenticels) | [58] |
| “Carmen” (P. communis) | 66 d before harvest (removed 13 d before harvesting) | paper bags: (1) white; (2) yellow; (3) black; (4) outside grey–inside yellow; (5) outside newspaper–inside yellow | red over-color formation removing bags before harvest, fruits were slightly smaller, improved quality of the skin | [59] |
| “Conference” (P. communis) | 30 d after full bloom | micro-perforated polyethylene | reduction in skin blemish and russet | [60] |
| “Cuiguan” (P. pyrifolia) | 20 d (changing the bag at day 45) or 35 d after full bloom | paper | fruits bagged earlier were brighter, with less russet, fewer dots and stone cells | [61] |
| “Cuiguan” (P. pyrifolia) | 20 d after full bloom | double-layer paper (yellow outside, red inside) | ascorbate decline | [64] |
| “Mantianhong” (P. pyrifolia) and “Cascade” (P. communis) | 20 d after full bloom (removed 10 d before harvest) | double layers of yellow–black paper | red skin coloration in response to light/UV irradiation | [88] |
| “Kalle” (P. communis) | 20 d after full bloom | white, yellow and double layered black paper | reduced skin color intensity, best performance with white bags | [89] |
| “Meirensu” (P. pyrifolia) | 40 d after full bloom (removed 10 d before harvest) | double-layered yellow–black paper | anthocyanin accumulation and expression of miR156 and its target PpSPL genes, | [90] |
| 27 different cultivars (P. pyrifolia, P. communis, P. bretschneideri, P. ussuriensis) | 40 d after full bloom, harvest 10 d before commercial maturity, then treatment with artificial light | double-layered paper (outer layer yellow outside and black inside, inner layer red) | increasing levels of anthocyanin under artificial light conditions. | [91] |
| “Chili” (P. bretschneideri) | 77 d after full bloom | polyethylene and non-woven fabric | prevention of scald with non-woven fabric, higher scald with polyethylene | [92] |
| “Pingguo” (P. bretschneideri) | 40 d after full bloom (removed 9 or 2 d before or at harvesting time) | paper | anthocyanin increase and up-regulation of MYB genes at day 9 after bag removal | [93] |
| Crop/Cultivar | Bagging Start | Bagging Material | Effect | Ref. |
|---|---|---|---|---|
| Peach “Hujingmilu” and “Yulu” | 42 days after full bloom | yellow paper | UV-light induction of anthocyanin biosynthesis | [74] |
| Peach “Janghowon Hwangdo” | after final thinning (early June) | coated white paper, coated yellow paper, white paper, yellow paper and newspaper | improvement in the appearance and in the accumulation of anthocyanins | [10] |
| Peach “Hakuho” | before pit hardening, and 15 days before harvest | orange paper or orange triple and single parchment paper, 15%, 50%, 80% transmittance | decrease of the color intensity proportionally to the light reduction. Increase in aroma volatile content. | [62] |
| Peach “3D-8” and “C18” | 50 d after full bloom, harvest at 70, 80 and 90 d after full bloom | double-layer paper (yellow outside and black inside) | reduced content in total carotenoids, low quality | [65] |
| Peach “Hujingmilu” and “Yulu” | 96–100 days after full bloom, harvest at commercial maturity or 106-139 days after full bloom | yellow paper, and black, white, blue and grey nonwoven polypropylene bags | non-woven polypropylene bags determined the highest anthocyanin content in peel. | [94] |
| Peach “Hujingmilu” | 50 days after flowering, bags removed at 90 or 105 days | paper single-layer, yellow | a short bagging period improves and stabilizes peel anthocyanin content reducing peel brightness and chlorophyll | [95] |
| Grape “Cabernet Sauvignon” | 3 weeks after full bloom (with different timing) to harvest | two-layer paper (yellow outside, black coated with wax inside), with a bent straw | limited effects on berry quality positive correlation of phenolics to different light regimes | [96] |
| Grape “Shenhua” and “Shenfeng” | 45 days after full bloom | white (light 25%) or shading light bags (light 0%) | incomplete color development, lower content of soluble sugar | [97] |
| Grape “Italia”, “Autumn Royal”, and “Regal Seedless” | berries at pea size (bagged at least 90 days) | paper | increased yield for the three cultivars and increased berry hardness for “Autumn Royal”, and “Regal Seedless” | [98] |
| Grape “Muscat Hamburg” | after fruit set | non-woven UV stabilized polypropylene of different colors | improved yield (both in summer and winter season) | [99] |
| Grape “Kyoho” (V. vinifera × V. labrusca) | 5 weeks after full bloom | white, green, blue and red paper | promotion of accumulation of esters, inhibition of synthesis of aldehydes, alcohols, terpenes, ketones and acids | [100] |
| Grape “Cabernet Sauvignon” and “Carignan” | from fruit set | fruit bags with a black double-layer inside | promotion of melatonin biosynthesis in berry skins, delayed fruit coloring and ripening | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.M.; Anwar, R.; Yousef, A.F.; Li, B.; Luvisi, A.; De Bellis, L.; Aprile, A.; Chen, F. Influence of Bagging on the Development and Quality of Fruits. Plants 2021, 10, 358. https://doi.org/10.3390/plants10020358
Ali MM, Anwar R, Yousef AF, Li B, Luvisi A, De Bellis L, Aprile A, Chen F. Influence of Bagging on the Development and Quality of Fruits. Plants. 2021; 10(2):358. https://doi.org/10.3390/plants10020358
Chicago/Turabian StyleAli, Muhammad Moaaz, Raheel Anwar, Ahmed F. Yousef, Binqi Li, Andrea Luvisi, Luigi De Bellis, Alessio Aprile, and Faxing Chen. 2021. "Influence of Bagging on the Development and Quality of Fruits" Plants 10, no. 2: 358. https://doi.org/10.3390/plants10020358
APA StyleAli, M. M., Anwar, R., Yousef, A. F., Li, B., Luvisi, A., De Bellis, L., Aprile, A., & Chen, F. (2021). Influence of Bagging on the Development and Quality of Fruits. Plants, 10(2), 358. https://doi.org/10.3390/plants10020358