Exogenous Glycine Betaine Application Improves Freezing Tolerance of Cabbage (Brassica oleracea L.) Leaves
Abstract
1. Introduction
2. Results
2.1. Effect of Exogenous GB on Leaf-Growth
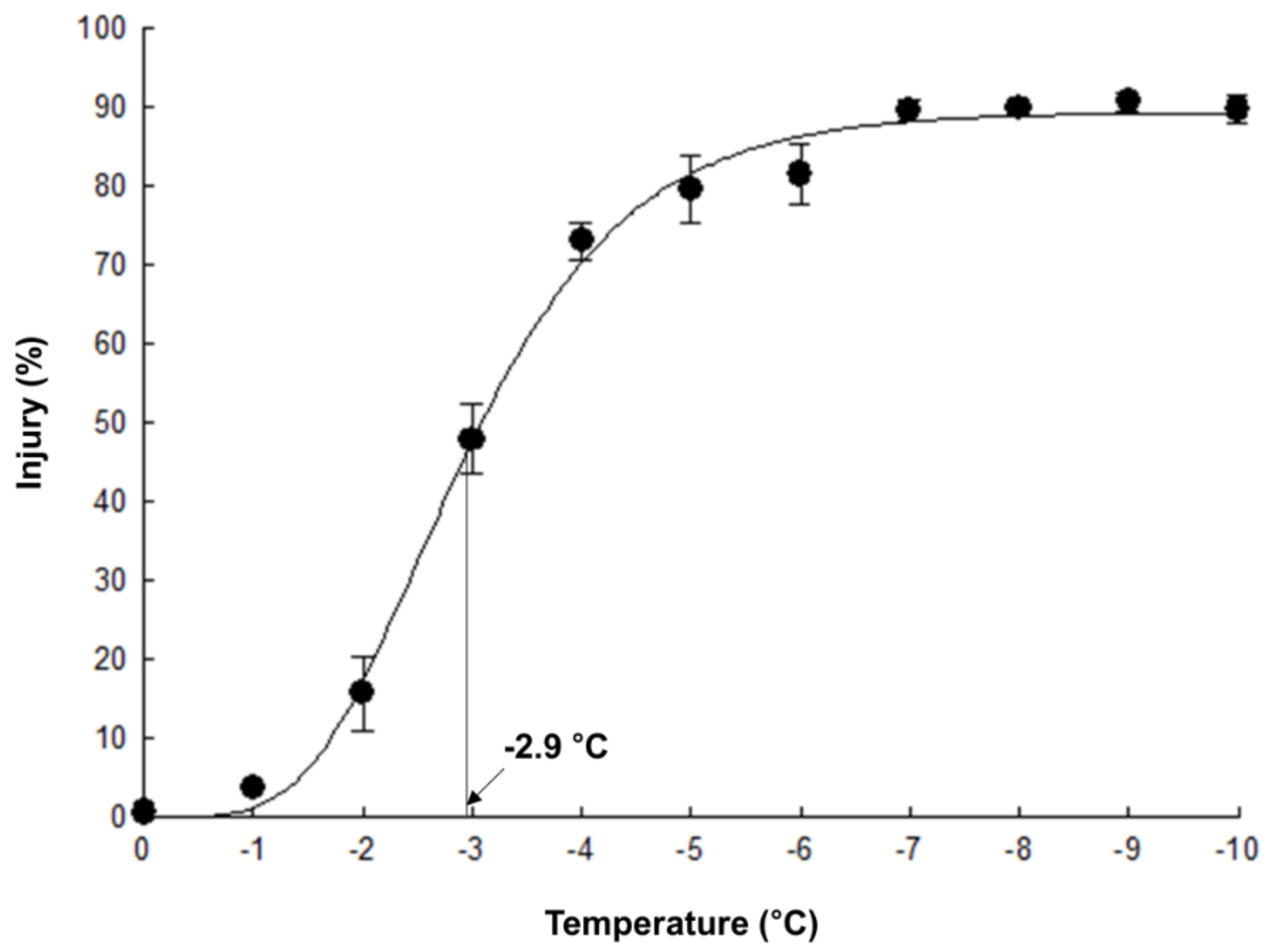
2.2. LT50 of Cabbage Leaves
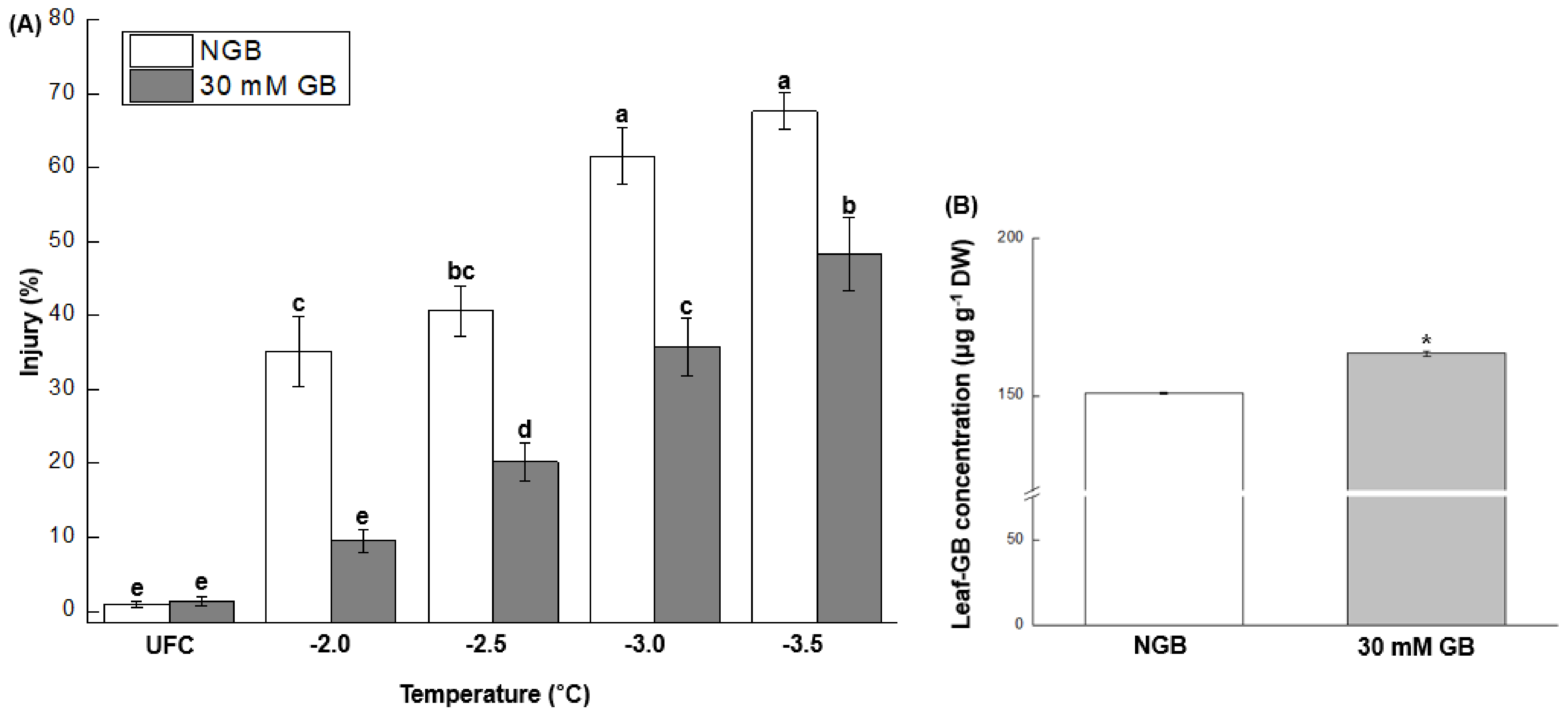
2.3. Effect of Exogenous GB on FT and GB Concentration
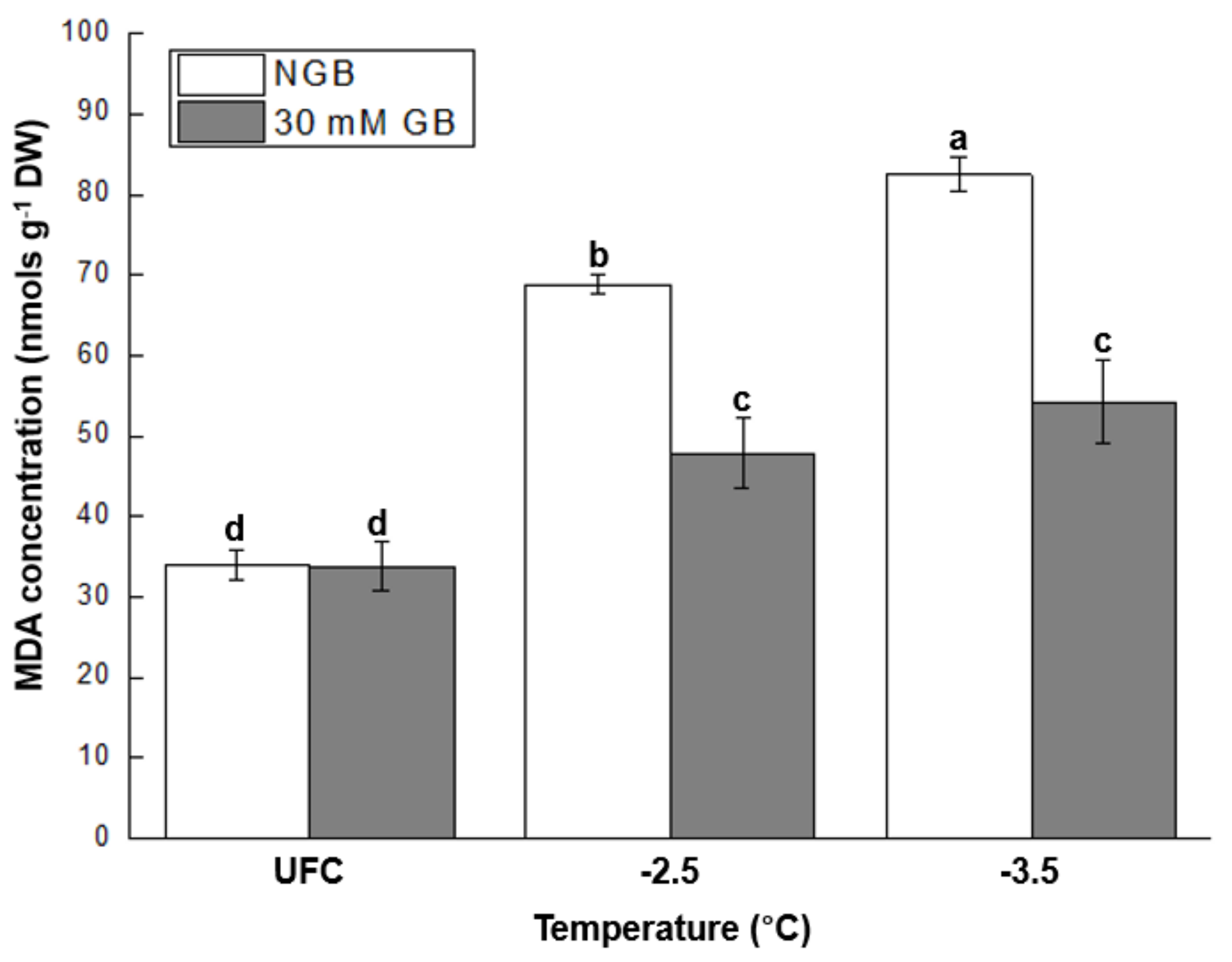
2.4. MDA Concentration
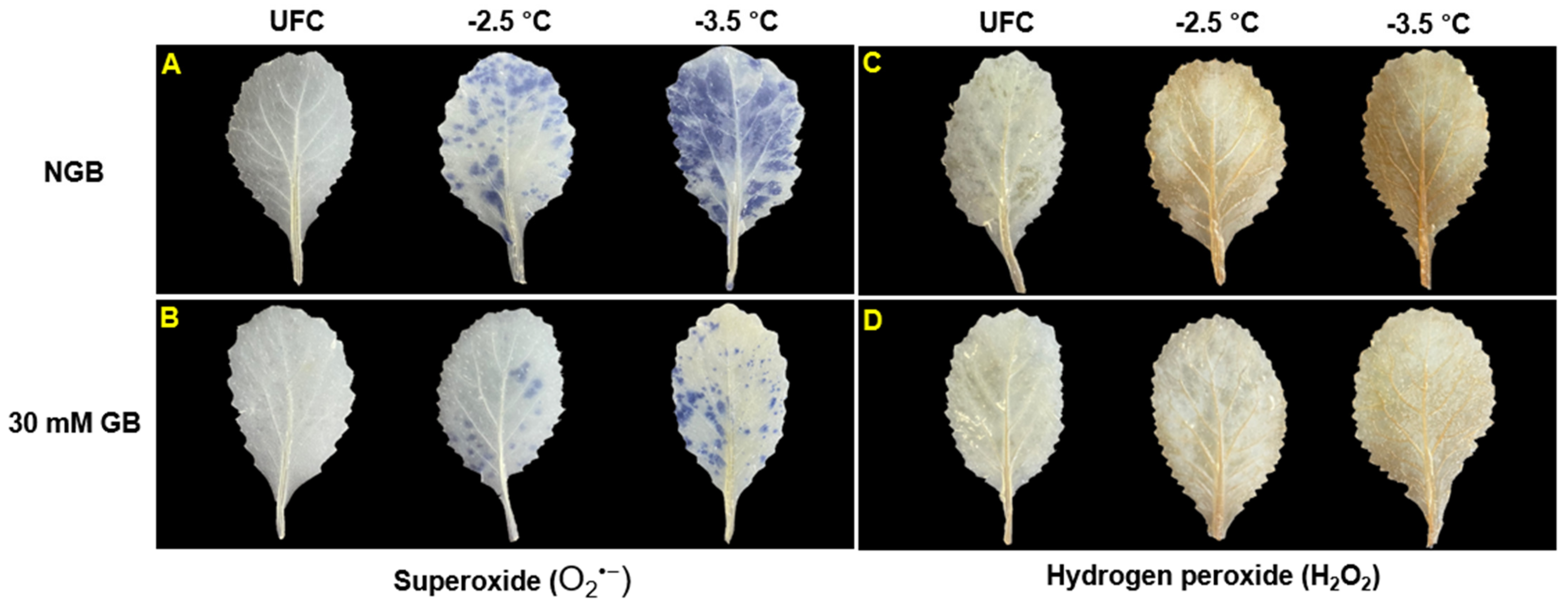
2.5. ROS (O2•− and H2O2) Staining
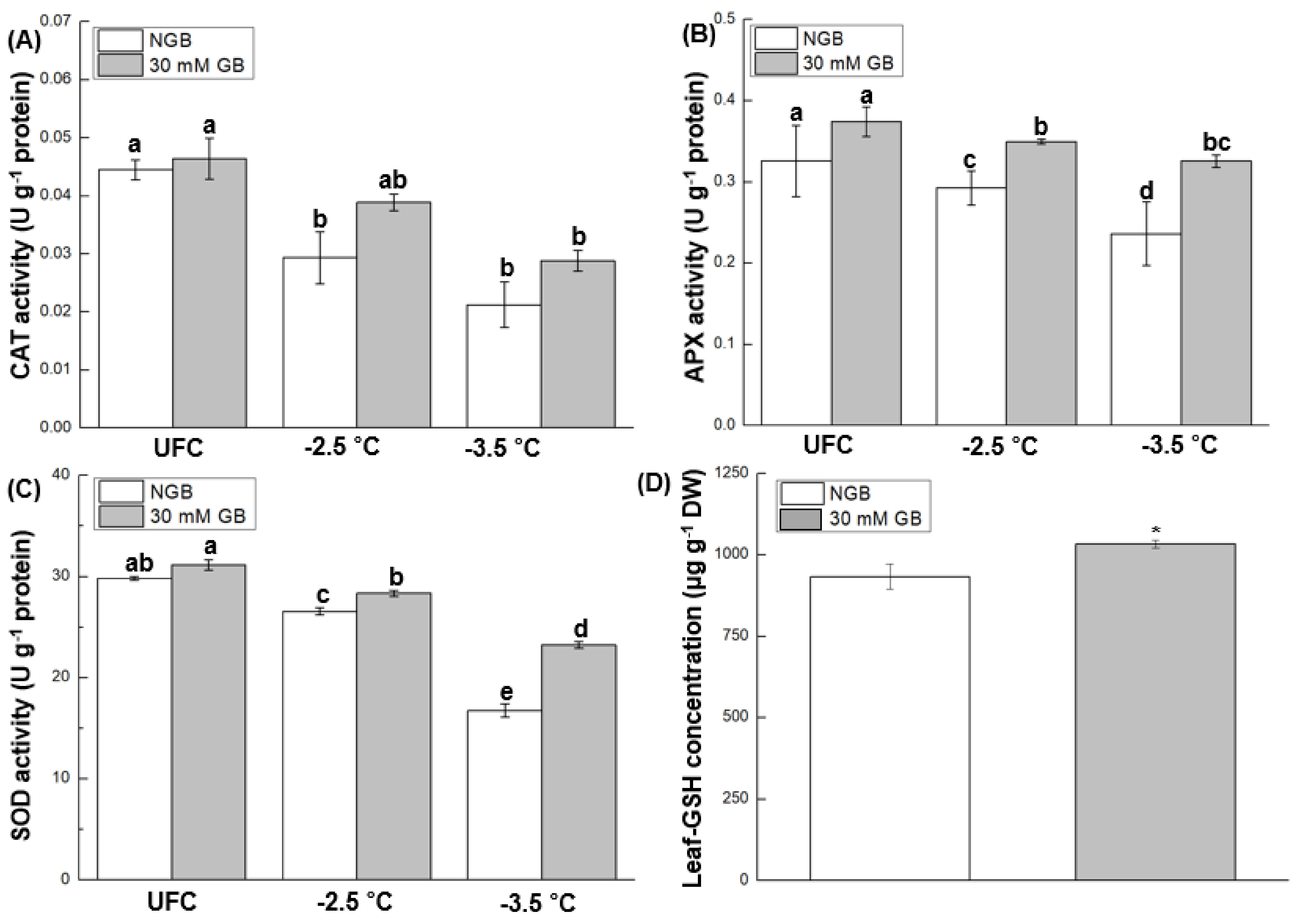
2.6. Antioxidant Enzyme Activities and Leaf-GSH Concentration
2.7. Proline Concentration
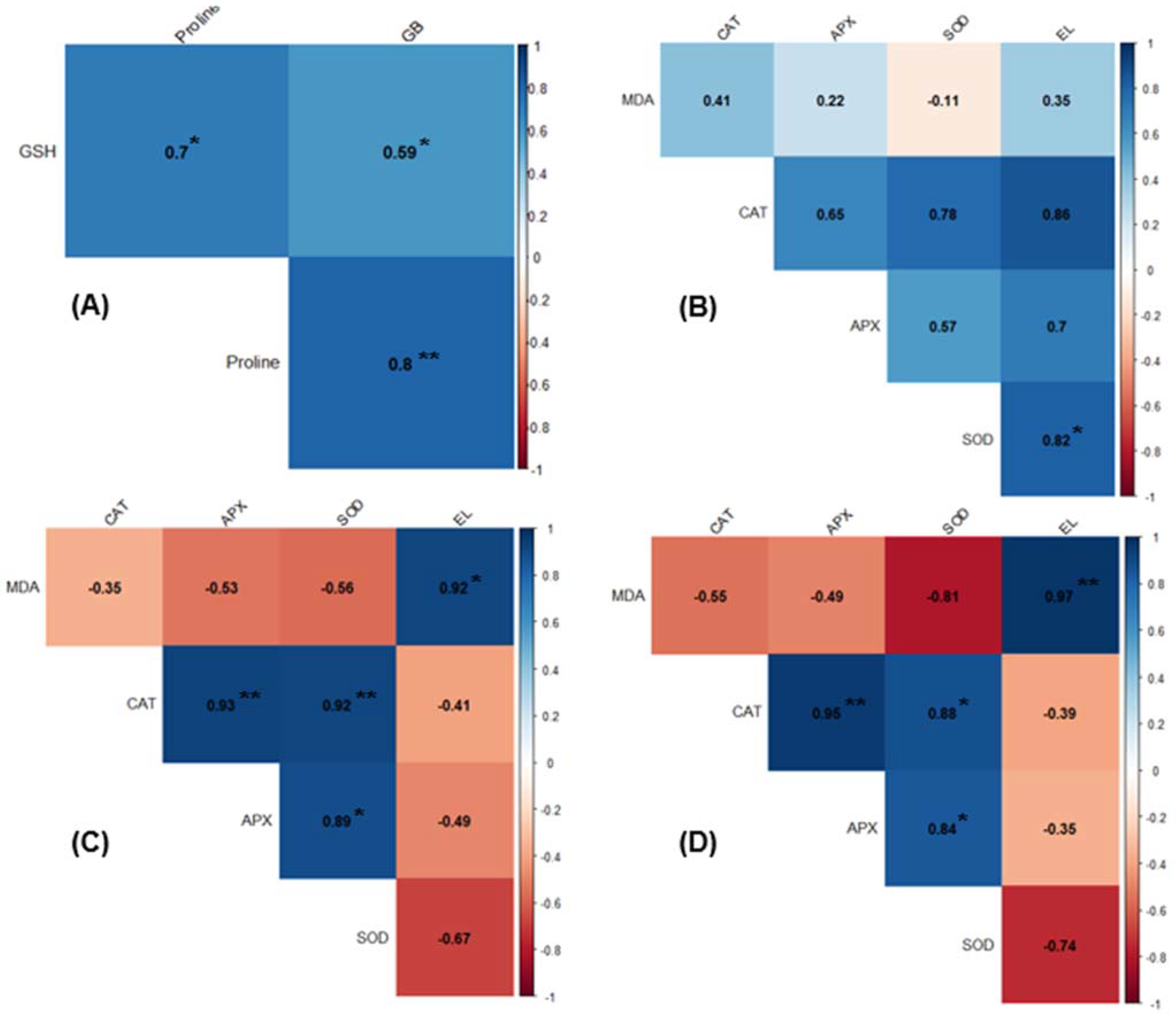
2.8. Correlation Analysis of Physiological and Biochemical Traits
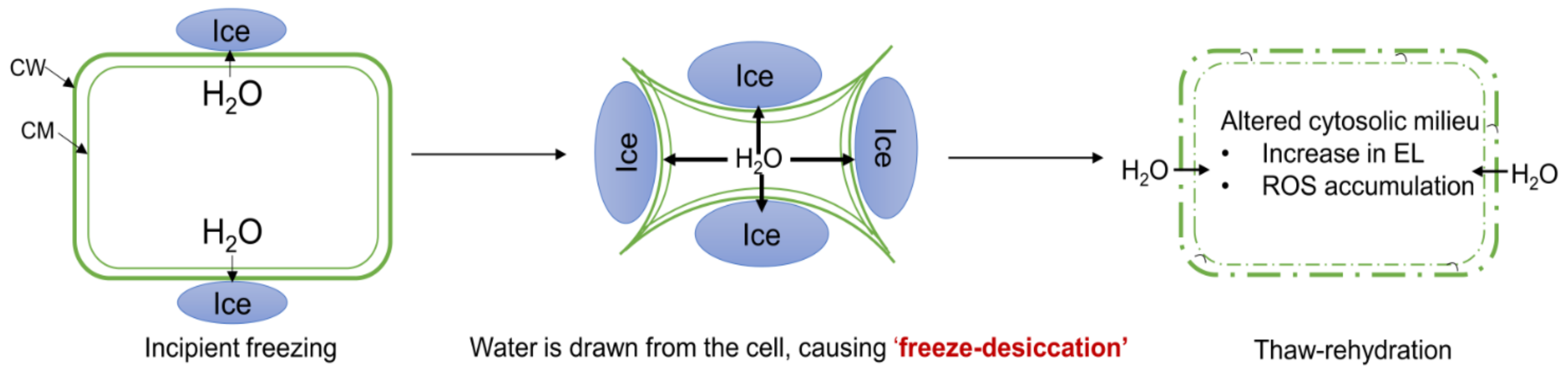
3. Discussion
3.1. GB Application and Leaf-Growth
3.2. GB Application Improves FT by Mitigating Membrane Damage
3.3. GB Application Enhances Antioxidant System
3.4. GB Application Accumulates Proline
4. Materials and Methods
4.1. Plant Material
4.2. Leaf Growth Measurement
4.3. Freezing Tolerance (FT) Determination
4.4. Determination of GB Concentration
4.5. Determination of Malondialdehyde (MDA) Concentration
4.6. ROS Staining
4.7. Measurement of Antioxidant Enzyme Activities
4.8. Determination of Glutathione (GSH) Concentration
4.9. Determination of Proline Concentration
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arora, R. Mechanism of freeze-thaw injury and recovery: A cool retrospective and warming up to new ideas. Plant Sci. 2018, 270, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Warmund, M.R.; Guinan, P.; Fernandez, G. Temperatures and cold damage to small fruit crops across the eastern United States associated with the April 2007 freeze. HortScience 2008, 43, 1643–1647. [Google Scholar] [CrossRef]
- Zohner, C.M.; Mo, L.; Renner, S.S.; Svenning, J.C.; Vitasse, Y.; Benito, B.M.; Ordonez, A.; Baumgarten, F.; Bastin, J.F.; Sebald, V.; et al. Late-spring frost risk between 1959 and 2017 decreased in North America but increased in Europe and Asia. Proc. Natl. Acad. Sci. USA 2020, 117, 12192–12200. [Google Scholar] [CrossRef] [PubMed]
- Moral, R.; Perez-Murcia, M.D.; Perez-Espinosa, A.; Moreno-Caselles, J.; Paredes, C.; Rufete, B. Salinity, organic content, micronutrients and heavy metals in pig slurries from South-eastern Spain. Waste Manag. 2008, 28, 367–371. [Google Scholar] [CrossRef]
- Gu, L.; Hanson, P.J.; Post, W.M.; Kaiser, D.P.; Yang, B.; Nemani, R.; Pallardy, S.G.; Meyers, T. The 2007 eastern US spring freeze: Increased cold damage in a warming world? Bioscience 2008, 58, 253–262. [Google Scholar] [CrossRef]
- Kendall, E.J.; McKersie, B.D. Free radical and freezing injury to cell membranes of winter wheat. Physiol. Plant. 1989, 76, 86–94. [Google Scholar] [CrossRef]
- Min, K.; Chen, K.; Arora, R. Effect of short-term versus prolonged freezing on freeze-thaw injury and post-thaw recovery in spinach: Importance in laboratory freeze-thaw protocols. Environ. Exp. Bot. 2014, 106, 124–131. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Le, M.Q.; Engelsberger, W.R.; Hincha, D.K. Natural genetic variation in acclimation capacity at sub-zero temperatures after cold acclimation at 4 °C in different Arabidopsis thaliana accessions. Cryobiology 2008, 57, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Molecular basis of plant cold acclimation: Insights gained from studying the CBF cold response pathway. Plant Physiol. 2010, 154, 571–577. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L.; Molecular, P.; Program, C.B.; et al. Exploring the Temperature-Stress Metabolome. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef]
- Xin, Z.; Browse, J. Cold comfort farm: The acclimation of plants to freezing temperatures. Plant Cell Environ. 2000, 23, 893–902. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef]
- Uemura, M.; Tominaga, Y.; Nakagawara, C.; Shigematsu, S.; Minami, A.; Kawamura, Y. Responses of the plasma membrane to low temperatures. Physiol. Plant. 2006, 126, 81–89. [Google Scholar] [CrossRef]
- Hincha, D.K. High concentrations of the compatible solute glycinebetaine destabilize model membranes under stress conditions. Cryobiology 2006, 53, 58–68. [Google Scholar] [CrossRef]
- Papageorgiou, G.C.; Murata, N. The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving Photosystem II complex. Photosynth. Res. 1995, 44, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Maximova, E.; Fuggi, A.; Carillo, P. Durum wheat roots adapt to salinity remodeling the cellular content of nitrogen metabolites and sucrose. Front. Plant Sci. 2017, 7, 2035. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Liang, C.; Wang, G.P.; Luo, Y.; Wang, W. The protection of wheat plasma membrane under cold stress by glycine betaine overproduction. Biol. Plant. 2010, 54, 83–88. [Google Scholar] [CrossRef]
- Basu, S.; Roychoudhury, A.; Saha, P.P.; Sengupta, D.N. Comparative analysis of some biochemical responses of three indica rice varieties during polyethylene glycol-mediated water stress exhibits distinct varietal differences. Acta Physiol. Plant. 2010, 32, 551–563. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Paramithiotis, S.; Shin, H.S. Kimchi and other widely consumed traditional fermented foods of Korea: A review. Front. Microbiol. 2016, 7, 1493. [Google Scholar] [CrossRef]
- Manley, R.C.; Hummel, R.L. Mefluidide does not consistently enhance the freezing tolerance of cabbage. HortScience 1996, 31, 402–404. [Google Scholar] [CrossRef]
- Sasaki, H.; Ichimura, K.; Imada, S.; Oda, M. Loss of freezing tolerance associated with decrease in sugar concentrations by short-term deacclimation in cabbage seedlings. J. Jpn. Soc. Hortic. Sci. 2001, 70, 294–298. [Google Scholar] [CrossRef][Green Version]
- Kishitani, S.; Takanami, T.; Suzuki, M.; Oikawa, M.; Yokoi, S.; Ishitani, M.; Alvarez-Nakase, A.M.; Takabe, T.; Takabe, T. Compatibility of glycinebetaine in rice plants: Evaluation using transgenic rice plants with a gene for peroxisomal betaine aldehyde dehydrogenase from barley. Plant Cell Environ. 2000, 23, 107–114. [Google Scholar] [CrossRef]
- Allard, F.; Houde, M.; Kröl, M.; Ivanov, A.; Huner, N.P.A.; Sarhan, F. Betaine Improves Freezing Tolerance in Wheat. Plant Cell Physiol. 1998, 39, 1194–1202. [Google Scholar] [CrossRef]
- Zhao, Y.; Aspinall, D.; Paleg, L.G. Protection of Membrane Integrity in Medicago sativa L. by Glycinebetaine against the Effects of Freezing. J. Plant Physiol. 1992, 140, 541–543. [Google Scholar] [CrossRef]
- Coughlan, S.J.; Heber, U. The role of glycinebetaine in the protection of spinach thylakoids against freezing stress. Planta 1982, 156, 62–69. [Google Scholar] [CrossRef]
- Rajashekar, C.B.; Zhou, H.; Marcum, K.B.; Prakash, O. Glycine betaine accumulation and induction of cold tolerance in strawberry (Fragaria X ananassa Duch.) plants. Plant Sci. 1999, 148, 175–183. [Google Scholar] [CrossRef]
- Xing, W.; Rajashekar, C.B. Glycine betaine involvement in freezing tolerance and water stress in Arabidopsis thaliana. Environ. Exp. Bot. 2001, 46, 21–28. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Hu, L.; Hu, T.; Zhang, X.; Pang, H.; Fu, J. Exogenous glycine betaine Ameliorates the adverse effect of salt stress on perennial ryegrass. J. Am. Soc. Hortic. Sci. 2012, 137, 38–46. [Google Scholar] [CrossRef]
- Osman, H.S. Enhancing antioxidant–yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Ann. Agric. Sci. 2015, 60, 389–402. [Google Scholar] [CrossRef]
- Osman, H.S.; Salim, B.B.M. Influence of exogenous application of some phytoprotectants on growth, yield and pod quality of snap bean under NaCl salinity. Ann. Agric. Sci. 2016, 61, 1–13. [Google Scholar] [CrossRef][Green Version]
- Teh, C.; Shaharuddin, N.; Ho, C.; Mahmood, M. Exogenous application of glycine betaine alleviates salt induced damages more efficiently than ascorbic acid in in vitro rice shoots. Aust. J. Basic Appl. Sci. 2016, 10, 58–65. [Google Scholar]
- Yang, X.; Lu, C. Photosynthesis is improved by exogenous glycinebetaine in salt-stressed maize plants. Physiol. Plant. 2005, 124, 343–352. [Google Scholar] [CrossRef]
- Arora, R.; Palta, J.P. A Loss in the plasma membrane ATPase activity and its recovery coincides with incipient freeze-thaw injury and postthaw recovery in onion bulb scale tissue. Plant Physiol. 1991, 95, 846–852. [Google Scholar] [CrossRef]
- Ryyppo, A.; Sutinen, S.; Maenpaa, M.; Vapaavuori, E.; Repo, T. Frost damage and recovery of Scots pine seedlings at the end of the growing season. Can. J. For. Res. 1997, 27, 1376–1382. [Google Scholar] [CrossRef]
- Uemura, M.; Yoshida, S. Studies on Freezing Injury in Plant Cells. Plant Physiol. 1986, 80, 187–195. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Bichemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, T.; Wang, M.; Liu, Y.; Brestic, M.; Chen, T.H.H.; Yang, X. Genetic engineering of the biosynthesis of glycine betaine modulates phosphate homeostasis by regulating phosphate acquisition in tomato. Front. Plant Sci. 2019, 9, 1995. [Google Scholar] [CrossRef]
- Nawaz, M.; Wang, Z. Abscisic Acid and Glycine Betaine Mediated Tolerance Mechanisms under Drought Stress and Recovery in Axonopus compressus: A New Insight. Sci. Rep. 2020, 10, 6942. [Google Scholar]
- Baek, K.-H.; Skinner, D.Z. Production of reactive oxygen species by freezing stress and the protective roles of antioxidant enzymes in plants. J. Agric. Chem. Environ. 2012, 1, 34–40. [Google Scholar] [CrossRef]
- Min, K.; Chen, K.; Arora, R. A metabolomics study of ascorbic acid-induced in situ freezing tolerance in spinach (Spinacia oleracea L.). Plant Direct 2020, 4, e00202. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Min, K.; Arora, R. Exogenous salicylic acid improves freezing tolerance of spinach (Spinacia oleracea L.) leaves. Cryobiology 2018, 81, 192–200. [Google Scholar] [CrossRef]
- Asada, K.; Allen, J.; Foyer, C.H.; Matthijs, H.C.P. The water-water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, I.; Cvetkovic, J.; Zuther, E.; Hincha, D.K.; Baier, M. Natural variation of cold deacclimation correlates with variation of cold-acclimation of the plastid antioxidant system in Arabidopsis thaliana accessions. Front. Plant Sci. 2016, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Hoque, M.A.; Okuma, E.; Banu, M.N.A.; Shimoishi, Y.; Nakamura, Y.; Murata, Y. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J. Plant Physiol. 2009, 166, 1587–1597. [Google Scholar] [CrossRef]
- Malekzadeh, P. Influence of exogenous application of glycinebetaine on antioxidative system and growth of salt-stressed soybean seedlings (Glycine max L.). Physiol. Mol. Biol. Plants 2015, 21, 225–232. [Google Scholar] [CrossRef]
- Sofy, M.R.; Elhawat, N.; Alshaal, T. Glycine betaine counters salinity stress by maintaining high K+/Na+ ratio and antioxidant defense via limiting Na+ uptake in common bean (Phaseolus vulgaris L.). Ecotoxicol. Environ. Saf. 2020, 200, 110732. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Fang, P.; Zeng, W.; Ding, Y.; Zhuang, Z.; Peng, Y. Comparing transcriptome expression profiles to reveal the mechanisms of salt tolerance and exogenous glycine betaine mitigation in maize seedlings. PLoS ONE 2020, 15, e0233616. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1455–1466. [Google Scholar] [CrossRef]
- Sun, H.; Luo, M.; Zhou, X.; Zhou, Q.; Sun, Y.; Ge, W.; Wei, B.; Cheng, S.; Ji, S. Exogenous glycine betaine treatment alleviates low temperature-induced pericarp browning of ‘Nanguo’ pears by regulating antioxidant enzymes and proline metabolism. Food Chem. 2020, 306, 125626. [Google Scholar] [CrossRef]
- Yao, W.; Xu, T.; Farooq, S.U.; Jin, P.; Zheng, Y. Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biol. Technol. 2018, 144, 20–28. [Google Scholar] [CrossRef]
- Lim, C.C.; Arora, R.; Townsend, E.C. Comparing Gompertz and Richards functions to estimate freezing injury in Rhododendron using electrolyte leakage. J. Am. Soc. Hortic. Sci. 1998, 123, 246–252. [Google Scholar] [CrossRef]
- Valadez-Bustos, M.G.; Aguado-Santacruz, G.A.; Tiessen-Favier, A.; Robledo-Paz, A.; Muñoz-Orozco, A.; Rascón-Cruz, Q.; Santacruz-Varela, A. A reliable method for spectrophotometric determination of glycine betaine in cell suspension and other systems. Anal. Biochem. 2016, 498, 47–52. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Dynamics of the antioxidant system during seed osmopriming, post-priming germination, and seedling establishment in Spinach (Spinacia oleracea). Plant Sci. 2011, 180, 212–220. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Understanding the cellular mechanism of recovery from freeze-thaw injury in spinach: Possible role of aquaporins, heat shock proteins, dehydrin and antioxidant system. Physiol. Plant. 2014, 150, 374–387. [Google Scholar] [CrossRef]
- Esen, A. A simple method for quantitative, semiquantitative, and qualitative assay of protein. Anal. Biochem. 1978, 89, 264–273. [Google Scholar] [CrossRef]
- Patton, A.J.; Cunningham, S.M.; Volenec, J.J.; Reicher, Z.J. Differences in freeze tolerance of zoysiagrasses: II. Carbohydrate and proline accumulation. Crop Sci. 2007, 47, 2170–2181. [Google Scholar] [CrossRef]

| Growth Parameters | Treatment | |
|---|---|---|
| NGB | 30 mM GB | |
| DW/FW y | 0.06 ± 0.002 | 0.07 ± 0.002 z |
| Leaf area (cm2) y | 8.8 ± 0.6 | 9.2 ±0.7 z |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, K.; Cho, Y.; Kim, E.; Lee, M.; Lee, S.-R. Exogenous Glycine Betaine Application Improves Freezing Tolerance of Cabbage (Brassica oleracea L.) Leaves. Plants 2021, 10, 2821. https://doi.org/10.3390/plants10122821
Min K, Cho Y, Kim E, Lee M, Lee S-R. Exogenous Glycine Betaine Application Improves Freezing Tolerance of Cabbage (Brassica oleracea L.) Leaves. Plants. 2021; 10(12):2821. https://doi.org/10.3390/plants10122821
Chicago/Turabian StyleMin, Kyungwon, Yunseo Cho, Eunjeong Kim, Minho Lee, and Sang-Ryong Lee. 2021. "Exogenous Glycine Betaine Application Improves Freezing Tolerance of Cabbage (Brassica oleracea L.) Leaves" Plants 10, no. 12: 2821. https://doi.org/10.3390/plants10122821
APA StyleMin, K., Cho, Y., Kim, E., Lee, M., & Lee, S.-R. (2021). Exogenous Glycine Betaine Application Improves Freezing Tolerance of Cabbage (Brassica oleracea L.) Leaves. Plants, 10(12), 2821. https://doi.org/10.3390/plants10122821







