High-Frequency Plant Regeneration, Genetic Uniformity, and Flow Cytometric Analysis of Regenerants in Rutachalepensis L.
Abstract
1. Introduction
2. Results
2.1. Effect of Cytokinins
2.2. Effect of Auxins and Cytokinins
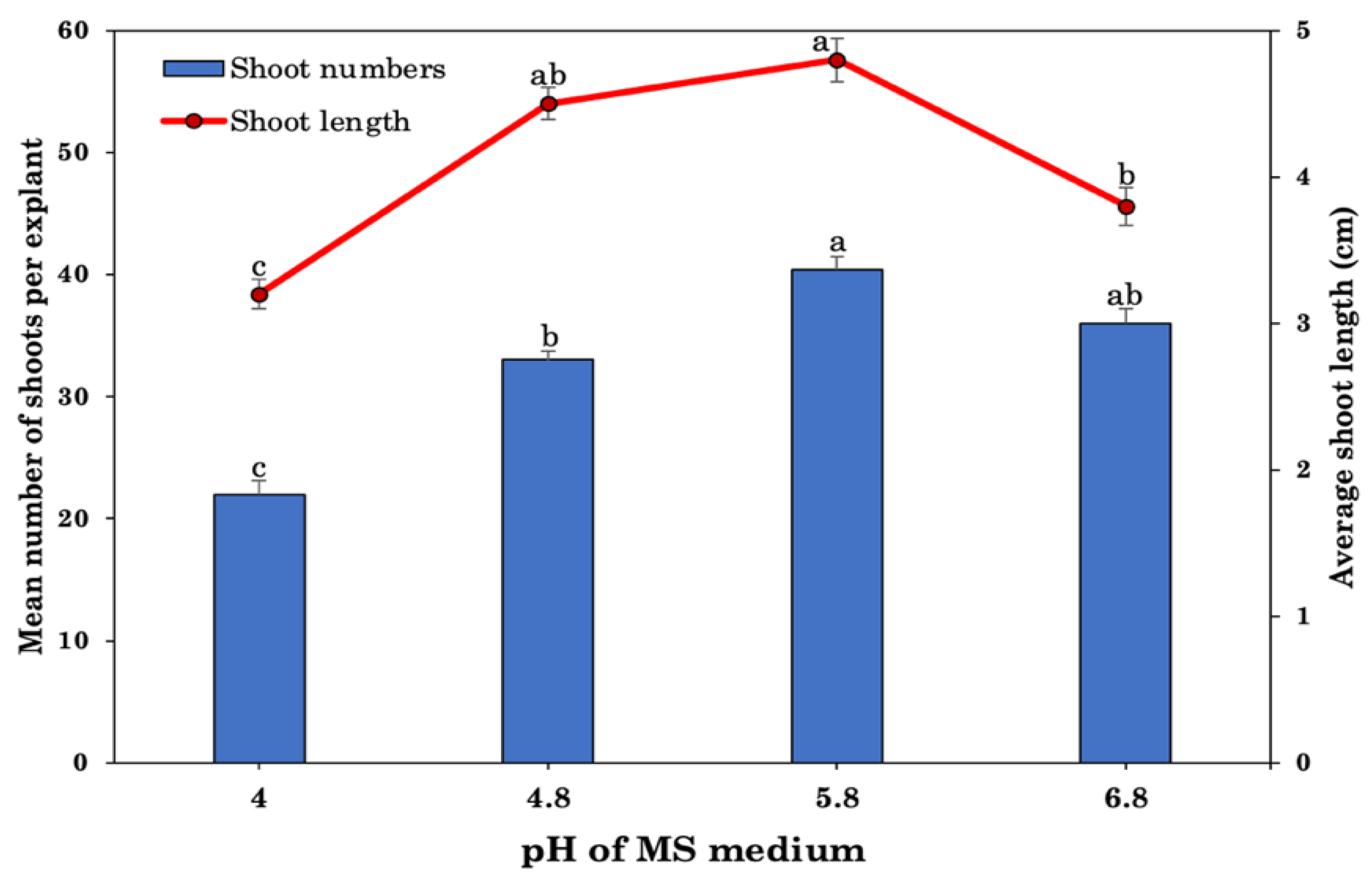
2.3. Effect of Different Medium and pH Levels
2.4. Effects of Carbon Sources
2.5. Rooting of Shootlets
2.6. Effect MS Basal Media Strengths on Rooting
2.7. Acclimatization
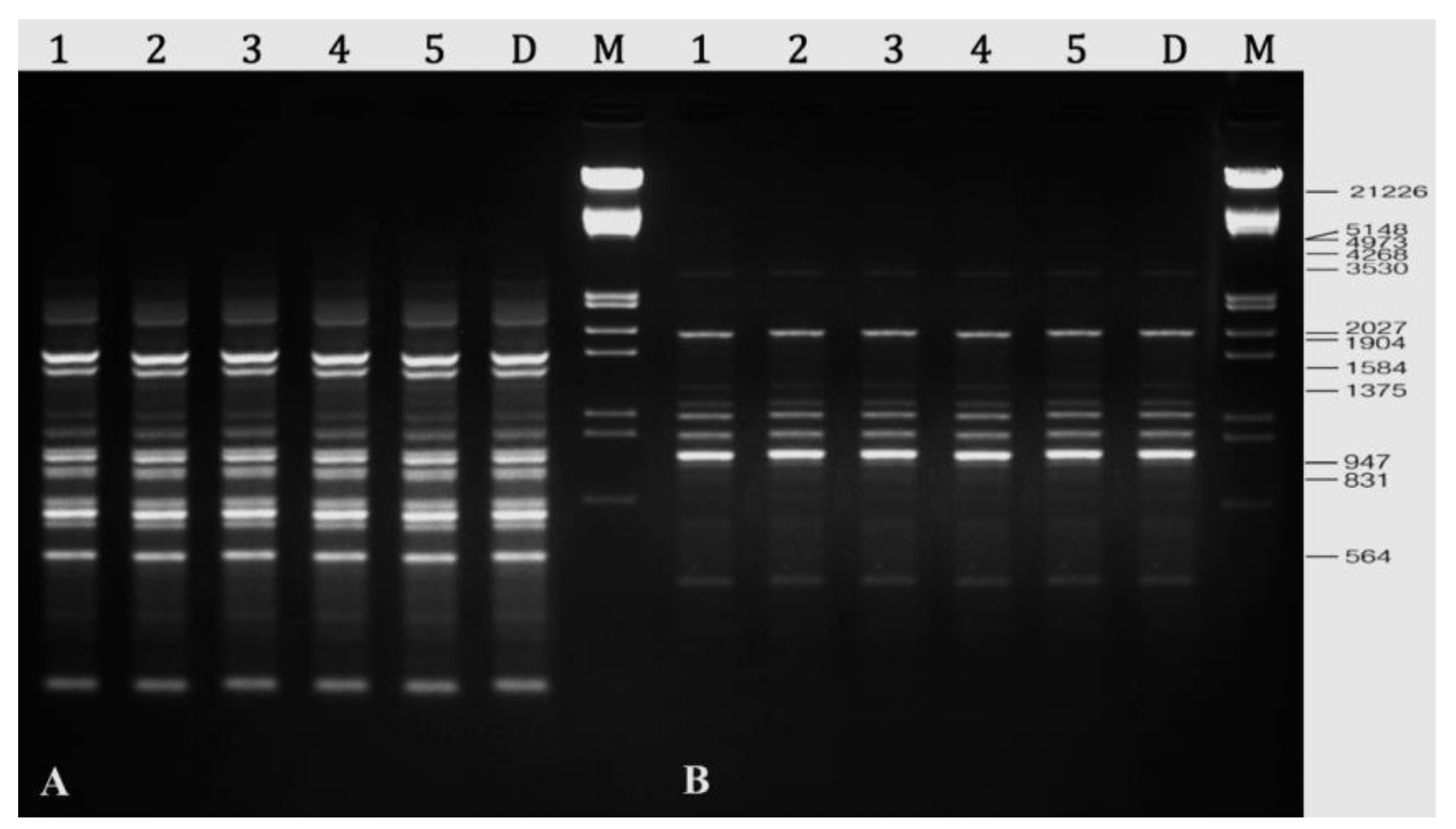
2.8. Assessment of Genetic Stability
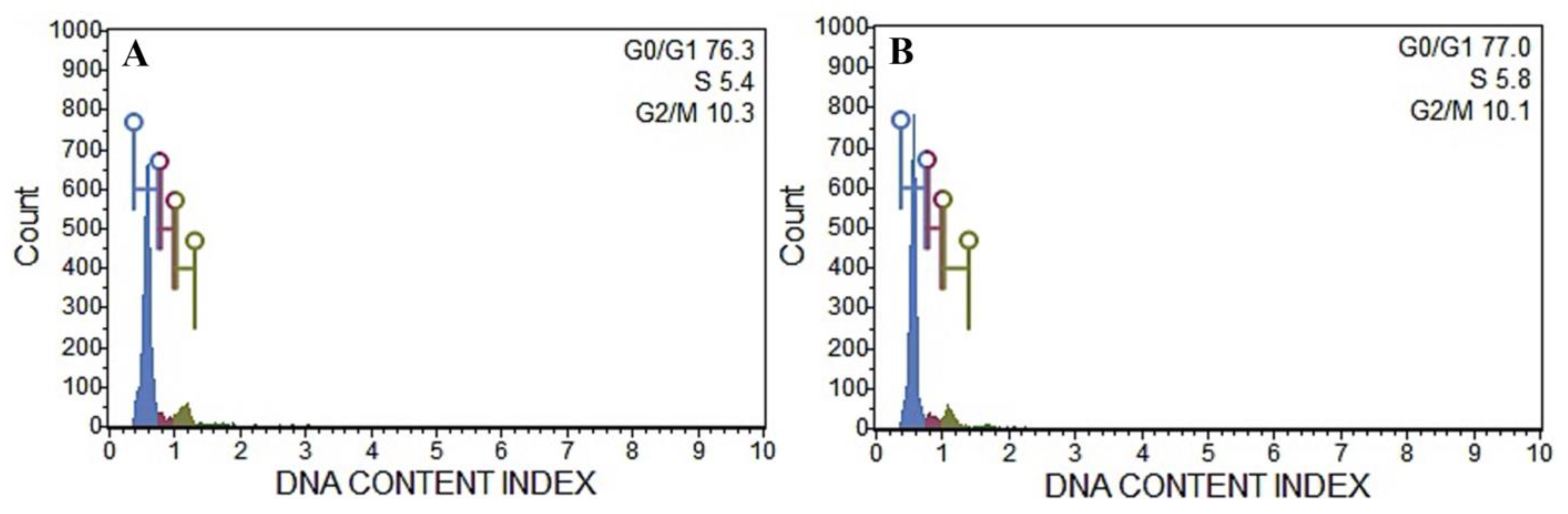
2.9. Flow Cytometric Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Surface Sterilization
4.2. Preparation of Media and Culture Conditions
4.3. In vitro Shoot Initiation and Proliferation
4.4. Effect of Various Media and pH
4.5. Effects Carbon Sources
4.6. Rooting of Shootlets
4.7. Acclimatization
4.8. Flow Cytometric Analysis
4.9. DNA Extraction and PCR Amplification
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iauk, L.; Mangano, K.; Rapisarda, A.; Ragusa, S.; Maiolino, L.; Musumeci, R.; Costanzo, R.; Serra, A.; Speciale, A. Protection against murine endotoxemia by treatment with Ruta chalepensis L., a plant with anti-inflammatory properties. J. Ethnopharmacol. 2004, 90, 267–272. [Google Scholar] [CrossRef]
- Günaydin, K.; Savci, S. Phytochemical studies on Ruta chalepensİs (LAM.) lamarck. Nat. Prod. Res. 2005, 19, 203–210. [Google Scholar] [CrossRef]
- Bnina, E.B.; Hammami, S.; Daamii-remadi, M.; Jannet, H.B.; Mighri, Z. Chemical composition and antimicrobial effects of Tunisian Ruta chalepensis L. essential oils. J. Société Chim. Tunis. 2010, 12, 1–9. [Google Scholar]
- Jaradat, N.; Adwan, L.; K’aibni, S.; Zaid, A.N.; Shtaya, M.J.; Shraim, N.; Assali, M. Variability of chemical compositions and antimicrobial and antioxidant activities of Ruta chalepensis leaf essential oils from three Palestinian regions. BioMed Res. Int. 2017, 2017, 2672689. [Google Scholar] [CrossRef]
- Gonzalez-Trujano, M.; Carrera, D.; Ventura-Martinez, R.; Cedillo-Portugal, E.; Navarrete, A. Neuropharmacological profile of an ethanol extract of Ruta chalepensis L. in mice. J. Ethnopharmacol. 2006, 106, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Iauk, L.; Sorrenti, V.; Lanteri, R.; Santangelo, R.; Licata, A.; Licata, F.; Vanella, A.; Malaguarnera, M.; Ragusa, S. Oxidative profile in patients with colon cancer: Effects of Ruta chalepensis L. Eur. Rev. Med. Pharm. Sci. 2011, 15, 181–191. [Google Scholar]
- Al-Majmaie, S.; Nahar, L.; Sharples, G.P.; Wadi, K.; Sarker, S.D. Isolation and antimicrobial activity of rutin and its derivatives from Ruta chalepensis (Rutaceae) growing in Iraq. Rec. Nat. Prod. 2019, 13, 64–70. [Google Scholar] [CrossRef]
- Ouerghemmi, I.; Bettaieb Rebey, I.; Rahali, F.Z.; Bourgou, S.; Pistelli, L.; Ksouri, R.; Marzouk, B.; Saidani Tounsi, M. Antioxidant and antimicrobial phenolic compounds from extracts of cultivated and wild-grown Tunisian Ruta chalepensis. J. Food Drug Anal. 2017, 25, 350–359. [Google Scholar] [CrossRef]
- Haddouchi, F.; Chaouche, T.M.; Zaouali, Y.; Ksouri, R.; Attou, A.; Benmansour, A. Chemical composition and antimicrobial activity of the essential oils from four Ruta species growing in Algeria. Food Chem. 2013, 141, 253–258. [Google Scholar] [CrossRef]
- Khlifi, D.; Sghaier, R.M.; Amouri, S.; Laouini, D.; Hamdi, M.; Bouajila, J. Composition and anti-oxidant, anti-cancer and anti-inflammatory activities of Artemisia herba-alba, Ruta chalpensis L. and Peganum harmala L. Food Chem. Toxicol. 2013, 55, 202–208. [Google Scholar] [CrossRef]
- Akkari, H.; Ezzine, O.; Dhahri, S.; B’chir, F.; Rekik, M.; Hajaji, S.; Darghouth, M.A.; Jamâa, M.L.B.; Gharbi, M. Chemical composition, insecticidal and in vitro anthelmintic activities of Ruta chalepensis (Rutaceae) essential oil. Ind. Crop. Prod. 2015, 74, 745–751. [Google Scholar] [CrossRef]
- Babu-Kasimala, M.; Tukue, M.; Ermias, R. Phytochemical screening and antibacterial activity of two common terresterial medicinal plants Ruta chalepensis and Rumex nervosus. Bali Med. J. 2014, 3, 116–121. [Google Scholar] [CrossRef]
- Boudjelal, A.; Henchiri, C.; Sari, M.; Sarri, D.; Hendel, N.; Benkhaled, A.; Ruberto, G. Herbalists and wild medicinal plants in M’Sila (North Algeria): An ethnopharmacology survey. J. Ethnopharmacol. 2013, 148, 395–402. [Google Scholar] [CrossRef]
- Rout, G.R.; Mohapatra, A.; Jain, S.M. Tissue culture of ornamental pot plant: A critical review on present scenario and future prospects. Biotechnol. Adv. 2006, 24, 531–560. [Google Scholar] [CrossRef]
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef]
- Dakah, A.; Zaid, S.; Suleiman, M.; Abbas, S.; Wink, M. In vitro propagation of the medicinal plant Ziziphora tenuior L. and evaluation of its antioxidant activity. Saudi J. Biol. Sci. 2014, 21, 317–323. [Google Scholar] [CrossRef]
- Pant, B. Application of Plant Cell and Tissue Culture for the Production of Phytochemicals in Medicinal Plants. Adv. Exp. Med. Biol. 2014, 808, 25–39. [Google Scholar] [PubMed]
- Faisal, M.; Ahmad, N.; Anis, M.; Alatar, A.A.; Qahtan, A.A. Auxin-cytokinin synergism in vitro for producing genetically stable plants of Ruta graveolens using shoot tip meristems. Saudi J. Biol. Sci. 2018, 25, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, T.A. Chapter 1—History of Plant Cell Culture. In Plant Tissue Culture, 3rd ed.; Smith, R.H., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 1–22. [Google Scholar] [CrossRef]
- Shahzad, A.; Sharma, S.; Parveen, S.; Saeed, T.; Shaheen, A.; Akhtar, R.; Yadav, V.; Upadhyay, A.; Ahmad, Z. Historical Perspective and Basic Principles of Plant Tissue Culture. In Plant Biotechnology: Principles and Applications; Abdin, M.Z., Kiran, U., Kamaluddin, A., Eds.; Springer: Singapore, 2017; pp. 1–36. [Google Scholar] [CrossRef]
- Yan, M.-M.; Xu, C.; Kim, C.-H.; Um, Y.-C.; Bah, A.A.; Guo, D.-P. Effects of explant type, culture media and growth regulators on callus induction and plant regeneration of Chinese jiaotou (Allium chinense). Sci. Hortic. 2009, 123, 124–128. [Google Scholar] [CrossRef]
- Dobránszki, J.; Teixeira da Silva, J.A. Micropropagation of apple—A review. Biotechnol. Adv. 2010, 28, 462–488. [Google Scholar] [CrossRef] [PubMed]
- Lipavská, H.; Konrádová, H. Somatic embryogenesis in conifers: The role of carbohydrate metabolism. Vitr. Cell. Dev. Biol. Plant 2004, 40, 23–30. [Google Scholar] [CrossRef]
- Shirin, F.; Parihar, N.S.; Shah, S.N. Effect of Nutrient Media and KNO3 on in vitro Plant Regeneration in Saraca asoca (Roxb.) Willd. Am. J. Plant Sci. 2015, 6, 3282–3292. [Google Scholar] [CrossRef][Green Version]
- Ahmad, N.; Anis, M. An efficient in vitro process for recurrent production of cloned plants of Vitex negundo L. Eur. J. For. Res. 2011, 130, 135–144. [Google Scholar] [CrossRef]
- Perveen, S.; Varshney, A.; Anis, M.; Aref, I.M. Influence of cytokinins, basal media and pH on adventitious shoot regeneration from excised root cultures of Albizia lebbeck. J. For. Res. 2011, 22, 47–52. [Google Scholar] [CrossRef]
- Roy, A.; Kundu, K.; Saxena, G.; Kumar, L.; Bharadvaja, N. Effect of different media and growth hormones on shoot multiplication of in vitro grown Centella asiatica accessions. Adv. Tech. Biol. Med. 2016, 4, 1–4. [Google Scholar] [CrossRef]
- Sharafi, A.; Hashemi Sohi, H.; Sharafi, A.A.; Azadi, P.; Mousavi, A. Tissue culture and regeneration of an antimalarial plant, Artemisia sieberi Besser. Res. J. Pharmacogn. 2014, 1, 15–20. [Google Scholar]
- Nathar, V.N.; Yatoo, G.M. Micropropagation of an antidiabetic medicinal plant, Artemisia pallens. Turk. J. Bot. 2014, 38, 491–498. [Google Scholar] [CrossRef]
- Ahmed, R.; Anis, M. Rapid in vitro propagation system through shoot tip cultures of Vitex trifolia L.—An important multipurpose plant of the Pacific traditional Medicine. Physiol. Mol. Biol. Plants 2014, 20, 385–392. [Google Scholar] [CrossRef]
- Al-Mahdawe, M.; Al-Mallah, M. Regeneration of the Medicinal Plant Ruta graveolens L. from Hypocotyl. Eur. Acad. Res. 2015, 2, 16256–16263. [Google Scholar]
- Bakhtiar, Z.; Mirjalili, M.H.; Sonboli, A. In vitro callus induction and micropropagation of Thymus persicus (Lamiaceae), an endangered medicinal plant. Crop Breed. Appl. Biotechnol. 2016, 16, 48–54. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Anis, M.; Alatar, A.A.; Faisal, M. In vitro clonal propagation and evaluation of genetic fidelity using RAPD and ISSR marker in micropropagated plants of Cassia alata L.: A potential medicinal plant. Agrofor. Syst. 2017, 91, 637–647. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; El-Sheikh, M.A.; Abdel-Salam, E.M.; Qahtan, A.A. Thidiazuron induced in vitro morphogenesis for sustainable supply of genetically true quality plantlets of Brahmi. Ind. Crop. Prod. 2018, 118, 173–179. [Google Scholar] [CrossRef]
- Hussain, S.A.; Anis, M.; Alatar, A.A. Efficient In vitro Regeneration System for Tecoma stans L., Using Shoot Tip and Assessment of Genetic Fidelity Among Regenerants. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 90, 171–178. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmad, N.; Anis, M.; Hakeem, K.R. Development of an efficient micropropagation system for Tecoma stans (L.) Juss. ex Kunth using thidiazuron and effects on phytochemical constitution. Vitr. Cell. Dev. Biol. Plant 2019, 55, 442–453. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; Ahmad, N.; Anis, M.; Hegazy, A.K. An Efficient and Reproducible Method for in vitro Clonal Multiplication of Rauvolfia tetraphylla L. and Evaluation of Genetic Stability using DNA-Based Markers. Appl. Biochem. Biotechnol. 2012, 168, 1739–1752. [Google Scholar] [CrossRef]
- Thorpe, T.; Stasolla, C.; Yeung, E.; De Klerk, G.; Roberts, A.; George, E. The components of plant tissue culture media II: Organic additions, osmotic and pH effects, and support systems. In Plant Propagation by Tissue Culture; George, E.F., Hall, M.A., De Klerk, G.-J., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 115–173. [Google Scholar]
- Bhatia, P.; Ashwath, N. Effect of medium pH on shoot regeneration from the cotyledonary explants of tomato. Biotechnology 2005, 4, 7–10. [Google Scholar]
- Naik, P.M.; Manohar, S.H.; Praveen, N.; Murthy, H.N. Effects of sucrose and pH levels on in vitro shoot regeneration from leaf explants of Bacopa monnieri and accumulation of bacoside A in regenerated shoots. Plant Cell Tissue Organ Cult. 2010, 100, 235–239. [Google Scholar] [CrossRef]
- Sharma, M.K.; Chaudhary, R.; Kureel, R.; Sengar, R. Effects of culture media pH on In Vitro shoot multiplication in sugarcane. Int. J. Chem. Stud. 2018, 6, 1308–1310. [Google Scholar]
- Rashid, R.; Bhat, J.A.; Bhat, M.I.; Bhat, B.A. Effect of pH on Callus Induction and Shoot Regeneration from Cotyledon and Leaf and Hypocotyl Explants of Tomato. Int. J. Pure Appl. Biosci. 2018, 6, 806–809. [Google Scholar]
- Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M.; Qahtan, A.A. Effects of 4-CPPU on in vitro multiplication and sustainable generation of Hibiscus rosa-sinensis L. ‘White Butterfly’. Saudi J. Biol. Sci. 2020, 27, 412–416. [Google Scholar] [CrossRef]
- Neto, V.B.D.P.; Otoni, W.C. Carbon sources and their osmotic potential in plant tissue culture: Does it matter? Sci. Hortic. 2003, 97, 193–202. [Google Scholar] [CrossRef]
- Sotiropoulos, T.; Molassiotis, A.; Mouhtaridou, G.; Papadakis, I.; Dimassi, K.; Therios, I.; Diamantidis, G. Sucrose and sorbitol effects on shoot growth and proliferation in vitro, nutritional status and peroxidase and catalase isoenzymes of M 9 and MM 106 apple (Malus domestica Borkh.) rootstocks. Eur. J. Hortic. Sci. 2006, 71, 114. [Google Scholar]
- Du Toit, E.; Robbertse, P.; Niederwieser, J. Plant carbohydrate partitioning of Lachenalia cv. Ronina during bulb production. Sci. Hortic. 2004, 102, 433–440. [Google Scholar] [CrossRef]
- Karami, O.; Deljou, A.; Esna-Ashari, M.; Ostad-Ahmadi, P. Effect of sucrose concentrations on somatic embryogenesis in carnation (Dianthus caryophyllus L.). Sci. Hortic. 2006, 110, 340–344. [Google Scholar] [CrossRef]
- Chen, C.-C.; Bates, R.; Carlson, J. Effect of environmental and cultural conditions on medium pH and explant growth performance of Douglas-fir (Pseudotsuga menziesii) shoot cultures. F1000Research 2014, 3, 298. [Google Scholar] [CrossRef]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef]
- Fuentes, S.R.; Calheiros, M.B.; Manetti-Filho, J.; Vieira, L.G. The effects of silver nitrate and different carbohydrate sources on somatic embryogenesis in Coffea canephora. Plant Cell Tissue Organ Cult. 2000, 60, 5–13. [Google Scholar] [CrossRef]
- Ahmad, T.; Abbasi, N.A.; Hafiz, I.A.; Ali, A. Comparison of sucrose and sorbitol as main carbon energy sources in microprogation of peach rootstock GF-677. Pak. J. Bot. 2007, 39, 1269–1275. [Google Scholar]
- Alatar, A.A. Thidiazuron induced efficient in vitro multiplication and ex vitro conservation of Rauvolfia serpentina—A potent antihypertensive drug producing plant. Biotechnol. Biotechnol. Equip. 2015, 29, 489–497. [Google Scholar] [CrossRef]
- Kamle, M.; Baek, K.-H. Somatic embryogenesis in guava (Psidium guajava L.): Current status and future perspectives. 3 Biotech 2017, 7, 203. [Google Scholar] [CrossRef]
- Bolyard, M. In vitro regeneration of Artemisia abrotanum L. by means of somatic organogenesis. Vitr. Cell. Dev. Biol. Plant 2018, 54, 127–130. [Google Scholar] [CrossRef]
- Jain, N.; Bairu, M.; Stirk, W.; Van Staden, J. The effect of medium, carbon source and explant on regeneration and control of shoot-tip necrosis in Harpagophytum procumbens. South Afr. J. Bot. 2009, 75, 117–121. [Google Scholar] [CrossRef][Green Version]
- Yadav, K.; Aggarwal, A.; Singh, N. Evaluation of genetic fidelity among micropropagated plants of Gloriosa superba L. using DNA-based markers—A potential medicinal plant. Fitoterapia 2013, 89, 265–270. [Google Scholar] [CrossRef]
- Asthana, P.; Jaiswal, V.S.; Jaiswal, U. Micropropagation of Sapindus trifoliatus L. and assessment of genetic fidelity of micropropagated plants using RAPD analysis. Acta Physiol. Plant. 2011, 33, 1821–1829. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmad, N.; Anis, M. Synergetic effect of TDZ and BA on minimizing the post-exposure effects on axillary shoot proliferation and assessment of genetic fidelity in Rauvolfia tetraphylla (L.). Rend. Lincei. Sci. Fis. E Nat. 2018, 29, 109–115. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Rawat, J.M.; Rawat, B.; Agnihotri, R.K.; Chandra, A.; Nautiyal, S. In vitro propagation, genetic and secondary metabolite analysis of Aconitum violaceum Jacq.: A threatened medicinal herb. Acta Physiol. Plant. 2013, 35, 2589–2599. [Google Scholar] [CrossRef]
- Fatima, N.; Ahmad, N.; Ahmad, I.; Anis, M. Interactive Effects of Growth Regulators, Carbon Sources, pH on Plant Regeneration and Assessment of Genetic Fidelity Using Single Primer Amplification Reaction (SPARS) Techniques in Withania somnifera L. Appl. Biochem. Biotechnol. 2015, 177, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Sebastian, J.K.; Jain, J.R.; Hanamanthagouda, M.S.; Murthy, H.N. Efficient in vitro propagation of Artemisia nilagirica var. nilagirica (Indian wormwood) and assessment of genetic fidelity of micropropagated plants. Physiol. Mol. Biol. Plants 2016, 22, 595–603. [Google Scholar] [CrossRef]
- Purohit, S.; Jugran, A.K.; Bhatt, I.D.; Palni, L.M.S.; Bhatt, A.; Nandi, S.K. In vitro approaches for conservation and reducing juvenility of Zanthoxylum armatum DC: An endangered medicinal plant of Himalayan region. Trees 2017, 31, 1101–1108. [Google Scholar] [CrossRef]
- Rohela, G.K.; Jogam, P.; Bylla, P.; Reuben, C. Indirect regeneration and assessment of genetic fidelity of acclimated plantlets by SCoT, ISSR, and RAPD markers in Rauvolfia tetraphylla L.: An endangered medicinal plant. Biomed. Res. Int. 2019, 2019, 3698742. [Google Scholar] [CrossRef]
- Upadhyay, A.; Shahzad, A.; Ahmad, Z. In vitro propagation and assessment of genetic uniformity along with chemical characterization in Hildegardia populifolia (Roxb.) Schott & Endl.: A critically endangered medicinal tree. Vitr. Cell. Dev. Biol. 2020, 56, 803–816. [Google Scholar] [CrossRef]
- Mishra, M.K.; Pandey, S.; Misra, P.; Niranjan, A. In vitro propagation, genetic stability and alkaloids analysis of acclimatized plantlets of Thalictrum foliolosum. Plant Cell Tissue Organ Cult. 2020, 142, 441–446. [Google Scholar] [CrossRef]
- Galbraith, D.W. Simultaneous flow cytometric quantification of plant nuclear DNA contents over the full range of described angiosperm 2C values. Cytom. A 2009, 75, 692–698. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; Hegazy, A.K.; Alharbi, S.A.; El-Sheikh, M.; Okla, M.K. Thidiazuron induced in vitro multiplication of Mentha arvensis and evaluation of genetic stability by flow cytometry and molecular markers. Ind. Crop. Prod. 2014, 62, 100–106. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Basak, S.; Ramesh, A.M.; Rangan, L. Nuclear DNA content of Pongamia pinnata L. and genome size stability of in vitro-regenerated plantlets. Protoplasma 2014, 251, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Bose, B.; Kumaria, S.; Choudhury, H.; Tandon, P. Assessment of genetic homogeneity and analysis of phytomedicinal potential in micropropagated plants of Nardostachys jatamansi, a critically endangered, medicinal plant of alpine Himalayas. Plant Cell Tissue Organ Cult. 2016, 124, 331–349. [Google Scholar] [CrossRef]
- Jena, S.; Ray, A.; Sahoo, A.; Sahoo, S.; Dash, B.; Kar, B.; Nayak, S. Rapid plant regeneration in industrially important Curcuma zedoaria revealing genetic and biochemical fidelity of the regenerants. 3 Biotech 2019, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.P.; De Campos, J.M.S.; Viccini, L.F.; Alkimim, E.R.; de Oliveira Santos, M. Micropropagation and ploidy stability of Lippia lacunosa Mart. & Schauer: An endangered brazilian medicinal plant. J. Neotrop. Agric. 2019, 6, 7–11. [Google Scholar] [CrossRef]
- Ahmad, N.; Faisal, M.; Anis, M.; Aref, I. In vitro callus induction and plant regeneration from leaf explants of Ruta graveolens L. South Afr. J. Bot. 2010, 76, 597–600. [Google Scholar] [CrossRef]
- Bohidar, S.; Thirunavoukkarasu, M.; Rao, T. Effect of Plant Growth Regulators on in vitro micropropagation of “Garden Rue” (Ruta graveolens L.). Int. J. Integr. Biol. 2008, 3, 36. [Google Scholar]
- Faisal, M.; Ahmad, N.; Anis, M. In vitro regeneration and mass propagation of Ruta graveolens L.—A multipurpose shrub. Hortic. Sci. 2005, 40, 1478–1480. [Google Scholar] [CrossRef]
- Kengar, A.; Paratkar, G. Effect of Plant Growth Regulators on Indirect Organogenesis in Ruta graveolens L. Int. J. Adv. Res. 2015, 3, 1113–1119. [Google Scholar]
- Van Staden, J.; Zazimalova, E.; George, E.F. Plant Growth Regulators II: Cytokinins, their Analogues and Antagonists. In Plant Propagation by Tissue Culture: The Background; George, E.F., Hall, M.A., Klerk, G.-J.D., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 1, pp. 205–226. [Google Scholar]
- Magyar-Tábori, K.; Dobránszki, J.; Teixeira da Silva, J.A.; Bulley, S.M.; Hudák, I. The role of cytokinins in shoot organogenesis in apple. Plant Cell Tissue Organ Cult. 2010, 101, 251–267. [Google Scholar] [CrossRef]
- Naaz, A.; Hussain, S.A.; Naz, R.; Anis, M.; Alatar, A.A. Successful plant regeneration system via de novo organogenesis in Syzygium cumini (L.) Skeels: An important medicinal tree. Agrofor. Syst. 2019, 93, 1285–1295. [Google Scholar] [CrossRef]
- Zhu, W.-T.; Du, J.-Z.; Sun, H.-B.; Jiang, S.-Y.; Chen, X.-J.; Sun, H.; Zhou, Y.; Wang, H.-L. In vitro propagation of an endemic and endangered medicinal plant Notopterygium incisum. Plant Cell Tissue Organ Cult. 2018, 135, 559–563. [Google Scholar] [CrossRef]
- Patricia, D.; Stephen, B.; John, A. Shoot organogenesis from leaf discs of the African ginger (Mondia whitei (Hook.f.) Skeels), an endangered medicinal plant. Vitr. Cell. Dev. Biol. Plant 2021, 57, 493–498. [Google Scholar] [CrossRef]
- Dwivedi, N.; Indiradevi, A.; Asha, K.; Nair, R.A.; Suma, A. A protocol for micropropagation of Aloe vera L. (Indian Aloe)—A miracle plant. Res. Biotechnol. 2014, 5, 1–5. [Google Scholar]
- Ab Rahman, Z.; Noor, E.S.M.; Ali, M.S.M.; Mirad, R.; Othman, A.N. In Vitro Micropropagation of a Valuable Medicinal Plant, Plectranthus amboinicus. Am. J. Plant Sci. 2015, 6, 1091. [Google Scholar] [CrossRef][Green Version]
- Fatima, N.; Ahmad, N.; Anis, M. Enhanced in vitro regeneration and change in photosynthetic pigments, biomass and proline content in Withania somnifera L. (Dunal) induced by copper and zinc ions. Plant Physiol. Biochem. 2011, 49, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Swain, D.; Lenka, S.; Hota, T.; Rout, G.R. Micro-propagation of Hypericum gaitii Haines, an endangered medicinal plant: Assessment of genetic fidelity. Nucleus 2016, 59, 7–13. [Google Scholar] [CrossRef]
- Coenen, C.; Lomax, T.L. Auxin-cytokinin interactions in higher plants: Old problems and new tools. Trends Plant Sci. 1997, 2, 351–356. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; Klerk, G.-J.D. Plant Growth Regulators I: Introduction; Auxins, their Analogues and Inhibitors. In Plant Propagation by Tissue Culture: The Background; George, E.F., Hall, M.A., Klerk, G.-J.D., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 1, pp. 175–204. [Google Scholar] [CrossRef]
- Owen, H.R.; Wengerd, D.; Miller, A.R. Culture medium pH is influenced by basal medium, carbohydrate source, gelling agent, activated charcoal, and medium storage method. Plant Cell Rep. 1991, 10, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Skirvin, R.M.; Chu, M.C.; Mann, M.L.; Young, H.; Sullivan, J.; Fermanian, T. Stability of tissue culture medium pH as a function of autoclaving, time, and cultured plant material. Plant Cell Rep. 1986, 5, 292–294. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Effect of various culture conditions on shoot multiplication and GC–MS analysis of Plumbago zeylanica accessions for plumbagin production. Acta Physiol. Plant. 2018, 40, 190. [Google Scholar] [CrossRef]
- Jagiełło-Kubiec, K.; Nowakowska, K.; Ilczuk, A.; Łukaszewska, A.J. Optimizing micropropagation conditions for a recalcitrant ninebark (Physocarpus opulifolius L. maxim.) cultivar. Vitr. Cell. Dev. Biol. Plant 2021, 57, 281–295. [Google Scholar] [CrossRef]
- Heidt, L.J.; Southam, F.W.; Sullivan, E.A. Autocatalyzed hydrolysis of sucrose by acid. J. Am. Chem. Soc. 1952, 74, 2377–2378. [Google Scholar] [CrossRef]
- Wann, S.R.; Veazey, R.L.; Kaphammer, J. Activated charcoal does not catalyze sucrose hydrolysis in tissue culture media during autoclaving. Plant Cell Tiss. Org. Cult. 1997, 50, 221–224. [Google Scholar] [CrossRef]
- Jayaraman, S.; Daud, N.H.; Halis, R.; Mohamed, R. Effects of plant growth regulators, carbon sources and pH values on callus induction in Aquilaria malaccensis leaf explants and characteristics of the resultant calli. J. For. Res. 2014, 25, 535–540. [Google Scholar] [CrossRef]
- Ahmad, A. In Vitro Morphogenesis and Assessment of Genetic Diversity in Pterocarpus marsupium Roxb. Using Molecular Markers. Ph.D. Thesis, Aligarh Muslim University, Aligarh, India, 2019. [Google Scholar]
- Aslam, M.M.; Karanja, J.K.; Zhang, Q.; Lin, H.; Xia, T.; Akhtar, K.; Liu, J.; Miao, R.; Xu, F.; Xu, W. In Vitro Regeneration Potential of White Lupin (Lupinus albus) from Cotyledonary Nodes. Plants 2020, 9, 318. [Google Scholar] [CrossRef]
- Yadav, V.; Shahzad, A.; Ahmad, Z.; Sharma, S.; Parveen, S. Synthesis of nonembryonic synseeds in Hemidesmus indicus R. Br.: Short term conservation, evaluation of phytochemicals and genetic fidelity of the regenerants. Plant Cell Tissue Organ Cult. 2019, 138, 363–376. [Google Scholar] [CrossRef]
- Al-Qurainy, F.; Nadeem, M.; Khan, S.; Alansi, S.; Tarroum, M. Micropropagation and evaluation of genetic fidelity of Maerua oblongifolia (forssk.) A. Rich: A rare medicinal plant from Saudi Arabia. Fresenius Environ. Bull. 2018, 27, 165–171. [Google Scholar]
- Jogam, P.; Sandhya, D.; Shekhawat, M.S.; Alok, A.; Manokari, M.; Abbagani, S.; Allini, V.R. Genetic stability analysis using DNA barcoding and molecular markers and foliar micro-morphological analysis of in vitro regenerated and in vivo grown plants of Artemisia vulgaris L. Ind. Crop. Prod. 2020, 151, 112476. [Google Scholar] [CrossRef]
- Dilkalal, A.; Annapurna, A.S.; Umesh, T.G. In vitro regeneration, antioxidant potential, and genetic fidelity analysis of Asystasia gangetica (L.) T.Anderson. Vitr. Cell. Dev. Biol. Plant 2021, 57, 447–459. [Google Scholar] [CrossRef]
- Elmongy, M.S.; Cao, Y.; Zhou, H.; Xia, Y. Root Development Enhanced by Using Indole-3-butyric Acid and Naphthalene Acetic Acid and Associated Biochemical Changes of In Vitro Azalea Microshoots. J. Plant Growth Regul. 2018, 37, 813–825. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Indole-3-butyric acid in plant growth and development. Plant Growth Regul. 2000, 32, 219–230. [Google Scholar] [CrossRef]
- Rahman, M.; Rajiora, O. Microsatellite DNA somaclonal variation in micropropagated trembling aspen (Populus tremuloides). Plant Cell Rep. 2001, 20, 531–536. [Google Scholar] [CrossRef]
- Sharma, U.; Rai, M.K.; Shekhawat, N.S.; Kataria, V. Genetic homogeneity revealed in micropropagated Bauhinia racemosa Lam. using gene targeted markers CBDP and SCoT. Physiol. Mol. Biol. Plants 2019, 25, 581–588. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Qiao, Y.; Chen, C. Plant regeneration from root segments of Anthurium andraeanum and assessment of genetic fidelity of in vitro regenerates. Vitr. Cell. Dev. Biol. Plant 2021. [Google Scholar] [CrossRef]
- Sahijram, L.; Soneji, J.R.; Bollamma, K.T. Analyzing somaclonal variation in micropropagated bananas (Musa spp.). Vitr. Cell. Dev. Biol. Plant 2003, 39, 551–556. [Google Scholar] [CrossRef]
- Fatima, N.; Ahmad, N.; Anis, M.; Ahmad, I. An improved in vitro encapsulation protocol, biochemical analysis and genetic integrity using DNA based molecular markers in regenerated plants of Withania somnifera L. Ind. Crop. Prod. 2013, 50, 468–477. [Google Scholar] [CrossRef]
- Prameela, J.; Ramakrishnaiah, H.; Krishna, V.; Deepalakshmi, A.P.; Naveen Kumar, N.; Radhika, R.N. Micropropagation and assessment of genetic fidelity of Henckelia incana: An endemic and medicinal Gesneriad of South India. Physiol. Mol. Biol. Plants 2015, 21, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Alatar, A.A.; Faisal, M.; Hegazy, A.K.; Alwathnani, H.A.; Okla, M.K. Clonal in vitro multiplication of grey mangrove and assessment of genetic fidelity using single primer amplification reaction (SPAR) methods. Biotechnol. Biotechnol. Equip. 2015, 29, 1069–1074. [Google Scholar] [CrossRef]
- Abdelsalam, A.; Mahran, E.; Chowdhury, K.; Boroujerdi, A. Metabolic profiling, in vitro propagation, and genetic assessment of the endangered rare plant Anarrhinum pubescens. J. Genet. Eng. Biotechnol. 2021, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Niazian, M.; Noori, S.A.S.; Galuszka, P.; Tohidfar, M.; Mortazavian, S.M.M. Genetic stability of regenerated plants via indirect somatic embryogenesis and indirect shoot regeneration of Carum copticum L. Ind. Crop. Prod. 2017, 97, 330–337. [Google Scholar] [CrossRef]
- Sadat-Hosseini, M.; Vahdati, K.; Leslie, C.A. Germination of Persian Walnut Somatic Embryos and Evaluation of their Genetic Stability by ISSR Fingerprinting and Flow Cytometry. HortScience 2019, 54, 1576–1580. [Google Scholar] [CrossRef]
- Viehmannova, I.; Cepkova, P.H.; Vitamvas, J.; Streblova, P.; Kisilova, J. Micropropagation of a giant ornamental bromeliad Puya berteroniana through adventitious shoots and assessment of their genetic stability through ISSR primers and flow cytometry. Plant Cell Tissue Organ Cult. 2016, 125, 293–302. [Google Scholar] [CrossRef]
- Alatar, A.A.; Faisal, M.; Abdel-Salam, E.M.; Canto, T.; Saquib, Q.; Javed, S.B.; El-Sheikh, M.A.; Al-Khedhairy, A.A. Efficient and reproducible in vitro regeneration of Solanum lycopersicum and assessment genetic uniformity using flow cytometry and SPAR methods. Saudi J. Biol. Sci. 2017, 24, 1430–1436. [Google Scholar] [CrossRef]
- Raji, M.R.; Lotfi, M.; Tohidfar, M.; Zahedi, B.; Carra, A.; Abbate, L.; Carimi, F. Somatic embryogenesis of muskmelon (Cucumis melo L.) and genetic stability assessment of regenerants using flow cytometry and ISSR markers. Protoplasma 2018, 255, 873–883. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.c.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Int. Plant Propag. Soc. Proc. 1980, 30, 421–427. [Google Scholar]
- White, P.R. A Handbook of Plant Tissue Culture; J. Cattell Press: Lancaster, PA, USA, 1943; Volume 56. [Google Scholar]
- Nitsch, J.P.; Nitsch, C. Haploid Plants from Pollen Grains. Science 1969, 163, 85. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]





| Cytokinins (µM) | Response% | Mean no. of Shoots | Mean Shoot Length (cm) | |
|---|---|---|---|---|
| BA | Kin | |||
| 0 | 0 | 0 ± 0 f | 0 ± 0 e | 0 ± 0 i |
| 1.0 | 68.43 ± 1.64 f | 16.45 ± 1 c | 2.92 ± 0.11 d | |
| 2.5 | 77.5 ± 0.92 b,c | 19.14 ± 0.71 b | 3.16 ± 0.16 d | |
| 5.0 | 85.82 ± 0.72 a | 23.42 ± 0.81 a | 4.38 ± 0.29 a | |
| 7.5 | 78.44 ± 0.98 b | 19.81 ± 0.86 b | 4.1 ± 0.1 b | |
| 10 | 73.83 ± 1.1 d,e | 18.04 ± 0.72 b,c | 3.76 ± 0.18 c | |
| 1.0 | 55.47 ± 0.89 h | 10.26 ± 0.8 e | 2.78 ± 0.1 d | |
| 2.5 | 65.91 ± 0.84 g | 10.72 ± 15 e | 2.96 ± 0.15 d | |
| 5.0 | 75.25 ± 0.79 c,d | 13.6 ± 0.4 d | 3.88 ± 0.17 c | |
| 7.5 | 72.28 ± 0.75 e | 10.42 ± 0.51 e | 2.92 ± 0.05 d | |
| 10 | 69.01 ± 0.66 f | 9.6 ± 0.81 e | 2.86 ± 0.05 d | |
| Cytokinins (µM) | Auxins (µM) | Response % | Mean no. of Shoots | Mean Shoot Length (cm) | |||
|---|---|---|---|---|---|---|---|
| BA | Kin | NAA | IAA | IBA | |||
| 5.0 | - | - | - | - | 85.82 ± 0.72 c | 23.42 ± 0.81 f | 4.38 ± 0.29 b,d |
| 5.0 | - | 0.5 | - | - | 80.71 ± 2.34 e | 25.43 ± 1.2 d,e | 3.78 ± 0.15 e,f,g |
| 5.0 | - | 1.0 | - | - | 96.33 ± 2.02 a | 40.37 ± 1.45 a | 4.8 ± 0.15 a,b |
| 5.0 | - | 1.5 | - | - | 83.44 ± 1.67 d | 30.44 ± 1.4 c | 4.32 ± 0.17 b,d |
| 5.0 | - | 2.0 | - | - | 70.02 ± 1.73 k | 21.12 ± 1.16 g,h | 3.28 ± 0.17 g,h,i,j |
| 5.0 | - | - | 0.5 | - | 79.36 ± 1.76 e,g | 22.7 ± 1.20 i,j,k | 3.34 ± 0.15 g,h,i,j |
| 5.0 | - | - | 1.0 | - | 90.04 ± 2.45 b | 32.66 ± 1.46 b | 4.66 ± 0.09 a,b |
| 5.0 | - | - | 1.5 | - | 80.11 ± 2.33 e,f | 25.41 ± 1.1 d | 4.3 ± 0.16 b,d |
| 5.0 | - | - | 2.0 | - | 70.05 ± 2.35 k | 20.31 ± 18 g,i | 3.12 ± 0.19 h,i,l |
| 5.0 | - | - | - | 0.5 | 75.16 ± 0.64 i | 20.63 ± 0.51 g,i | 2.76 ± 0.08 l |
| 5.0 | - | - | - | 1.0 | 86.37 ± 0.66 c | 26.23 ± 0.58 d | 3.16 ± 0.14 h,i,l |
| 5.0 | - | - | - | 1.5 | 78.51 ± 0.79 f,g | 22.24 ± 0.86 f g | 2.92 ± 0.11 i,l |
| 5.0 | - | - | - | 2.0 | 66.4 ± 0.76 m | 18.87 ± 0.86 i,j,k | 2.78 ± 0.06 k,i |
| - | 5.0 | 0.5 | - | - | 78.62 ± 0.86 f,g | 16.4 ± 0.68 l,m,n | 3.42 ± 0.23 f,i |
| - | 5.0 | 1.0 | - | - | 90.31 ± 0.36 b | 25.61 ± 18 b | 4.9 ± 0.25 a |
| - | 5.0 | 1.5 | - | - | 80.23 ± 0.51 e,f | 20.43 ± 0.87 g,i | 3.78 ± 0.18 e,f,g |
| - | 5.0 | 2.0 | - | - | 68.32 ± 0.5 i | 14.21 ± 1.28 o | 2.84 ± 0.23 j,l |
| - | 5.0 | - | 0.5 | - | 76.44 ± 0.63 h,i | 14.82 ± 0.66 m,o | 2.84 ± 0.23 j,l |
| - | 5.0 | - | 1.0 | - | 86.01 ± 0.56 c | 19.6 ± 0.51 h,i,j | 4.18 ± 0.22 c,d,e |
| - | 5.0 | - | 1.5 | - | 77.74 ± 0.74 g,h | 17.26 ± 0.37 k,l | 3.62 ± 0.14 f,h |
| - | 5.0 | - | 2.0 | - | 67.55 ± 0.6 l,m | 14.02 ± 0.71 o | 3.16 ± 0.14 h,i,l |
| - | 5.0 | - | - | 0.5 | 71.22 ± 0.65 j,k | 13.4 ± 0.51 o | 2.72 ± 0.19 l |
| - | 5.0 | - | - | 1.0 | 80.6 ± 0.56 e | 17.82 ± 0.74 j,l | 3.88 ± 0.12 d,f |
| - | 5.0 | - | - | 1.5 | 72.74 ± 0.67 i | 16.45 ± 0.93 l,m,n | 3.18 ± 0.42 e,f,g |
| - | 5.0 | - | - | 2.0 | 64.67 ± 0.96 n | 14.47 ± 0.93 n,o | 3.04 ± 0.15 i,l |
| Auxins (μM) | Response% | No. of Roots/Microshoots | Root Length (cm) | ||
|---|---|---|---|---|---|
| IAA | NAA | IBA | |||
| 0 | 0 | 0 | 0 ± 0 f | 0 ± 0 f | 0 ± 0 f |
| 0.1 | 57.61 ± 1.45 d | 1.4 ± 0.3 f,g | 1.54 ± 0.11 e | ||
| 0.5 | 81.33 ± 2.03 b | 2.6 ± 0.33 c.d | 2.14 ± 0.11 c,d,e | ||
| 1.0 | 70.4 ± 1.33 c | 1.9 ± 0.19 d,e,f | 1.82 ± 06 d,e | ||
| 2.0 | 43.02 ± 1.67 f | 1.19 ± 0.17 g | 1.56 ± 0.16 e | ||
| 0.1 | 704 ± 1.15 c | 2.51 ± 0.34 c.d.e | 1.98 ± 0.37 b,c | ||
| 0.5 | 90.31 ± 2.15 a | 4.34 ± 0.33 a,b | 2.3 ± 0.13 b,c | ||
| 1.0 | 702 ± 1.45 c | 2.32 ± 0.31 c,d,e | 2.04 ± 0.14 b,c | ||
| 2.0 | 52.04 ± 2.01 e | 1.55 ± 0.28 e,f,g | 1.90 ± 0.15 c | ||
| 0.1 | 711 ± 2.33 c | 4.04 ± 0.57 b | 2.96 ± 0.69 b,c | ||
| 0.5 | 91.63 ± 2.88 a | 5.38 ± 0.51 a | 4.91 ± 0.39 a | ||
| 1.0 | 71.36 ± 2.64 c | 3.34 ± 0.34 b,c | 3.27 ± 0.69 b | ||
| 2.0 | 53.01 ± 1.85 d,e | 2.74 ± 0.29 c,d | 2.76 ± 0.28 b,c | ||
| Treatments | Response% | Number of Roots/Microshoots | Root Length (cm) |
|---|---|---|---|
| ¼ MS | 57.24 ± 0.39 d | 2.62 ± 0.74 b | 2.48 ± 0.35 b |
| ½ MS | 91.63 ± 2.88 a | 5.48 ± 0.51 a | 4.91 ± 0.39 a |
| ¾ MS | 71.80 ± 0.47 b | 3.44 ± 0.51 b | 2.66 ± 0.55 b |
| 1 MS | 66.24 ± 0.65 c | 2.81 ± 0.58 b | 2.56 ± 0.16 b |
| Name of Primers | Sequence 5′–3′ | Ta (°C) | No. of Bands |
|---|---|---|---|
| GL A-01 | CAGGCCCTTC | 33.6 | 13 |
| GL A-02 | TGCCGAGCTG | 33.6 | 3 |
| GL A-03 | AGTCAGCCAC | 29.5 | 14 |
| GL A-04 | AATCGGGCTG | 29.5 | 11 |
| GL A-05 | AGGGGTCTTG | 29.5 | 9 |
| GL A-06 | GGTCCCTGAC | 33.6 | 4 |
| GL A-07 | GAAACGGGTG | 29.5 | 13 |
| GL A-08 | GTGACGTAGG | 29.5 | 9 |
| GL A-09 | GGGTAACGCC | 33.6 | 6 |
| GL A-10 | GTGATCGCAG | 29.5 | 15 |
| GL B-01 | GTTTCGCTCC | 29.5 | 8 |
| GL B-02 | TGATCCCTGG | 29.5 | 2 |
| Total no. of bands | 107 | ||
| Average no. of bands/primers | 8.92 |
| Name of Primers | Sequence 5′–3′ | Ta (°C) | No. of Bands |
|---|---|---|---|
| HVR | GCTCCTCCCCTCCT | 50 | 10 |
| HBV3 | GGTGAAGCACAGGTG | 53 | 12 |
| HBV5 | GGTGTAGAGAGGGGT | 56 | 15 |
| M13 | GAGGGTGGCGGTTCT | 57 | 10 |
| 33.6 | GGAGGTGGGCA | 52 | 14 |
| Total number of bands | 61 | ||
| Average no. of bands/primers | 12.2 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qahtan, A.A.; Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M. High-Frequency Plant Regeneration, Genetic Uniformity, and Flow Cytometric Analysis of Regenerants in Rutachalepensis L. Plants 2021, 10, 2820. https://doi.org/10.3390/plants10122820
Qahtan AA, Faisal M, Alatar AA, Abdel-Salam EM. High-Frequency Plant Regeneration, Genetic Uniformity, and Flow Cytometric Analysis of Regenerants in Rutachalepensis L. Plants. 2021; 10(12):2820. https://doi.org/10.3390/plants10122820
Chicago/Turabian StyleQahtan, Ahmed A., Mohamad Faisal, Abdulrahman A. Alatar, and Eslam M. Abdel-Salam. 2021. "High-Frequency Plant Regeneration, Genetic Uniformity, and Flow Cytometric Analysis of Regenerants in Rutachalepensis L." Plants 10, no. 12: 2820. https://doi.org/10.3390/plants10122820
APA StyleQahtan, A. A., Faisal, M., Alatar, A. A., & Abdel-Salam, E. M. (2021). High-Frequency Plant Regeneration, Genetic Uniformity, and Flow Cytometric Analysis of Regenerants in Rutachalepensis L. Plants, 10(12), 2820. https://doi.org/10.3390/plants10122820









