Multicomponent Polyphenolic Extracts from Vaccinium corymbosum at Lab and Pilot Scale. Characterization and Effectivity against Nosocomial Pathogens
Abstract
:1. Introduction
2. Results
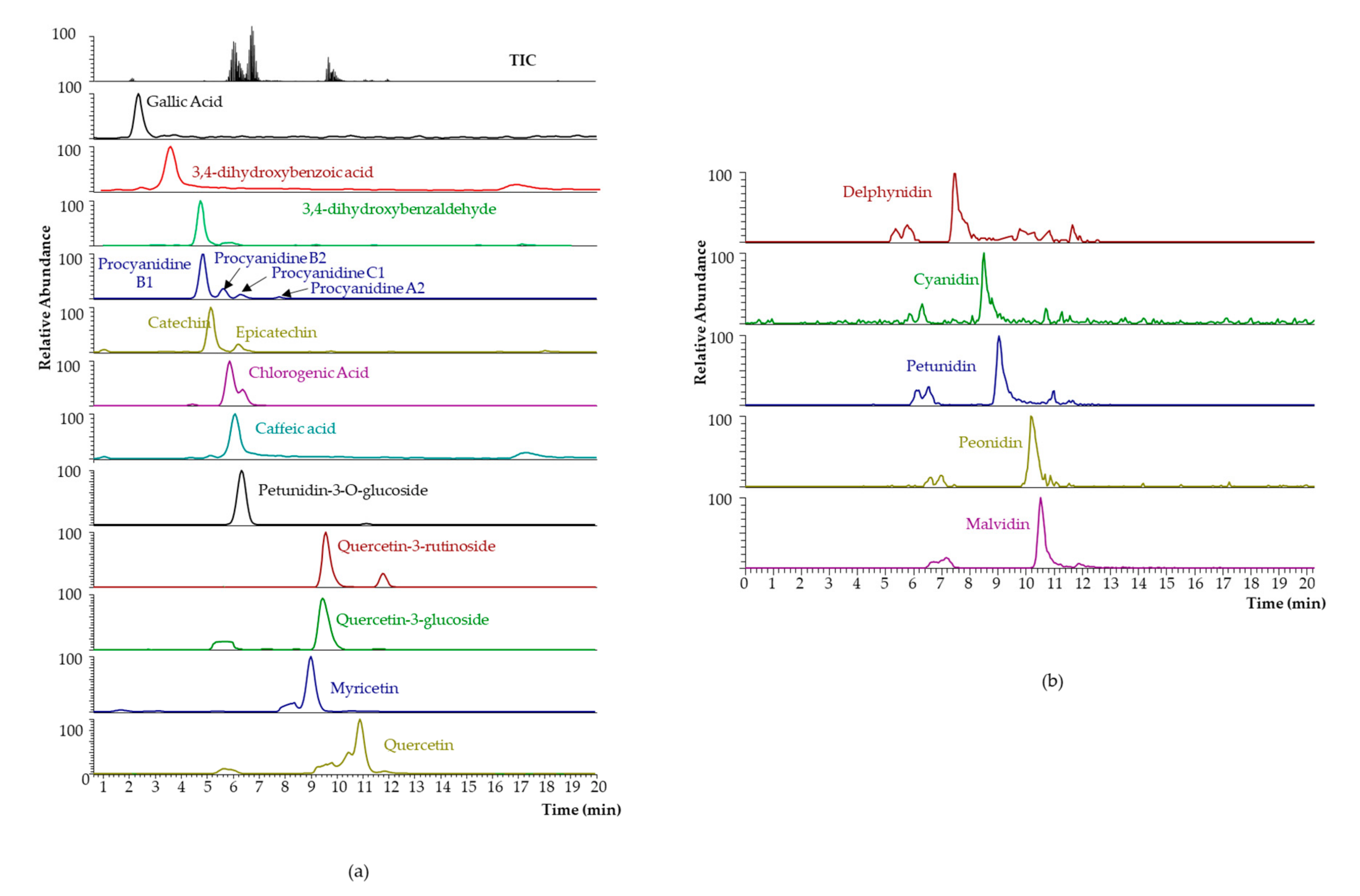
2.1. Analytical Characterization of MSATs Blueberry Extracts
2.2. Antibacterial Activities of Blueberry Extracts
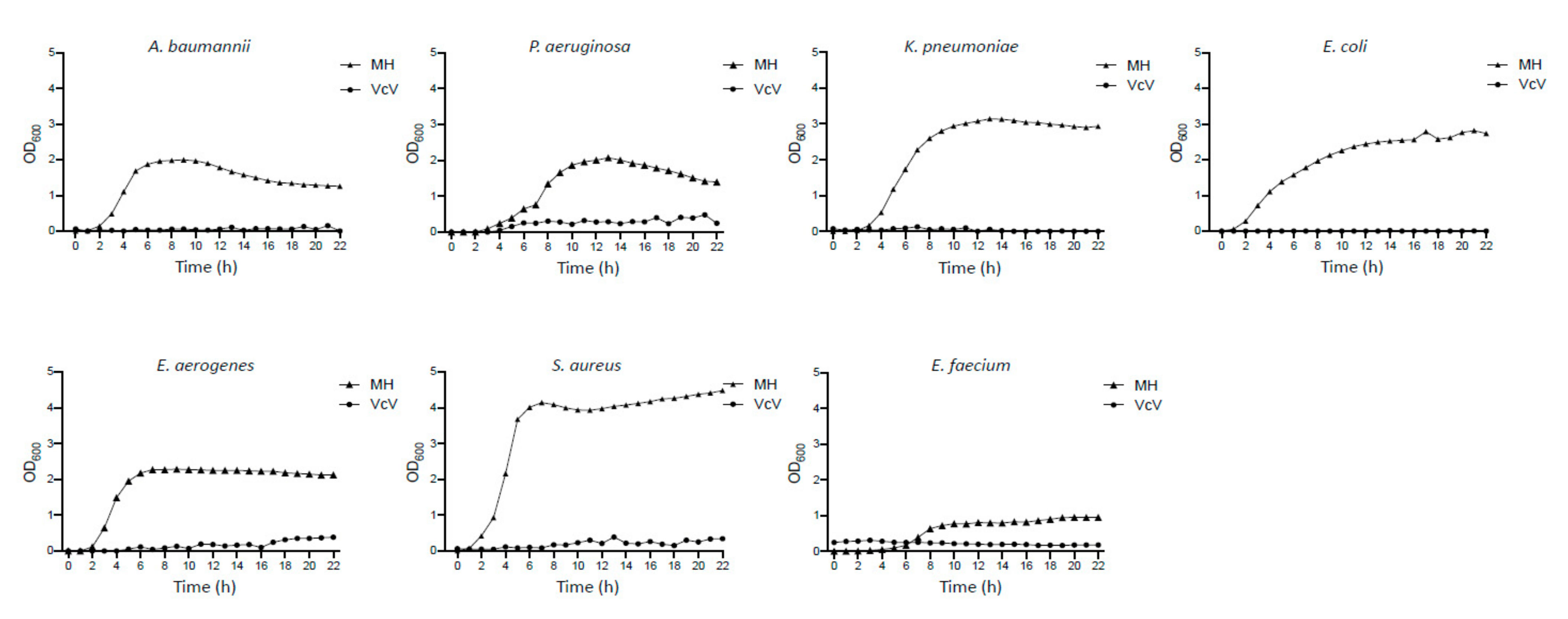
2.2.1. Antimicrobial Effect
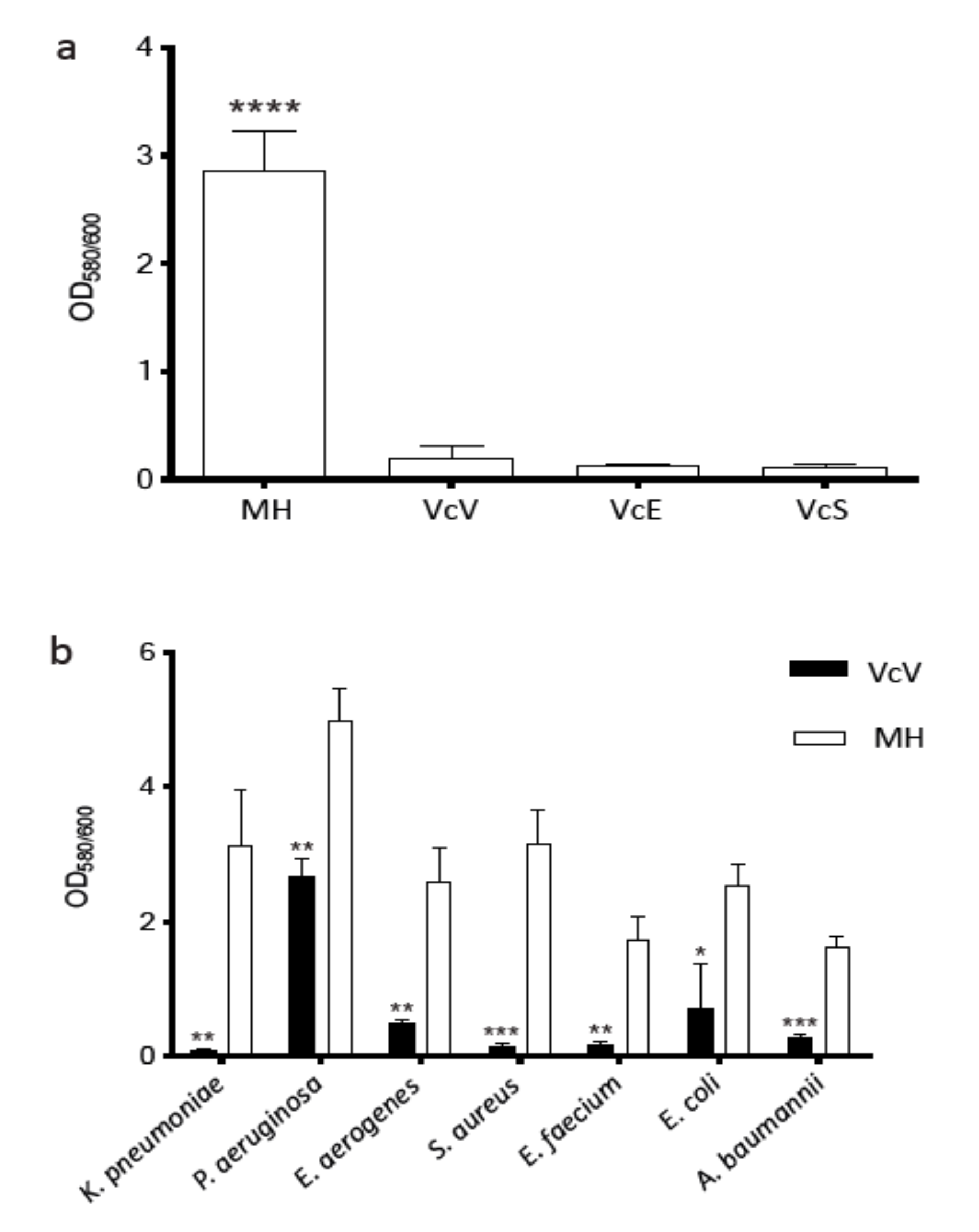
2.2.2. Antibiofilm Effect
2.3. Analytical Characterization of Pilot-Scale Blueberry Extracts
3. Discussion
4. Materials and Methods
4.1. Reagents, Solvents, and Materials
4.2. Samples and Sample Pre-Treatment
4.3. Extraction Systems at Medium and Pilot Scale
4.4. Analytical Characterization of the Blueberry Extracts
4.5. Evaluation of the Antimicrobial Activity
4.5.1. Bacterial Strains and Culture Conditions
4.5.2. Killing Curves Assay
4.5.3. Growth Curves Assay
4.5.4. Statistical Analysis
4.5.5. Quantitative Biofilm Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Centre for Disease Prevention and Control (ECDC). TESSy—The European Surveillance System; Antimicrobial Resistance (AMR) Reporting Protocol 2015; European Centre for Disease Prevention and Control: Brussels, Belgium, 2020. [Google Scholar]
- World Health Organization (WHO). WHO Publishes List of Bacteria for Which New Antibiotics are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 15 October 2021).
- Frieden, T. Antibiotic Resistance Threats in the United States. 2013. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 30 October 2021).
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [Green Version]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Alakomi, H.L.; Oksman-Caldentey, K.M. Bioactive berry compounds-novel tools against human pathogens. Appl. Microbiol. Biotechnol. 2005, 67, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B.; Reed, J.D.; Krueger, C.G.; Winterbottom, R.; Cunningham, D.G.; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Rane, H.S.; Bernardo, S.M.; Howell, A.B.; Lee, S.A. Cranberry-derived proanthocyanidins prevent formation of Candida albicans biofilms in artificial urine through biofilm-and adherence-specific mechanisms. J. Antimicrob. Chemother. 2014, 69, 428–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González de Llano, D.; Esteban-Fernández, A.; Sánchez-Patán, F.; Martínlvarez, P.J.; Moreno-Arribas, M.; Bartolomé, B. Anti-adhesive activity of cranberry phenolic compounds and their microbial-derived metabolites against uropathogenic Escherichia coli in bladder epithelial cell cultures. Int. J. Mol. Sci. 2015, 16, 12119–12130. [Google Scholar] [CrossRef] [PubMed]
- Uberos, J.; Rodríguez-Belmonte, R.; Rodríguez-Pérez, C.; Molina-Oya, M.; Blanca-Jover, E.; Narbona-López, E.; Muñoz-Hoyos, A. Phenolic acid content and antiadherence activity in the urine of patients treated with cranberry syrup (Vaccinium macrocarpon) vs. trimethoprim for recurrent urinary tract infection. J. Funct. Food. 2015, 18, 608–616. [Google Scholar] [CrossRef]
- Howell, A.B. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol. Nutr. Food Res. 2007, 51, 732–737. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Mendes, M.; Morais, R.M.; Calhau, C.A.O.; Pintado, M.M. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J. Appl. Microbiol. 2016, 121, 693–703. [Google Scholar] [CrossRef]
- Salaheen, S.; Peng, M.; Joo, J.; Teramoto, H.; Biswas, D. Eradication and sensitization of methicillin resistant Staphylococcus aureus to methicillin with bioactive extracts of berry pomace. Front. Microbiol. 2017, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Salaheen, S.; Jaiswal, E.; Joo, J.; Peng, M.; Ho, R.; Oconnor, D.; Adlerz, K.; Aranda-Espinoza, J.H.; Biswas, D. Bioactive extracts from berry byproducts on the pathogenicity of Salmonella Typhimurium. Int. J. Food Microbiol. 2016, 237, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Von Borowski, R.G.; Zimmer, K.R.; Leonardi, B.F.; Trentin, D.S.; Silva, R.C.; de Barros, M.P.; Macedo, A.J.; Gnoatto, S.C.B.; Gosmann, G.; Zimmer, A.R. Red pepper Capsicum baccatum: Source of antiadhesive and antibiofilm compounds against nosocomial bacteria. Ind. Crops Prod. 2019, 127, 148–157. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant natural products targeting bacterial virulence factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rama, J.L.; Mallo, N.; Biddau, M.; Fernandes, F.; de Miguel, T.; Sheiner, L.; Choupina, A.; Lores, M. Exploring the powerful phytoarsenal of white grape marc against bacteria and parasites causing significant diseases. Environ. Sci. Pollut. Res. 2021, 28, 24270–24278. [Google Scholar] [CrossRef] [Green Version]
- Gato, E.; Rosalowska, A.; Martinez-Guitian, M.; Lores, M.; Bou, G.; Perez, A. Anti-adhesive activity of a Vaccinium corymbosum polyphenolic extract targeting intestinal colonization by Klebsiella pneumoniae. Biomed. Pharmacother. 2020, 132, 110885. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yagiz, Y.; Hsu, W.Y.; Simonne, A.; Lu, J.; Marshall, M.R. Antioxidant, antibacterial, and antibiofilm properties of polyphenols from muscadine grape (Vitis rotundifolia Michx.) pomace against selected foodborne pathogens. J. Agric. Food Chem. 2014, 62, 6640–6649. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Fialová, S.; Rendeková, K.N.; Kovac, J.; Mucaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green chemistry. Frontiers 1998, 640, 1998. [Google Scholar]
- Dudonné, S.P.; Dubé, P.; Anhe, F.F.; Pilon, G.; Marette, A.; Lemire, M.; Harris, C.; Dewailly, E.; Desjardins, Y. Comprehensive analysis of phenolic compounds and abscisic acid profiles of twelve native Canadian berries. J. Food Compos. Anal. 2015, 44, 214–224. [Google Scholar] [CrossRef]
- Borges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J. Agric. Food Chem. 2010, 58, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, N.M.; Yaman, M. Determination of anthocyanins in cherry and cranberry by high-performance liquid chromatography-electrospray ionization-mass spectrometry. Eur. Food Res. Technol. 2016, 242, 127–135. [Google Scholar] [CrossRef]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium spp.) using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.A.; Wojdylo, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Food 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Pascu, M.; Pascu, D.E.; Cozea, A.; Bunaciu, A.A.; Miron, A.R.; Nechifor, C.A. Biologically active extracts with kidney affections applications. Appl. Surf. Sci. 2015, 358, 647–654. [Google Scholar] [CrossRef]
- Buran, T.J.; Sandhu, A.K.; Li, Z.; Rock, C.R.; Yang, W.W.; Gu, L. Adsorption/desorption characteristics and separation of anthocyanins and polyphenols from blueberries using macroporous adsorbent resins. J. Food Eng. 2014, 128, 167–173. [Google Scholar] [CrossRef]
- Klavins, L.; Kviesis, J.; Nakurte, I.; Klavins, M. Berry press residues as a valuable source of polyphenolics: Extraction optimisation and analysis. LWT 2018, 93, 583–591. [Google Scholar] [CrossRef]
- Loncaric, A.; Celeiro, M.; Jozinovic, A.; Jelinic, J.; Kovac, T.; Jokic, S.; Babic, J.; Moslavac, T.; Zavadlav, S.; Lores, M. Green extraction methods for extraction of polyphenolic compounds from blueberry pomace. Foods 2020, 9, 1521. [Google Scholar] [CrossRef] [PubMed]
- Lores, M.; Alvarez Casas, M.; Garcia-Jares, C.; Llompart, M. Polyphenol Extract from White-Grape Residue. EP Patent EP2875822A4, 15 June 2016. [Google Scholar]
- Lores, M.; Pájaro, M.; Álvarez-Casas, M.; Domínguez, J.; García-Jares, C. Use of ethyl lactate to extract bioactive compounds from Cytisus scoparius: Comparison of pressurized liquid extraction and medium scale ambient temperature systems. Talanta 2015, 140, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Gedas, A.; Simoes, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, A.; Wu, V.C.H. The potential of berries to serve as selective inhibitors of pathogens and promoters of beneficial microorganisms. Food Qual. Saf. 2017, 1, 3–12. [Google Scholar] [CrossRef]
- Alvesalo, J.; Vuorela, H.; Tammela, P.I.; Leinonen, M.; Saikku, P.; Vuorela, P. Inhibitory effect of dietary phenolic compounds on Chlamydia pneumoniae in cell cultures. Biochem. Pharmacol. 2006, 71, 735–741. [Google Scholar] [CrossRef]
- Heinonen, M. Antioxidant activity and antimicrobial effect of berry phenolics-a Finnish perspective. Mol. Nutr. Food Res. 2007, 51, 684–691. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Celeiro, M.; Lamas, J.P.; Arcas, R.; Lores, M. Antioxidants profiling of by-products from Eucalyptus Greenboards Manufacture. Antioxidants 2019, 8, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Plant Polyphenols Group | Blueberry cultivar | VcV | VcE | VcS |
|---|---|---|---|---|
| Compound Name | Concentration (µg g−1 dw) | |||
| Benzoic acids | Gallic acid | 2.4 ± 0.3 | 2.5 ± 0.6 | 1.9 ± 0.5 |
| Hydroxycinnamic acids | Caffeic acid | 2.6 ± 0.5 | 2.5 ± 0.4 | 9.6 ± 1.1 |
| Chlorogenic acid | 708 ± 22 | 154 ± 40 | 781 ± 72 | |
| Phenolic aldehydes | Protocatechualdehyde | 2.8 ± 0.2 | 1.6 ± 0.3 | 4.9 ± 0.4 |
| ∑ Non-Flavanoids | 716 | 161 | 797 | |
| Flavan-3-ols | Catechin | 2.7 ± 0.9 | 0.27 ± 0.08 | nd |
| Epicatechin | 0.5 ± 0.2 | nd | 0.2 ± 0.1 | |
| Epicatechin-gallate | 5.8 ± 0.9 | 4.6 ± 2.0 | 9.9 ± 6.4 | |
| ∑ Flavan-3-ols | 9 | 5 | 10 | |
| Flavan-3-ols oligomeric derivatives | Procyanidine A2 | 0.3 ± 0.1 | 7.6 ± 2.3 | 0.5 ± 0.2 |
| Procyanidine B1 | 3.5 ± 0.3 | 0.17 ± 0.09 | 0.20 ± 0.03 | |
| Procyanidine B2 | 3.5 ± 1.4 | 0.5 ± 0.3 | 0.5 ± 0.2 | |
| ∑ Procyanidines | 7.3 | 8.3 | 1.2 | |
| Flavonols | Quercetin | 760 ± 61 | 336 ± 49 | 10.6 ± 5.2 |
| Isoquercetin | 1300 ± 146 | 348 ± 40 | 592 ± 108 | |
| Rutin | 75 ± 13 | 7.7 ± 1.4 | 121 ± 24 | |
| ∑ Flavonols | 2135 | 692 | 724 | |
| Anthocyanidins 1 | Delphinidin | 153 ± 24 | nd | nd |
| Cyanidin | 269 ± 47 | 35 ± 7 | 84 ± 5 | |
| Petunidin | 812 ± 24 | 18.5 ± 2.4 | 122 ± 34 | |
| Peonidin | 121 ± 33 | 17 ± 5 | 114 ± 11 | |
| Malvidin | 2895 ± 861 | 339 ± 36 | 2113 ± 251 | |
| Anthocyanins | Petunidin-3-O-glucoside | 18,485 ± 468 | 3134 ± 283 | 6167 ± 363 |
| ∑ Anthocyani(di)ns | 22,735 | 3544 | 8599 | |
| ∑ Flavanoids | 24,886 | 4248 | 9334 | |
| ∑ Bioactive Polyphenols | 25,602 | 4409 | 10,133 | |
| Plant Polyphenols Group | Compound Name | MSATs | Pilot Scale |
|---|---|---|---|
| Concentration (µg g−1 dw) | |||
| Benzoic acids | Gallic acid | 2.6 ± 0.2 | 3.96 ± 0.07 |
| Protocatechuic acid | 0.61 ± 0.08 | 2.8 ± 0.4 | |
| Hydroxycinnamic acids | Caffeic acid | 4.45 ± 0.09 | 5.2 ± 0.1 |
| Chlorogenic acid | 475 ± 4 | 978 ± 3 | |
| Phenolic aldehydes | Protocatechualdehyde | 1.3 ± 0.2 | 1.0 ± 0.2 |
| ∑ Non-Flavanoids | 484 | 991 | |
| Flavan-3-ols | Catechin | 7.47 ± 0.06 | 30 ± 5 |
| Epicatechin | 1.7 ± 0.3 | 7 ± 2 | |
| ∑ Flavan-3-ols | 9 | 37 | |
| Flavan-3-ols oligomeric derivatives | Procyanidine A2 | - | 0.513 ± 0.03 |
| Procyanidine B1 | 20.8 ± 0.5 | 50 ± 2 | |
| Procyanidine B2 | 18.6 ± 0.1 | 42 ± 4 | |
| Procyanidine C1 | 302 ± 43 | 316 ± 39 | |
| ∑ Procyanidines | 341 | 408 | |
| Flavonols | Quercetin | 17 ± 1 | 27 ± 4 |
| Isoquercetin | 791 ± 25 | 846 ± 31 | |
| Rutin | 128 ± 4 | 130 ± 1 | |
| Myricetin | 8.9 ± 1.6 | 19 ± 4 | |
| ∑ Flavonols | 945 | 1022 | |
| Anthocyanidins 1 | Delphinidin | 150 ± 17 | 1983 ± 87 |
| Cyanidin | 75 ± 13 | 248 ± 10 | |
| Petunidin | 149 ± 29 | 1239 ± 16 | |
| Peonidin | 17 ± 4 | 60 ± 4 | |
| Malvidin | 1220 ± 67 | 7182 ± 68 | |
| Anthocyanins | Petunidin-3-O-glucoside | 3565 ± 35 | 7664 ± 155 |
| ∑ Anthocyani(di)ns | 5176 | 18,376 | |
| ∑ Flavanoids | 6471 | 19,843 | |
| ∑ Bioactive Polyphenols | 6955 | 20,834 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gato, E.; Perez, A.; Rosalowska, A.; Celeiro, M.; Bou, G.; Lores, M. Multicomponent Polyphenolic Extracts from Vaccinium corymbosum at Lab and Pilot Scale. Characterization and Effectivity against Nosocomial Pathogens. Plants 2021, 10, 2801. https://doi.org/10.3390/plants10122801
Gato E, Perez A, Rosalowska A, Celeiro M, Bou G, Lores M. Multicomponent Polyphenolic Extracts from Vaccinium corymbosum at Lab and Pilot Scale. Characterization and Effectivity against Nosocomial Pathogens. Plants. 2021; 10(12):2801. https://doi.org/10.3390/plants10122801
Chicago/Turabian StyleGato, Eva, Astrid Perez, Alicja Rosalowska, Maria Celeiro, German Bou, and Marta Lores. 2021. "Multicomponent Polyphenolic Extracts from Vaccinium corymbosum at Lab and Pilot Scale. Characterization and Effectivity against Nosocomial Pathogens" Plants 10, no. 12: 2801. https://doi.org/10.3390/plants10122801
APA StyleGato, E., Perez, A., Rosalowska, A., Celeiro, M., Bou, G., & Lores, M. (2021). Multicomponent Polyphenolic Extracts from Vaccinium corymbosum at Lab and Pilot Scale. Characterization and Effectivity against Nosocomial Pathogens. Plants, 10(12), 2801. https://doi.org/10.3390/plants10122801








