Phytochemical Characterization and Evaluation of Biological Activities of Egyptian Carob Pods (Ceratonia siliqua L.) Aqueous Extract: In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Carob Pod Aqueous Extract
2.2. Phytochemical Analysis of the Carob Extracts
2.2.1. Quantification of the Total Flavonoid Content
2.2.2. Quantification of the Total Phenolic Content
2.2.3. Quantification of the Total Alkaloid Content
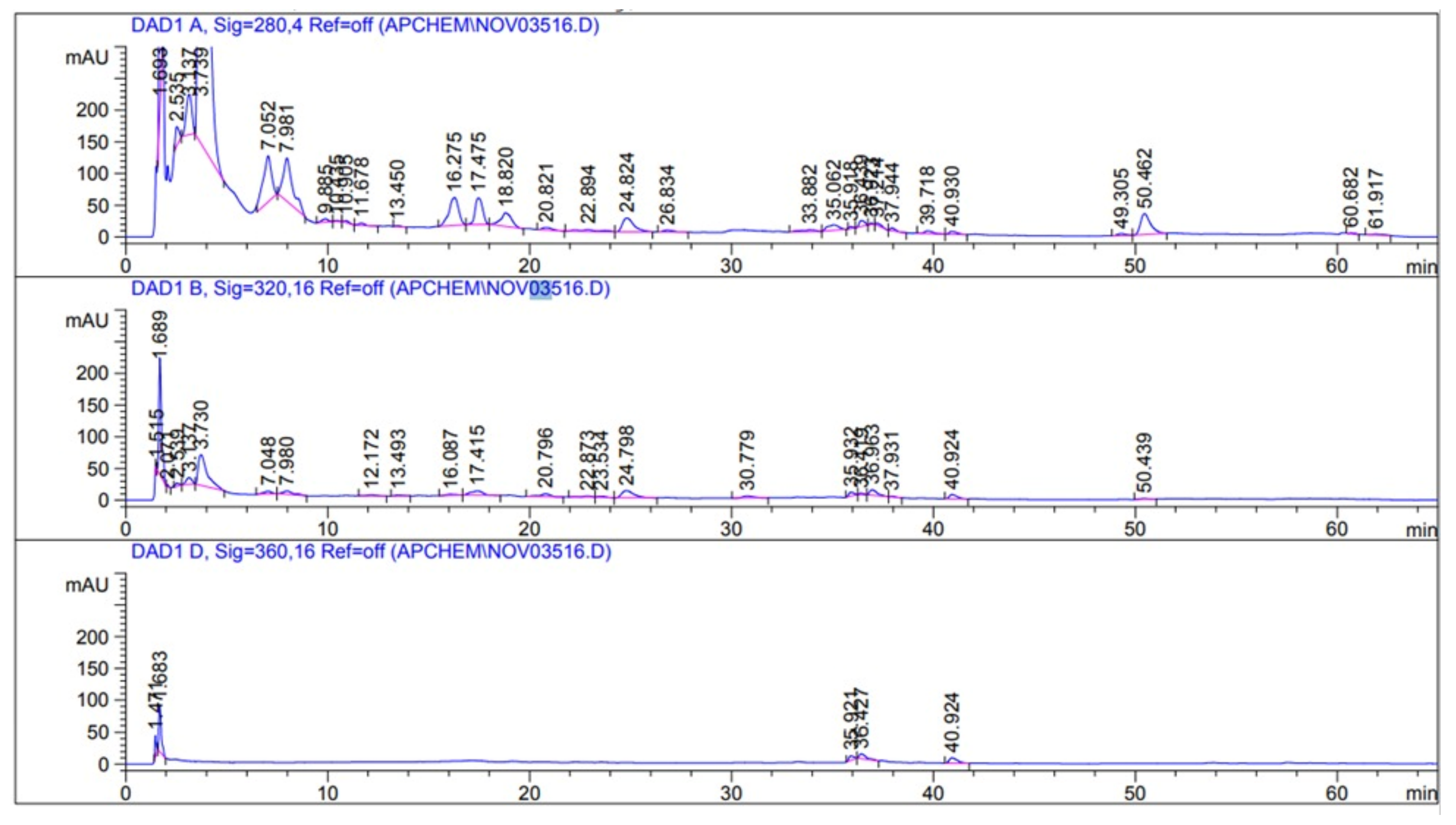
2.2.4. High-Performance Liquid Chromatography (HPLC) Analysis of CPAE
2.2.5. Quantification of the Total Carbohydrate Content
2.2.6. Quantification of the Total Amino Acid Content
2.3. Bio-Screening Analysis of CPAE
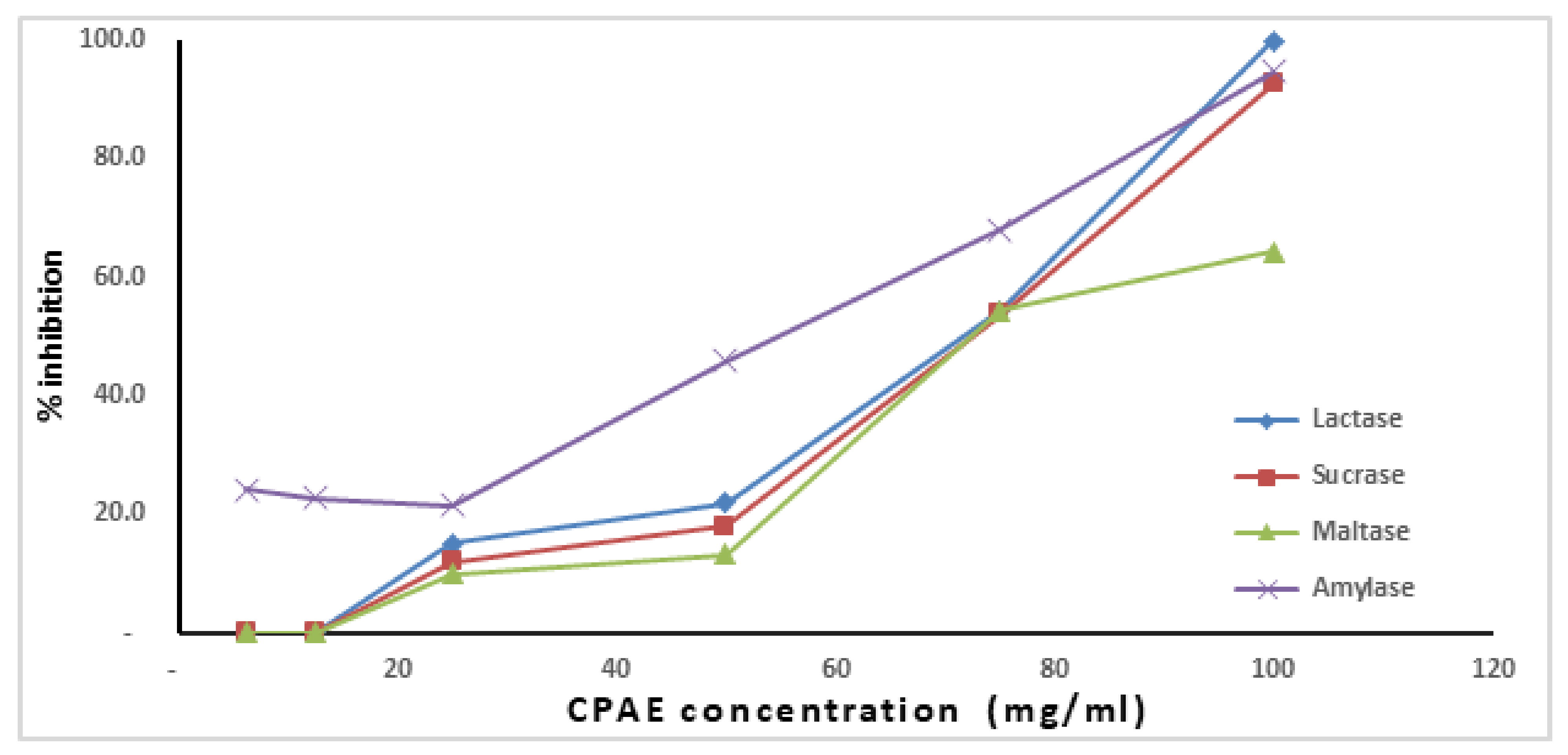
2.3.1. Determination of Anti-Digestive Enzyme Activities
Determination of α-Amylase Activity
Determination of Maltase, Sucrase and Lactase Activities
2.3.2. Determination of 2,2-Diphenyl-1-picryl hydrazyl (DPPH) Radical Scavenging Activity
2.3.3. Determination of Hydroxyl Radical Scavenging Activity
2.3.4. Determination of Ferric Reducing Antioxidant Power (FRAP)
2.3.5. Determination of Nitric Oxide (NO) Radical Scavenging Activity
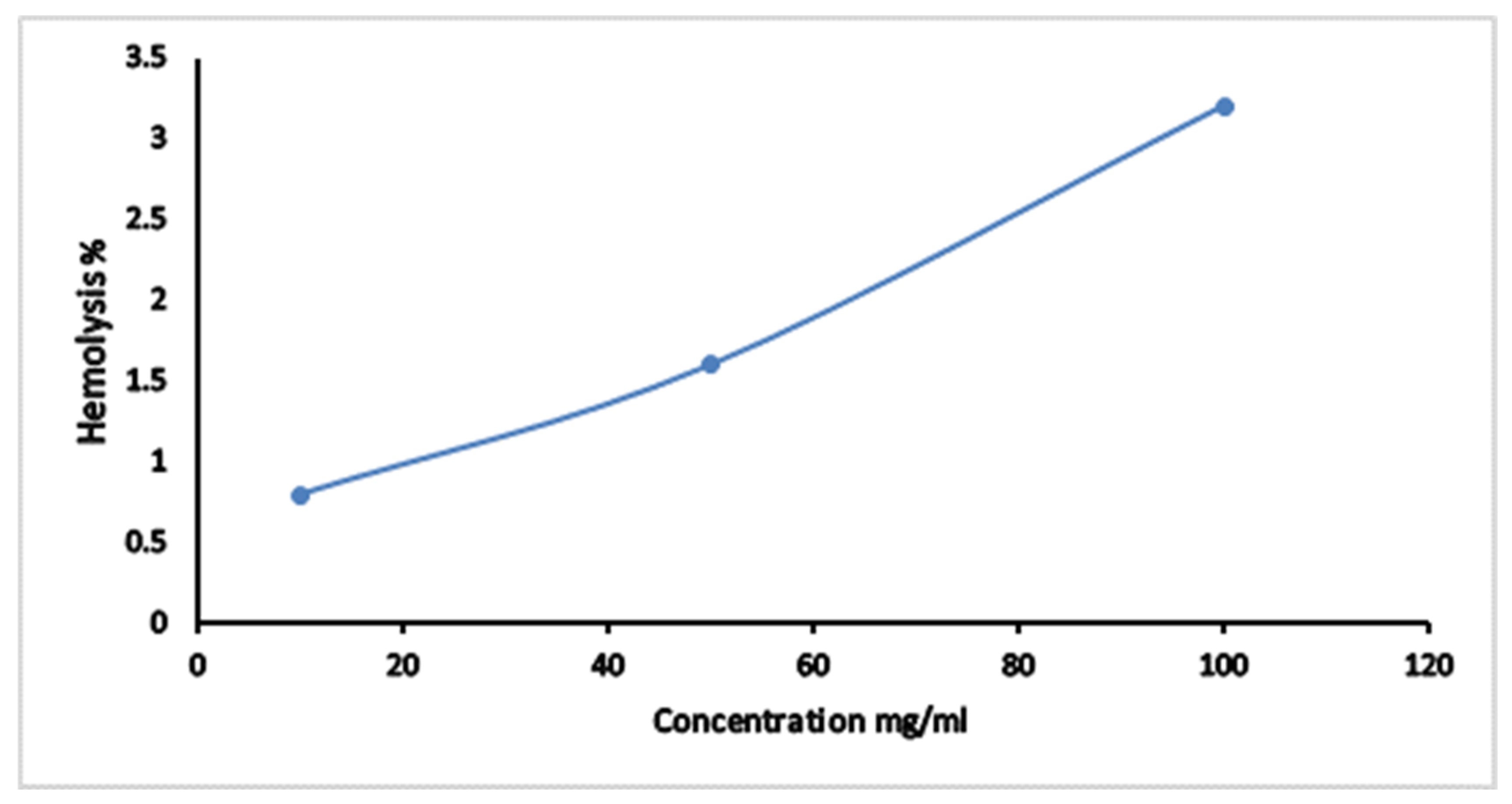
2.3.6. Hemolytic Activity Assay
2.4. Antimicrobial Activity
2.4.1. Antibacterial and Antifungal Method
2.4.2. Antiviral Method
Evaluation of the Maximum Non-Toxic Concentration (MNTC) of CPAE on Vero Cells
2.5. Statistical Analysis
3. Results
3.1. The Phytochemical Ingredients of the CPAE
3.2. The Antioxidant Effect of CPAE
3.3. Anti-Digestive Enzymes Effect of CPAE
3.4. The Hemolytic Effect of CPAE
3.5. Anti-Microbial Activity of CPAE
3.5.1. The Antibacterial Activity against Gram-Negative, Gram-Positive Bacteria and Antifungal Effect
3.5.2. Antiviral Activity of CPAE
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, F.; Sarker, M.M.R.; Ming, L.C.; Mohamed, I.N.; Zhao, C.; Sheikh, B.Y.; Tsong, H.F.; Rashid, M.A. Comprehensive review on phytochemicals, pharmacological and clinical potentials of gymnema sylvestre. Front. Pharmacol. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Fanzo, J.; Hunter, D.; Borelli, T.; Mattei, F. Diversifying Food and Diets: Using Agricultural Biodiversity to Improve Nutrition and Health; Routledge: London, UK, 2013; ISBN 1136461469. [Google Scholar]
- He, J.-H.; Chen, L.-X.; Li, H. Progress in the discovery of naturally occurring anti-diabetic drugs and in the identification of their molecular targets. Fitoterapia 2019, 134, 270–289. [Google Scholar] [CrossRef]
- Longhi, S.; Monteriù, A.; Freddi, A.; Aquilanti, L.; Ceravolo, M.G.; Carnevali, O.; Giordano, M.; Moroncini, G. The First Outstanding 50 Years of “Università Politecnica delle Marche”: Research Achievements in Life Sciences; Springer: Cham, Switzerland, 2020; ISBN 9783030338329. [Google Scholar]
- Sibanda, T.; Okoh, A.I. The challenges of overcoming antibiotic resistance: Plant extracts as potential sources of antimicrobial and resistance modifying agents. Afr. J. Biotechnol. 2007, 6, 1684–5315. [Google Scholar]
- Santonocito, D.; Granata, G.; Geraci, C.; Panico, A.; Siciliano, E.A.; Raciti, G.; Puglia, C. Carob seeds: Food waste or source of bioactive compounds? Pharmaceutics 2020, 12, 1090. [Google Scholar] [CrossRef]
- Fidan, H.; Stankov, S.; Petkova, N.; Petkova, Z.; Iliev, A.; Stoyanova, M.; Ivanova, T.; Zhelyazkov, N.; Ibrahim, S.; Stoyanova, A. Evaluation of chemical composition, antioxidant potential and functional properties of carob (Ceratonia siliqua L.) seeds. J. Food Sci. Technol. 2020, 57, 2404–2413. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, D.M.; El-Kersh, D.M.; Farag, M.A. Ceratonia siliqua (carob-locust bean) outgoing and potential trends of phytochemical, economic and medicinal merits. In Wild Fruits: Composition, Nutritional Value and Products; Springer: Cham, Switzerland, 2019; pp. 481–498. [Google Scholar]
- Zhu, B.-J.; Zayed, M.Z.; Zhu, H.-X.; Zhao, J.; Li, S.-P. Functional polysaccharides of carob fruit: A review. Chin. Med. 2019, 14, 1–10. [Google Scholar] [CrossRef]
- Zunft, H.J.F.; Lüder, W.; Harde, A.; Haber, B.; Graubaum, H.-J.; Gruenwald, J. Carob pulp preparation for treatment of hypercholesterolemia. Adv. Ther. 2001, 18, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Corsi, L.; Avallone, R.; Cosenza, F.; Farina, F.; Baraldi, C.; Baraldi, M. Antiproliferative effects of Ceratonia siliqua L. on mouse hepatocellular carcinoma cell line. Fitoterapia 2002, 73, 674–684. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Jabri, M.-A.; El-Benna, J.; Amri, M.; Marzouki, L.; Sebai, H. Protective effect of Ceratonia siliqua L. against a dextran sulfate sodium-induced alterations in liver and kidney in rat. J. Med. Food 2016, 19, 882–889. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Grami, D.; Amri, M.; Eto, B.; El-Benna, J.; Sebai, H.; Marzouki, L. Chemical constituents and pharmacological actions of carob pods and leaves (Ceratonia siliqua L.) on the gastrointestinal tract: A review. Biomed. Pharmacother. 2017, 93, 522–528. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Taga, M.S.; Miller, E.E.; Pratt, D.E. Chia seeds as a source of natural lipid antioxidants. J. Am. Oil Chem. Soc. 1984, 61, 928–931. [Google Scholar] [CrossRef]
- Ayoola, G.A.; Coker, H.A.; Adesegun, S.A.; Adepoju-Bello, A.A.; Obaweya, K.; Ezennia, E.C.; Atangbayila, T.O. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 2008, 7, 1019–1024. [Google Scholar]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Hedge, J.E.; Hofreiter, B.T. Determination of total carbohydrate by anthrone method. In Carbohydrate Chemistry; Academic Press: New York, NY, USA, 1962; Volume 17. [Google Scholar]
- Moore, S.; Stein, W.H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem. 1954, 211, 907–913. [Google Scholar] [CrossRef]
- Bernfeld, P. Enzymes of starch degradation and synthesis. Adv. Enzymol. Relat. Subj. Biochem. 1951, 12, 379–428. [Google Scholar] [PubMed]
- Denneberg, T.; Lindberg, T.; Berg, N.O.; Dahlqvist, A. Morphology, dipeptidases and disaccharidases of small intestinal mucosa in chronic renal failure. Acta Med. Scand. 1974, 195, 465–470. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Sutharsingh, R.; Kavimani, S.; Jayakar, B.; Uvarani, M.; Thangathirupathi, A. Quantitative phytochemical estimation and antioxidant studies on aerial parts of Naravelia zeylanica DC. Int. J. Pharm. Stud. Res. 2011, 2, 52–56. [Google Scholar]
- Garratt, D.C. The Quantitative Analysis of Drugs; Springer Science & Business Media: New York, NY, USA, 2012; ISBN 1461333806. [Google Scholar]
- Wikler, M.A. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standard. Clin. Lab. Stand. Inst. 2006, 26, M7-A7. [Google Scholar]
- Hu, J.M.; Hsiung, G.D. Evaluation of new antiviral agents: I. In vitro perspectives. Antivir. Res. 1989, 11, 217–232. [Google Scholar] [CrossRef]
- Ayache, S.B.; Saafi, E.B.; Emhemmed, F.; Flamini, G.; Achour, L.; Muller, C.D. Biological activities of aqueous extracts from Carob. Molecules 2020, 25, 1–16. [Google Scholar]
- Nawel, O. Phytochemical analysis and antioxidant activity of the flavonoids extracts from pods of Ceratonia siliqua L. J. Pharm. Pharm. 2017, 4, 159–165. [Google Scholar] [CrossRef]
- Al-Olayan, E.M.; El-Khadragy, M.F.; Alajmi, R.A.; Othman, M.S.; Bauomy, A.A.; Ibrahim, S.R.; Abdel Moneim, A.E. Ceratonia siliqua pod extract ameliorates Schistosoma mansoni-induced liver fibrosis and oxidative stress. BMC Complement. Altern. Med. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lakkab, I.; El Hajaji, H.; Lachkar, N.; Lefter, R.; Ciobica, A.; El Bali, B.; Lachkar, M. Ceratonia siliqua L. seed peels: Phytochemical profile, antioxidant activity, and effect on mood disorders. J. Funct. Foods 2019, 54, 457–465. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Torun, H.; Glew, R.H.; Bak, Z.D.; Chuang, L.T.; Presley, J.M.; Andrews, R. Nutrient content of carob pod (Ceratonia siliqua L.) flour prepared commercially and domestically. Plant Foods Hum. Nutr. 2009, 64, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Torun, H.; Ayaz, F.A.; Colak, N.; Grúz, J.; Strnad, M. Phenolic acid content and free radical-scavenging activity of two differently processed Carob tree (Ceratonia siliqua L.) Pod. Food Nutr. Sci. 2013, 4, 547–553. [Google Scholar] [CrossRef][Green Version]
- Rtibi, K.; Jabri, M.A.; Selmi, S.; Souli, A.; Sebai, H.; El-Benna, J.; Amri, M.; Marzouki, L. Carob pods (Ceratonia siliqua L.) inhibit human neutrophils myeloperoxidase and in vitro ROS-scavenging activity. RSC Adv. 2015, 5, 84207–84215. [Google Scholar] [CrossRef]
- Yang, J.H.; Zhang, L.; Li, J.S.; Chen, L.H.; Zheng, Q.; Chen, T.; Chen, Z.P.; Fu, T.M.; Di, L.Q. Enhanced oral bioavailability and prophylactic effects on oxidative stress and hepatic damage of an oil solution containing a rosmarinic acid-phospholipid complex. J. Funct. Foods 2015, 19, 63–73. [Google Scholar] [CrossRef]
- Goulas, V.; Georgiou, E. Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: Extraction optimization and application in food models. Foods 2020, 9, 20. [Google Scholar] [CrossRef]
- El Bouzdoudi, B.; El Ansari, Z.N.; Mangalagiu, I.; Mantu, D.; Badoc, A.; Lamarti, A. Determination of polyphenols content in carob pulp from wild and domesticated Moroccan trees. Am. J. Plant Sci. 2016, 7, 1937–1951. [Google Scholar] [CrossRef]
- Qasem, M.A.; Noordin, M.I.; Arya, A.; Alsalahi, A.; Jayash, S.N. Evaluation of the glycemic effect of Ceratonia siliqua pods (Carob) on a streptozotocin-nicotinamide induced diabetic rat model. PeerJ 2018, 2018, 29844959. [Google Scholar]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta-Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Abidar, S.; Boiangiu, R.S.; Dumitru, G.; Todirascu-Ciornea, E.; Amakran, A.; Cioanca, O.; Hritcu, L.; Nhiri, M. The aqueous extract from ceratonia siliqua leaves protects against 6-hydroxydopamine in zebrafish: Understanding the underlying mechanism. Antioxidants 2020, 9, 304. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Ouchemoukh, S.; Meziant, N.; Idiri, Y.; Hernanz, D.; Stinco, C.M.; Rodríguez-Pulido, F.J.; Heredia, F.J.; Madani, K.; Luis, J. Bioactive metabolites involved in the antioxidant, anticancer and anticalpain activities of Ficus carica L., Ceratonia siliqua L. and Quercus ilex L. extracts. Ind. Crops Prod. 2017, 95, 6–17. [Google Scholar] [CrossRef]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell. Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef]
- Tengan, C.H.; Moraes, C.T. NO control of mitochondrial function in normal and transformed cells. Biochim. Biophys. Acta (BBA)-Bioenerg. 2017, 1858, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Martín-Diana, A.B.; Martínez-Villaluenga, C.; Aguirre, L.; Silván, J.M.; Dueñas, M.; De Luis, D.A.; Lasa, A. In vitro approach for evaluation of carob by-products as source bioactive ingredients with potential to attenuate metabolic syndrome (MetS). Heliyon 2019, 5, e01175. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G.; Olabiy, A.A. Nutritional, antioxidant and inhibitory properties of cocoa powder enriched wheat-plantain biscuits on key enzymes linked to type 2 diabetes. Int. Food Res. J. 2018, 25, 793–803. [Google Scholar]
- Shim, Y.-J.; Doo, H.-K.; Ahn, S.-Y.; Kim, Y.-S.; Seong, J.-K.; Park, I.-S.; Min, B.-H. Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J. Ethnopharmacol. 2003, 85, 283–287. [Google Scholar] [CrossRef]
- Lopes, G.; Andrade, P.B.; Valentão, P. Phlorotannins: Towards new pharmacological interventions for diabetes mellitus type 2. Molecules 2017, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Patarra, J.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. In vitro antioxidant and inhibitory activity of water decoctions of carob tree (Ceratonia siliqua L.) on cholinesterases, α-amylase and α-glucosidase. Nat. Prod. Res. 2015, 29, 2155–2159. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Anis, B.H.; Mohamed, T.; Raoudha, M.J.; Mohamed, D.; Samir, J. Identification of phenolic compounds by high performance liquid chromatography/mass spectrometry (HPLC/MS) and in vitro evaluation of the antioxidant and antimicrobial activities of Ceratonia siliqua leaves extracts. J. Med. Plants Res. 2015, 9, 479–485. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phyther. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Tassou, C.C.; Drosinos, E.H.; Nychas, G.-J. Weak antimicrobial effect of carob (Ceratonia siliqua) extract against food-related bacteria in culture media and model food systems. World J. Microbiol. Biotechnol. 1997, 13, 479–481. [Google Scholar] [CrossRef]
- Shao, D.; Li, J.; Li, J.; Tang, R.; Liu, L.; Shi, J.; Huang, Q.; Yang, H. Inhibition of gallic acid on the growth and biofilm formation of Escherichia coli and Streptococcus mutans. J. Food Sci. 2015, 80, M1299–M1305. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential use of phenolic acids as anti-Candida agents: A review. Front. Microbiol. 2015, 6, 1420. [Google Scholar]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.M.S.; Shedeed, N.A.; Abdel-Kalek, H.H.; El-Din, M.H.A.S. Antioxidative, antibacterial and antifungal activities of tea infusions from berry leaves, carob and doum. Pol. J. Food Nutr. Sci. 2011, 61, 201–209. [Google Scholar] [CrossRef]
- Kratz, J.M.; Andrighetti-Fröhner, C.R.; Kolling, D.J.; Leal, P.C.; Cirne-Santos, C.C.; Yunes, R.A.; Nunes, R.J.; Trybala, E.; Bergström, T.; Frugulhetti, I.C.P.P. Anti-HSV-1 and anti-HIV-1 activity of gallic acid and pentyl gallate. Mem. Inst. Oswaldo Cruz 2008, 103, 437–442. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Concentration |
|---|---|
| Total flavonoids (Rutin/100 mg CPAE) | 0.752 ± 0.11 |
| Total phenolics (GA/100 mg CPAE) | 0.348 ± 0.07 |
| Total amino acids (mg/mL) | 43.28 ± 2.1 |
| Total alkaloids (mg/mL) | 15.3 ± 0.98 |
| Total carbohydrates (mg/mL) | 5.1 ± 0.23 |
| Compound | Concentration (µg/g) | Retention Time |
|---|---|---|
| Gallic acid | 833.751 | 3.74 |
| Catechin | 180.197 | 11.67 |
| Protocatechuic acid | 110.576 | 7.98 |
| Cinnamic acid | 23.335 | 39.7 |
| p-coumaric acid | 12.707 | 36.42 |
| Rutin | 10.983 | 35.9 |
| Gentisic acid | 3.487 | 7.04 |
| p-hydroxybenzoic acid | 2.731 | 9.88 |
| Vanillic acid | 2.261 | 17.47 |
| Ferulic acid | 2.038 | 20.79 |
| Parameters | CPAE IC50 (mg/mL) | Ascorbic Acid IC50 (mg/mL) |
|---|---|---|
| DPPH scavenging activity | 3.78 ± 0.08 * | 1.59 ± 0.01 |
| Ferric reducing activity | 6.30 ± 0.11 * | 4.21 ± 0.25 |
| NO scavenging activity | 4.33 ± 0.06 * | 3.48 ± 0.31 |
| •OH, scavenging activity | 7.07 ± 0.12 * | 1.32 ± 0.01 |
| Pathogenic Microorganism | Inhibition Zoon (mm) | |
|---|---|---|
| CPAE | Postive Control | |
| Bacillus subtilis (ATCC 6051) | 17.0 ± 0.12 * | 25.0 ± 0.3 |
| Staph. aureus (ATCC 6538) | 25.0 ± 0.2 * | 15.0 ± 0.11 |
| Escherichia coli (ATCC 8739) | 24.0 ± 0.3 * | 17.0 ± 0.09 |
| Pseudomonas aeruginosa (ATCC 90274) | N.A | 22.0 ± 0.12 |
| Candida albicans (ATCC 10221) | 15 ± 0.13 | N.A |
| Aspergillus falvus (ATCC 9643) | N.A | N.A |
| Concentration (µg/mL) | Viability % |
|---|---|
| 10,000 | 39.4 ± 0.01 a |
| 5000 | 96.5 ± 0.05 b |
| 2500 | 97.4 ± 0.02 b |
| 1250 | 99.0 ± 0.04 c |
| 625 | 99.8 ± 0.05 c |
| 312.5 | 100.0 ± 0.08 c |
| IC50 | 9154.22 |
| μg/mL | Viability | Antiviral Effect % |
|---|---|---|
| 0 | 37.94 | 0.00 |
| 1250 | 40.78 | 4.58 ± 0.01 a |
| 2500 | 50.40 | 20.08 ± 0.25 b |
| 5000 | 66.80 | 46.50 ± 1.5 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, W.S.; Khadr, A.E.S.; Kamel, M.A.E.N.; Abd Eldaim, M.A.; El Sayed, I.E.T.; Abdel-Bary, H.M.; Ullah, S.; Ghareeb, D.A. Phytochemical Characterization and Evaluation of Biological Activities of Egyptian Carob Pods (Ceratonia siliqua L.) Aqueous Extract: In Vitro Study. Plants 2021, 10, 2626. https://doi.org/10.3390/plants10122626
Darwish WS, Khadr AES, Kamel MAEN, Abd Eldaim MA, El Sayed IET, Abdel-Bary HM, Ullah S, Ghareeb DA. Phytochemical Characterization and Evaluation of Biological Activities of Egyptian Carob Pods (Ceratonia siliqua L.) Aqueous Extract: In Vitro Study. Plants. 2021; 10(12):2626. https://doi.org/10.3390/plants10122626
Chicago/Turabian StyleDarwish, Wael Sobhy, Abada El Sayed Khadr, Maher Abd El Naby Kamel, Mabrouk A. Abd Eldaim, Ibrahim El Tantawy El Sayed, Hamed Mohamed Abdel-Bary, Sami Ullah, and Doaa Ahmed Ghareeb. 2021. "Phytochemical Characterization and Evaluation of Biological Activities of Egyptian Carob Pods (Ceratonia siliqua L.) Aqueous Extract: In Vitro Study" Plants 10, no. 12: 2626. https://doi.org/10.3390/plants10122626
APA StyleDarwish, W. S., Khadr, A. E. S., Kamel, M. A. E. N., Abd Eldaim, M. A., El Sayed, I. E. T., Abdel-Bary, H. M., Ullah, S., & Ghareeb, D. A. (2021). Phytochemical Characterization and Evaluation of Biological Activities of Egyptian Carob Pods (Ceratonia siliqua L.) Aqueous Extract: In Vitro Study. Plants, 10(12), 2626. https://doi.org/10.3390/plants10122626








