Grey and Black Anti-Hail Nets Ameliorated Apple (Malus × domestica Borkh. cv. Golden Delicious) Physiology under Mediterranean Climate
Abstract
:1. Introduction
2. Results
2.1. Glomalin-Related Soil Proteins
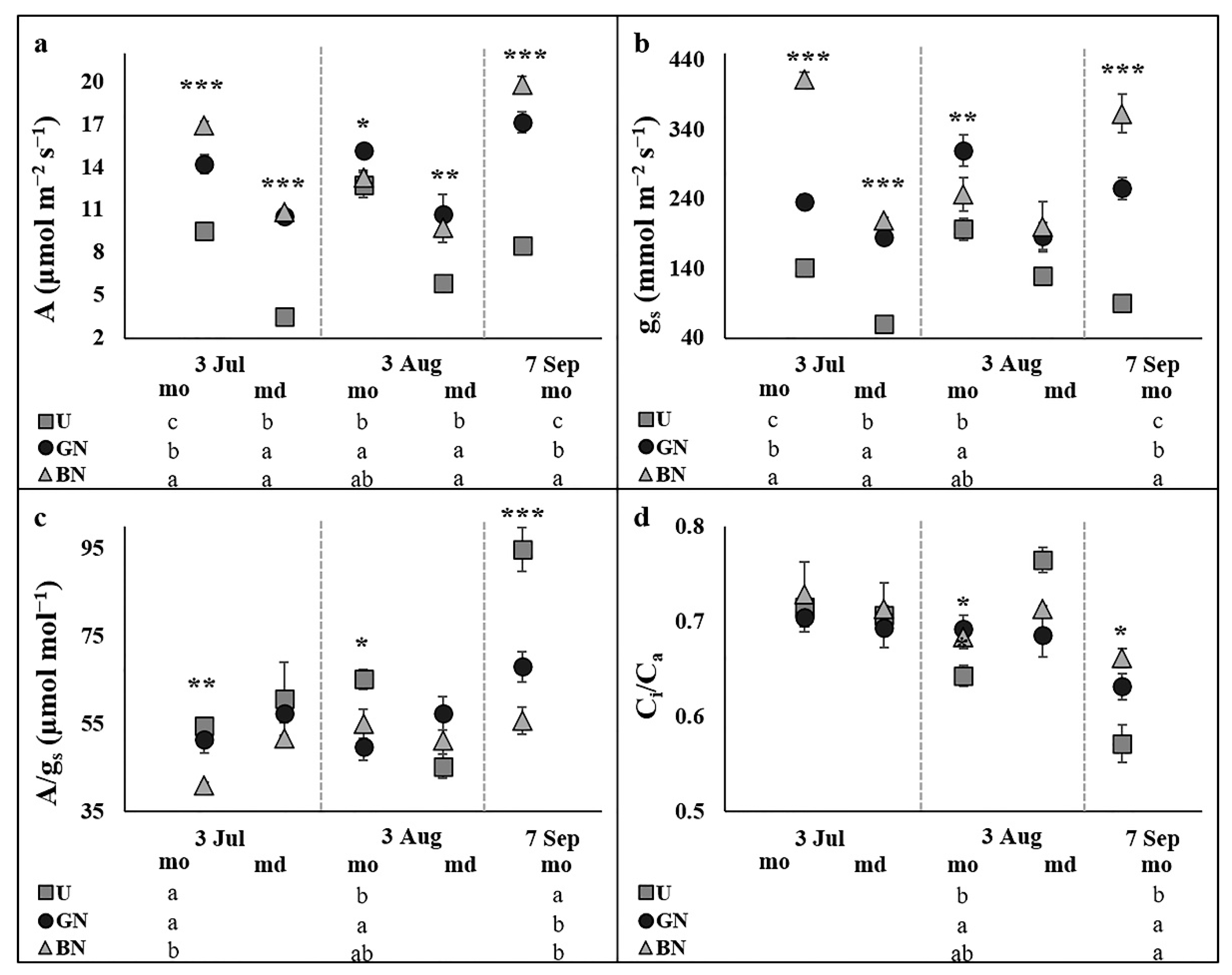
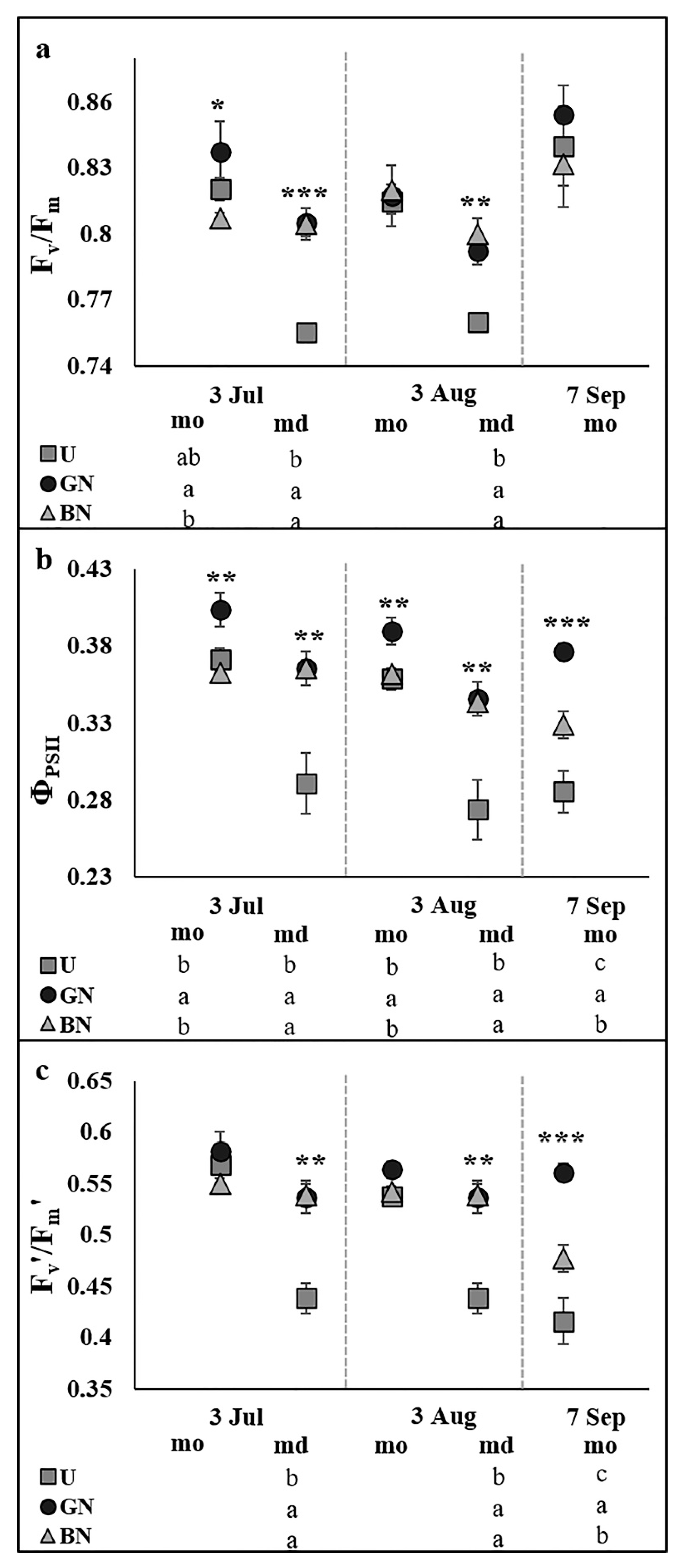
2.2. Leaf Gas Exchange and Chlorophyll a Fluorescence
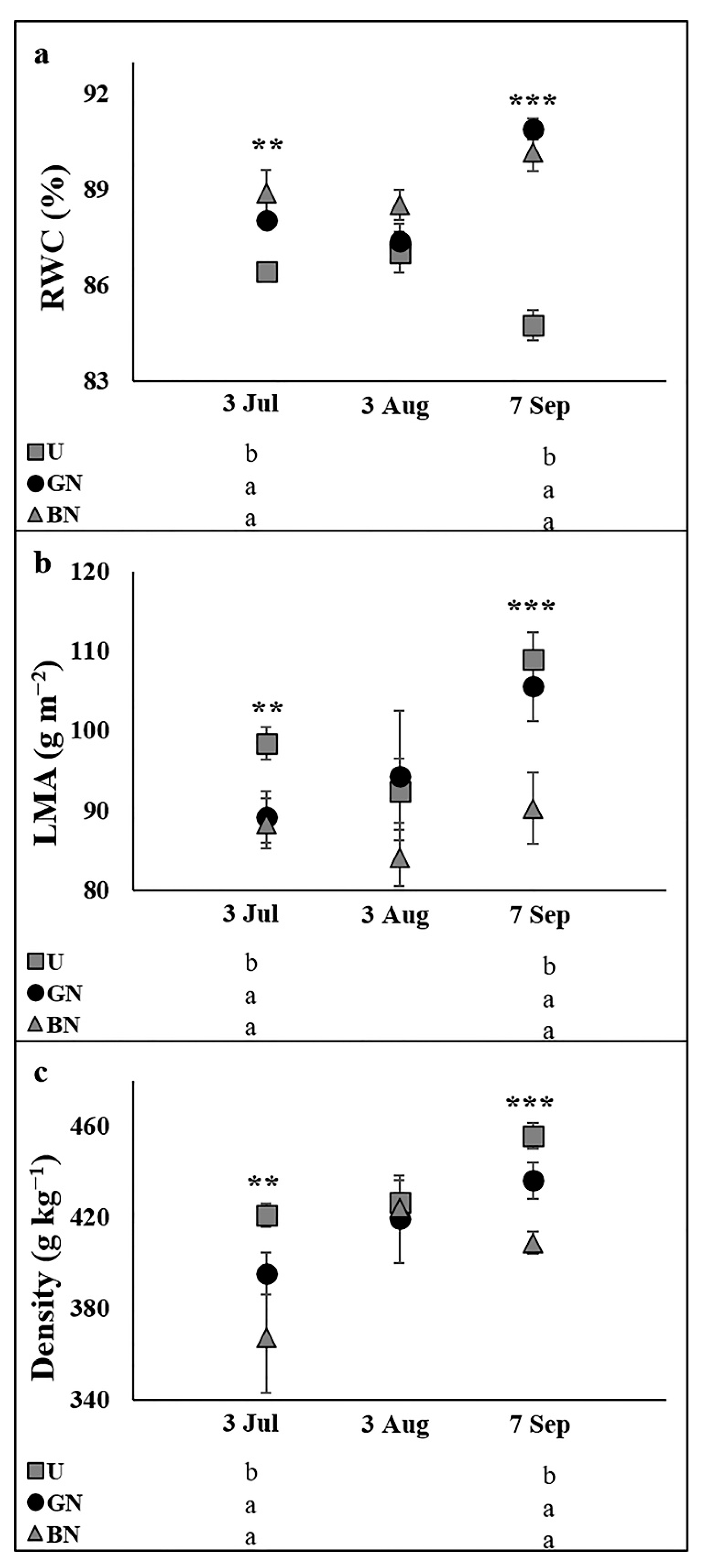
2.3. Leaf Water Status and Sclerophylly Indexes
2.4. Leaf Biochemical Analysis
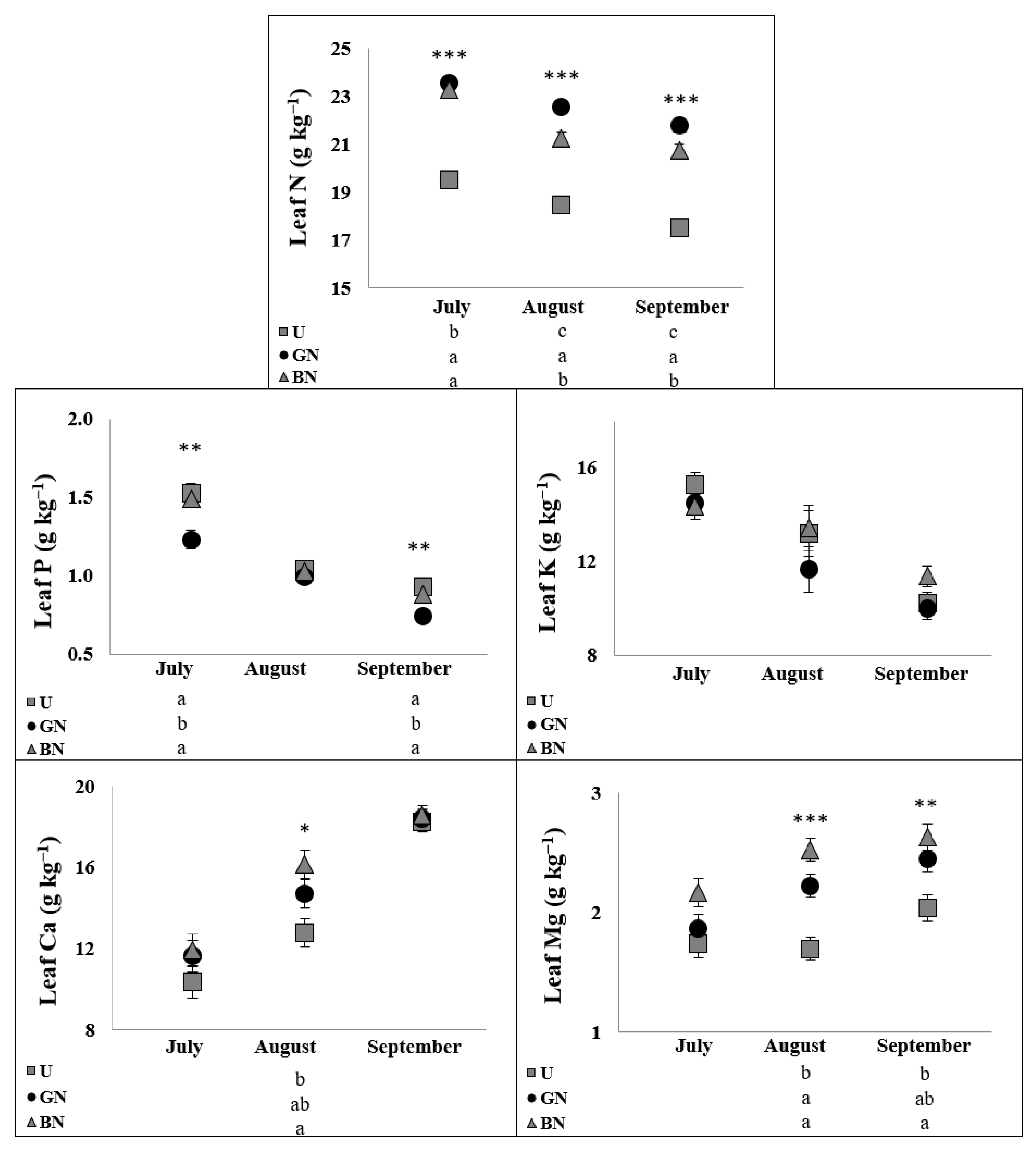
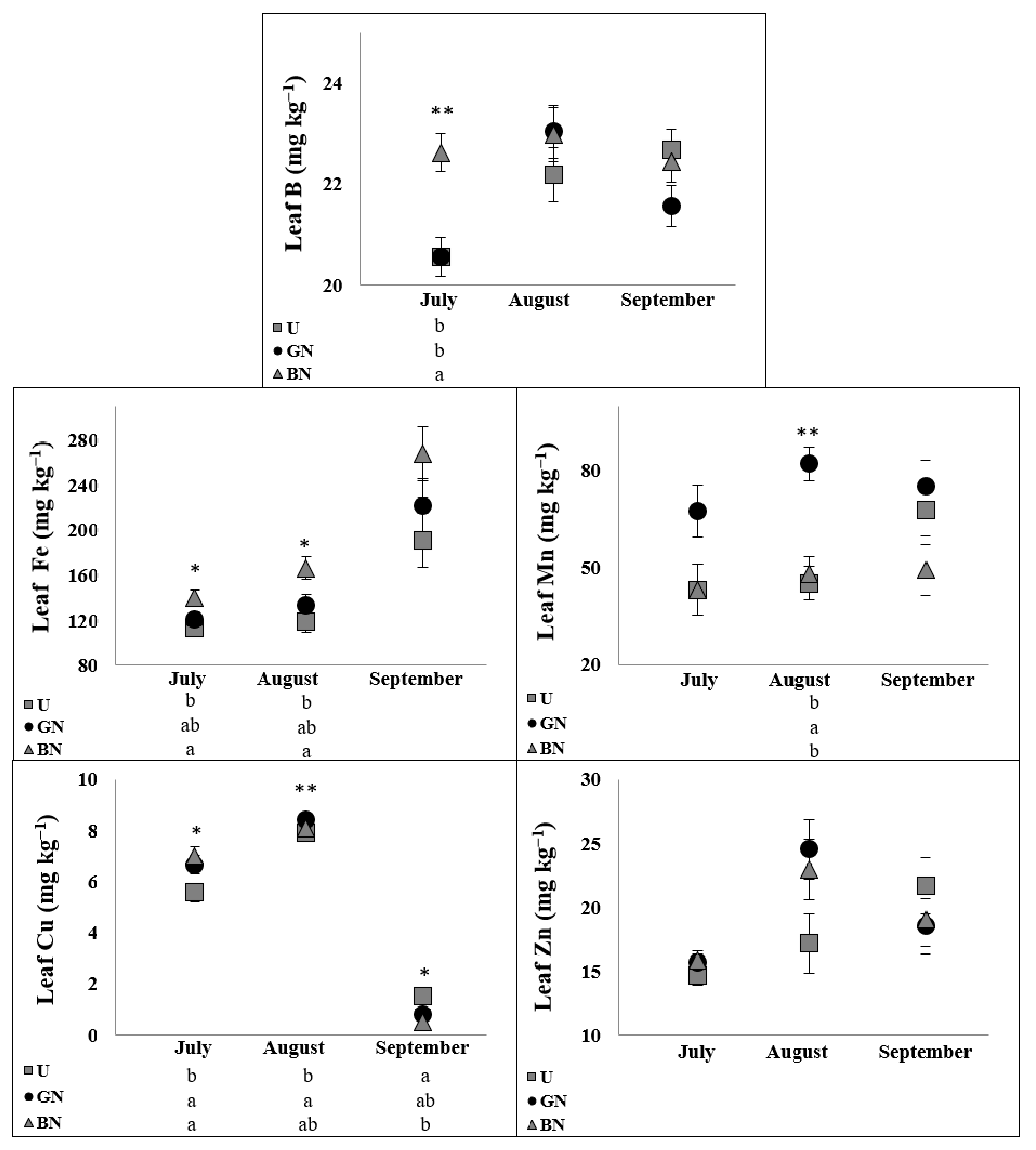
2.5. Leaf Ionome
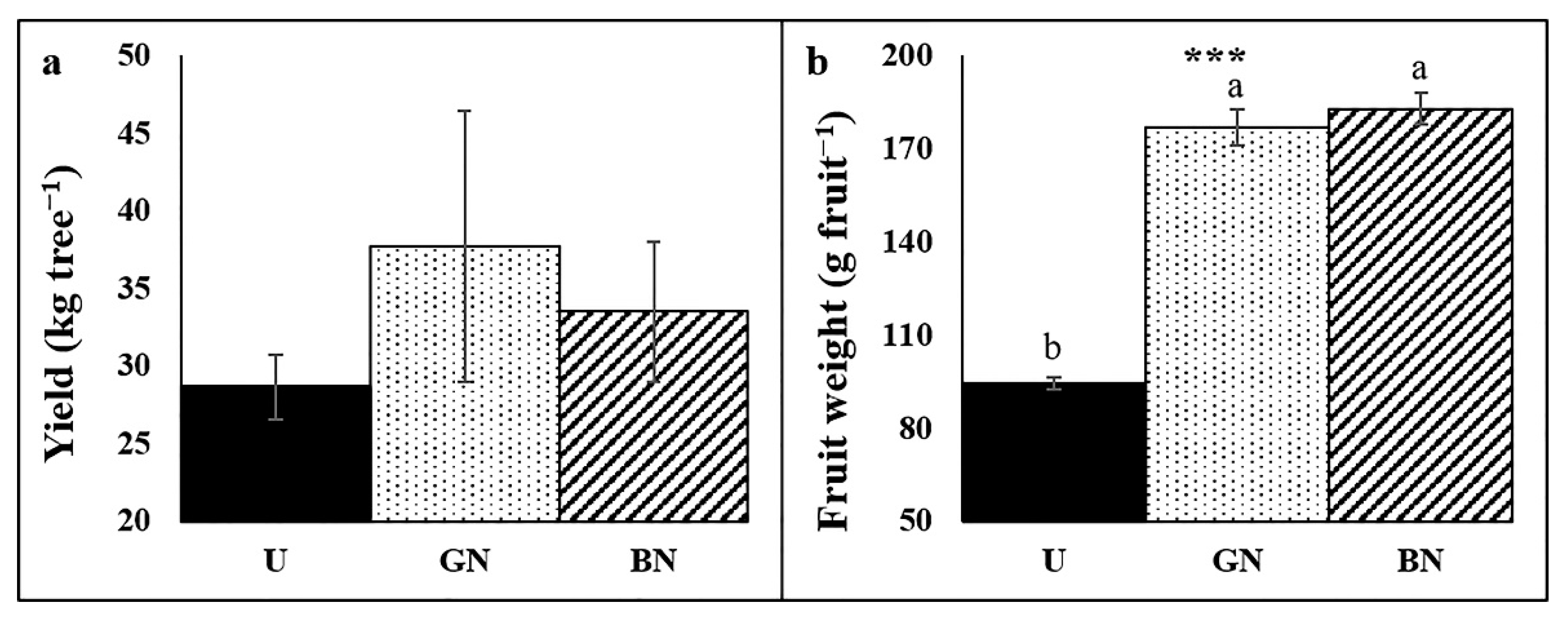
2.6. Trees Yield and Fruit Weight
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Experimental Design and Monitoring
4.3. Glomalin-Related Soil Proteins
4.4. Leaf Gas Exchange and Chlorophyll a Fluorescence
4.5. Leaf Water Status and Sclerophylly Indexes
4.6. Leaf Biochemical Analysis
4.7. Leaf Ionome
4.8. Yield and Fruit Weight
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of The United Nations—Statistics Division. 2021. Available online: http://www.fao.org/faostat/en/#home (accessed on 8 October 2021).
- Bosco, L.C.; Bergamaschi, H.; Cardoso, L.S.; de Paula, V.A.; Marodin, G.A.B.; Brauner, P.C. Microclimate alterations caused by agricultural hail net coverage and effects on apple tree yield in subtropical climate of Southern Brazil. Bragantia 2018, 77, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Kalcsits, L.; Musacchi, S.; Layne, D.R.; Schmidt, T.; Mupambi, G.; Serra, S.; Mendoza, M.; Asteggiano, L.; Jarolmasjed, S.; Sankaran, S.; et al. Above and below-ground environmental changes associated with the use of photoselective protective netting to reduce sunburn in apple. Agric. For. Meteorol. 2017, 237–238, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress—From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2012; pp. 1–33. [Google Scholar]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- do Amarante, C.V.T.; Steffens, C.A.; Argenta, L.C. Yield and fruit quality of ‘Gala’ and ‘Fuji’ apple trees protected by white anti-hail net. Sci. Hortic. 2011, 129, 79–85. [Google Scholar] [CrossRef]
- Racsko, J.; Schrader, L.E. Sunburn of Apple Fruit: Historical Background, Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2012, 31, 455–504. [Google Scholar] [CrossRef]
- Naor, A.; Naschitz, S.; Peres, M.; Gal, Y. Responses of apple fruit size to tree water status and crop load. Tree Physiol. 2008, 28, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Girona, J.; Behboudian, M.H.; Mata, M.; Del Campo, J.; Marsal, J. Effect of hail nets on the microclimate, irrigation requirements, tree growth, and fruit yield of peach orchards in Catalonia (Spain). J. Hortic. Sci. Biotechnol. 2012, 87, 545–550. [Google Scholar] [CrossRef]
- Bosco, L.C.; Bergamaschi, H.; Marodin, G.A.B. Solar radiation effects on growth, anatomy, and physiology of apple trees in a temperate climate of Brazil. Int. J. Biometeorol. 2020, 64, 1969–1980. [Google Scholar] [CrossRef]
- Mupambi, G.; Anthony, B.M.; Layne, D.R.; Musacchi, S.; Serra, S.; Schmidt, T.; Kalcsits, L.A. The influence of protective netting on tree physiology and fruit quality of apple: A review. Sci. Hortic. 2018, 236, 60–72. [Google Scholar] [CrossRef]
- Sever, M.B.; Tojnko, S.; Breznikar, A.; Babojelić, M.S.; Ivančič, A.; Sirk, M.; Unuk, T. The influence of differently coloured anti-hail nets and geomorphologic characteristics on microclimatic and light conditions in apple orchards. J. Cent. Eur. Agric. 2020, 21, 386–397. [Google Scholar] [CrossRef]
- Solomakhin, A.; Blanke, M. The microclimate under coloured hailnets affects leaf and fruit temperature, leaf anatomy, vegetative and reproductive growth as well as fruit colouration in apple. Ann. Appl. Biol. 2010, 156, 121–136. [Google Scholar] [CrossRef]
- McCaskill, M.R.; McClymont, L.; Goodwin, I.; Green, S.; Partington, D.L. How hail netting reduces apple fruit surface temperature: A microclimate and modelling study. Agric. For. Meteorol. 2016, 226–227, 148–160. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. (Eds.) IPCC: Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; in press. [Google Scholar]
- Singh, P.K.; Singh, M.; Tripathi, B.N. Glomalin: An arbuscular mycorrhizal fungal soil protein. Protoplasma 2013, 250, 663–669. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.; He, Y.; Chang, Y. Glomalin-related soil protein in the rhizosphere of Robinia pseudoacacia L. seedlings under higher air temperature combined with Cd-contaminated soil. Eur. J. Soil Sci. 2018, 69, 634–645. [Google Scholar] [CrossRef]
- Iglesias, I.; Alegre, S. The effect of anti-hail nets on fruit protection, radiation, temperature, quality and profitability of ‘Mondial Gala’ apples. J. Appl. Hortic. 2006, 8, 91–100. [Google Scholar] [CrossRef]
- Solomakhin, A.; Blanke, M.M. Coloured hailnets alter light transmission, spectra and phytochrome, as well as vegetative growth, leaf chlorophyll and photosynthesis and reduce flower induction of apple. Plant Growth Regul. 2008, 56, 211–218. [Google Scholar] [CrossRef]
- Lopez, G.; Boini, A.; Manfrini, L.; Torres-Ruiz, J.M.; Pierpaoli, E.; Zibordi, M.; Losciale, P.; Morandi, B.; Corelli-Grappadelli, L. Effect of shading and water stress on light interception, physiology and yield of apple trees. Agric. Water Manag. 2018, 210, 140–148. [Google Scholar] [CrossRef]
- Mészáros, M.; Bělíková, H.; Čonka, P.; Náměstek, J. Effect of hail nets and fertilization management on the nutritional status, growth and production of apple trees. Sci. Hortic. 2019, 255, 134–144. [Google Scholar] [CrossRef]
- Stroka, M.A.; Ayub, R.A.; da Silva, D.M.; Pessenti, I.L.; Pereira, A.B.; Barbosa, E.A.A. Effect of anti-hail nets with different colors on ‘Eva’ apple trees agronomical responses. Rev. Bras. Frutic. 2021, 43, e-157. [Google Scholar] [CrossRef]
- Dussi, M.C.; Giardina, G.; Sosa, D.; Junyent, R.G.; Zecca, A.; Reeb, P.R. Shade nets effect on canopy light distribution and quality of fruit and spur leaf on apple cv. Fuji. Span. J. Agric. Res. 2005, 3, 253–260. [Google Scholar] [CrossRef]
- Olivares-Soto, H.; Bastías, R.M. Photosynthetic efficiency of apples under protected shade nets. Chil. J. Agric. Res. 2018, 78, 126–138. [Google Scholar] [CrossRef]
- Nicolás, E.; Torrecillas, A.; Amico, J.D.; Alarcón, J.J. Sap flow, gas exchange, and hydraulic conductance of young apricot trees growing under a shading net and different water supplies. J. Plant Physiol. 2005, 162, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Duman, F. Uptake of mineral elements during abiotic stress. In Abiotic Stress Response in Plants—Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012; pp. 267–282. [Google Scholar]
- Shahak, Y.; Ratner, K.; Giller, Y.E.; Zur, N.; Or, E.; Gussakovsky, E.E.; Stern, R.; Sarig, P.; Raban, E.; Harcavi, E.; et al. Improving Solar Energy Utilization, Productivity and Fruit Quality in Orchards and Vineyards by Photoselective Netting. Acta Hortic. 2008, 772, 65–72. [Google Scholar] [CrossRef]
- Rillig, M.C.; Steinberg, P.D. Glomalin production by an arbuscular mycorrhizal fungus: A mechanism of habitat modification? Soil Biol. Biochem. 2002, 34, 1371–1374. [Google Scholar] [CrossRef]
- Purin, S.; Rillig, M.C. The arbuscular mycorrhizal fungal protein glomalin: Limitations, progress, and a new hypothesis for its function. Pedobiologia 2007, 51, 123–130. [Google Scholar] [CrossRef]
- Gadkar, V.; Rillig, M.C. The arbuscular mycorrhizal fungal protein glomalin is a putative homolog of heat shock protein 60. FEMS Microbiol. Lett. 2006, 263, 93–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, Z.; Azadi, N.; Rahimi, S.; Certini, G. The response of glomalin-related soil proteins to fire or tillage. Geoderma 2018, 329, 65–72. [Google Scholar] [CrossRef]
- Bhagat, K.P.; Kumar, R.A.; Ratnakumar, P.; Kumar, S.; Bal, S.K.; Agrawal, P.K. Photosynthesis and Associated Aspects Under Abiotic Stresses Environment. In Approaches to Plant Stress and Their Management; Gaur, R.K., Sharma, P., Eds.; Springer: New Delhi, India, 2014; pp. 191–205. [Google Scholar] [CrossRef]
- Tanny, J.; Cohen, S.; Grava, A.; Naor, A.; Lukyanov, V. The effect of shading screens on microclimate of apple orchards. Acta Hortic. 2009, 103–108. [Google Scholar] [CrossRef]
- Zhang, D.; Du, Q.; Zhang, Z.; Jiao, X.; Song, X.; Li, J. Vapour pressure deficit control in relation to water transport and water productivity in greenhouse tomato production during summer. Sci. Rep. 2017, 7, 43461. [Google Scholar] [CrossRef]
- Glenn, D.M.; Cooley, N.; Walker, R.; Clingeleffer, P.; Shellie, K. Impact of Kaolin Particle Film and Water Deficit on Wine Grape Water Use Efficiency and Plant Water Relations. HortScience 2010, 45, 1178–1187. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Ferreira, H.; Rocha, L.; Pavia, I.; Moutinho-Pereira, J.; Correia, C.M. Kaolin particle film modulates morphological, physiological and biochemical olive tree responses to drought and rewatering. Plant Physiol. Biochem. 2018, 133, 29–39. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Luzio, A.; Silva, E.; Gonçalves, A.; Meijón, M.; Escandón, M.; Arrobas, M.; Rodrigues, M.A.; Moutinho-Pereira, J.; et al. Kaolin and salicylic acid alleviate summer stress in rainfed olive orchards by modulation of distinct physiological and biochemical responses. Sci. Hortic. 2018, 246, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rom, C.R. Light Thresholds for Apple Tree Canopy Growth and Development. HortScience 1991, 26, 989–992. [Google Scholar] [CrossRef] [Green Version]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahak, Y.; Gussakovsky, E.E.; Gal, E.; Ganelevin, R. Colornets: Crop Protection and Light-Quality Manipulation in One Technology. Acta Hortic. 2004, 659, 143–151. [Google Scholar] [CrossRef]
- Bastías, R.M.; Manfrini, L.; Grappadelli, L.C. Exploring the potential use of photo-selective nets for fruit growth regulation in apple. Chileanjar 2012, 72, 224–231. [Google Scholar] [CrossRef]
- Zeng, G.; Guo, Y.; Xu, J.; Hu, M.; Zheng, J.; Wu, Z. Partial shade optimizes photosynthesis and growth in bayberry (Myrica rubra) trees. Hortic. Environ. Biotechnol. 2017, 58, 203–211. [Google Scholar] [CrossRef]
- Glenn, D.M.; Erez, A.; Puterka, G.J.; Gundrum, P. Particle Films Affect Carbon Assimilation and Yield in ‘Empire’ Apple. J. Am. Soc. Hortic. Sci. 2003, 128, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Jifon, J.L.; Syvertsen, J.P. Kaolin Particle Film Applications Can Increase Photosynthesis and Water Use Efficiency of ‘Ruby Red’ Grapefruit Leaves. J. Am. Soc. Hortic. Sci. 2003, 128, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Dinis, L.-T.; Bernardo, S.; Luzio, A.; Pinto, G.; Meijón, M.; Pintó-Marijuan, M.; Cotado, A.; Correia, C.; Moutinho-Pereira, J. Kaolin modulates ABA and IAA dynamics and physiology of grapevine under Mediterranean summer stress. J. Plant Physiol. 2018, 220, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-J.; Guo, Y.-P.; Shen, Y.-G.; Guo, D.-P.; Li, D.-Y. Midday depression of photosynthesis and effects of mist spray in citrus. Ann. Appl. Biol. 2009, 154, 143–155. [Google Scholar] [CrossRef]
- Lebese, T.C.; Stassen, P.J.C.; Midgley, S.J.E. Photosynthetic capacity and diurnal gas exchange of ‘Brookfield Gala’ apple leaves under three irrigation systems. S. Afr. J. Plant Soil 2011, 28, 55–63. [Google Scholar] [CrossRef]
- Bhusal, N.; Bhusal, S.J.; Yoon, T.-M. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Xu, D.Q.; Shen, Y.G. External and internal factors responsible for midday depression of photosynthesis. In Handbook of Photosynthesis; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 287–294. [Google Scholar]
- Hendry, G.A.F.; Price, A.H. Stress indicators: Chlorophylls and carotenoids. In Methods in Comparative Plant Ecology; Hendry, G.A.F., Grime, J.P., Eds.; Chapman & Hall: London, UK, 1993; pp. 148–152. [Google Scholar]
- Lichtenthaler, H.K.; Ac, A.; Marek, M.V.; Kalina, J.; Urban, O. Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; Laghari, H.L.; Bhabhan, G.M.; Talpur, K.H.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–218. [Google Scholar]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 307–321. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- de Wit, M.; Keuskamp, D.H.; Bongers, F.J.; Hornitschek, P.; Gommers, C.M.; Reinen, E.; Martínez-Cerón, C.; Fankhauser, C.; Pierik, R. Integration of Phytochrome and Cryptochrome Signals Determines Plant Growth during Competition for Light. Curr. Biol. 2016, 26, 3320–3326. [Google Scholar] [CrossRef] [Green Version]
- Basile, B.; Romano, R.; Giaccone, M.; Barlotti, E.; Colonna, V.; Cirillo, C.; Shahak, Y.; Forlani, M. Use of Photo-Selective Nets for Hail Protection of Kiwifruit Vines in Southern Italy. Acta Hortic. 2008, 770, 185–192. [Google Scholar] [CrossRef]
- Shahak, Y.; Kong, Y.; Ratner, K. The wonders of yellow netting. Acta Hortic. 2016, 1134, 327–334. [Google Scholar] [CrossRef]
- IPMA. Instituto Português do Mar e da Atmosfera. Available online: http://www.ipma.pt/pt/oclima/normais.clima/ (accessed on 8 October 2021).
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Ferreira, H.; Moutinho-Pereira, J.; Correia, C. The role of nighttime water balance on Olea europaea plants subjected to contrasting water regimes. J. Plant Physiol. 2018, 226, 56–63. [Google Scholar] [CrossRef]
- Bilger, W.; Schreiber, U. Energy-dependent quenching of dark-level chlorophyll fluorescence in intact leaves. Photosyn. Res. 1986, 10, 303–308. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sesták, Z.; Castky, J.; Jarvis, P.G. Plant photosynthetic production. In Manual of Methods; Dr. W. Junk Publishers: The Hague, The Netherlands, 1991. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; pp. 350–382. [Google Scholar]
- Irigoyen, J.J.; Einerich, D.W.; Sanchez-Diaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
| Total Extractable Glomalin-Related Soil Proteins | Easily Extractable Glomalin-Related Soil Proteins | |
|---|---|---|
| U | 2.12 ± 0.31 a | 0.736 ± 0.082 a |
| GN | 0.751 ± 0.073 b | 0.418 ± 0.048 b |
| BN | 0.461 ± 0.024 b | 0.289 ± 0.057 b |
| Sig | *** | *** |
| U | GN | BN | Sig | |
|---|---|---|---|---|
| TSS | 84.2 ± 2.5 | 79.9 ± 6.3 | 86.8 ± 5.6 | n.s. |
| Chl(a+b) | 3.33 ± 0.28 b | 4.38 ± 0.23 a | 4.29 ± 0.18 a | * |
| Chla/b | 2.83 ± 0.18 | 2.86 ± 0.10 | 2.68 ± 0.18 | n.s. |
| Car | 2.00 ± 0.19 | 1.87 ± 0.17 | 1.77 ± 0.132 | n.s. |
| Chl(a+b)/Car | 1.67 ± 0.09 b | 2.36 ± 0.14 a | 2.45 ± 0.11 a | ** |
| TSP | 9.92 ± 0.33 | 10.1 ± 0.5 | 9.36 ± 0.30 | n.s. |
| -SH | 2.49 ± 0.22 | 2.48 ± 0.09 | 2.27 ± 0.13 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, C.; Rodrigues, M.Â.; Pinto, L.; Gonçalves, A.; Silva, E.; Martins, S.; Rocha, L.; Pavia, I.; Arrobas, M.; Ribeiro, A.C.; et al. Grey and Black Anti-Hail Nets Ameliorated Apple (Malus × domestica Borkh. cv. Golden Delicious) Physiology under Mediterranean Climate. Plants 2021, 10, 2578. https://doi.org/10.3390/plants10122578
Brito C, Rodrigues MÂ, Pinto L, Gonçalves A, Silva E, Martins S, Rocha L, Pavia I, Arrobas M, Ribeiro AC, et al. Grey and Black Anti-Hail Nets Ameliorated Apple (Malus × domestica Borkh. cv. Golden Delicious) Physiology under Mediterranean Climate. Plants. 2021; 10(12):2578. https://doi.org/10.3390/plants10122578
Chicago/Turabian StyleBrito, Cátia, Manuel Ângelo Rodrigues, Luís Pinto, Alexandre Gonçalves, Ermelinda Silva, Sandra Martins, Luis Rocha, Ivo Pavia, Margarida Arrobas, António Castro Ribeiro, and et al. 2021. "Grey and Black Anti-Hail Nets Ameliorated Apple (Malus × domestica Borkh. cv. Golden Delicious) Physiology under Mediterranean Climate" Plants 10, no. 12: 2578. https://doi.org/10.3390/plants10122578
APA StyleBrito, C., Rodrigues, M. Â., Pinto, L., Gonçalves, A., Silva, E., Martins, S., Rocha, L., Pavia, I., Arrobas, M., Ribeiro, A. C., Moutinho-Pereira, J., & Correia, C. M. (2021). Grey and Black Anti-Hail Nets Ameliorated Apple (Malus × domestica Borkh. cv. Golden Delicious) Physiology under Mediterranean Climate. Plants, 10(12), 2578. https://doi.org/10.3390/plants10122578













