Obtaining Salt Stress-Tolerant Eggplant Somaclonal Variants from In Vitro Selection
Abstract
1. Introduction
2. Results
2.1. Establishment of In Vitro Salt-Tolerant Lines
2.2. Shoot Induction and Acclimatization
2.3. Salt Stress Test
2.3.1. Evaluation of Growth and Biochemical Parameters in Callus Tissue
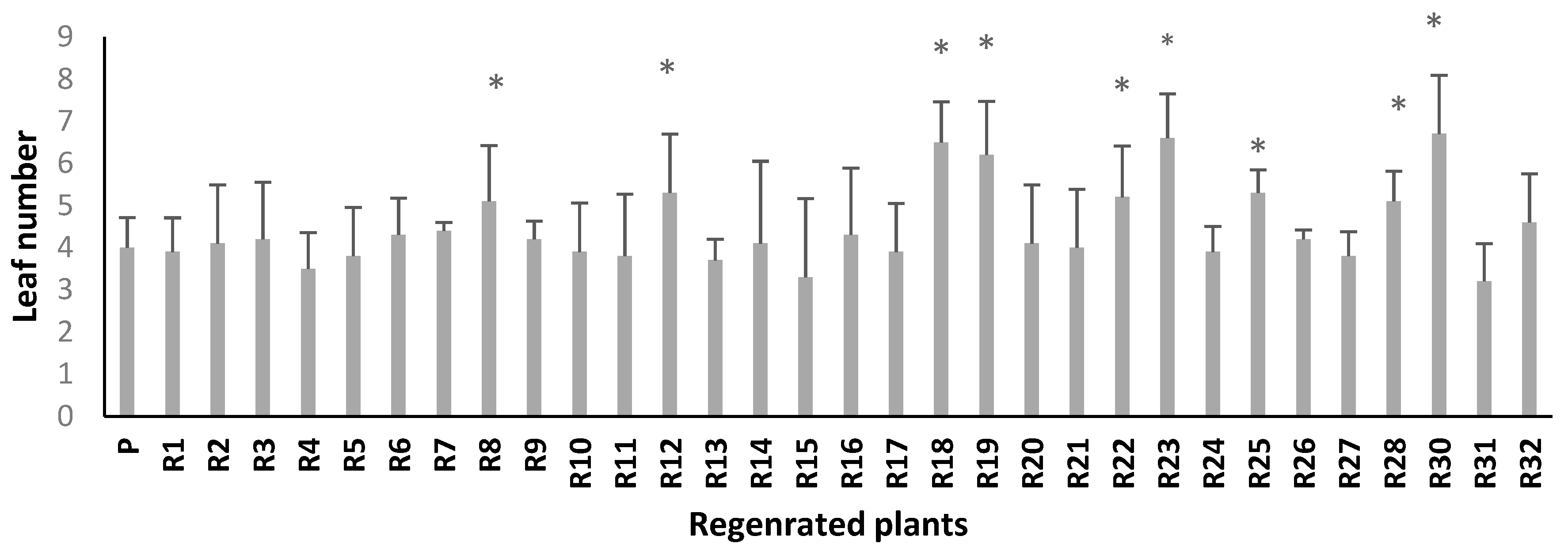
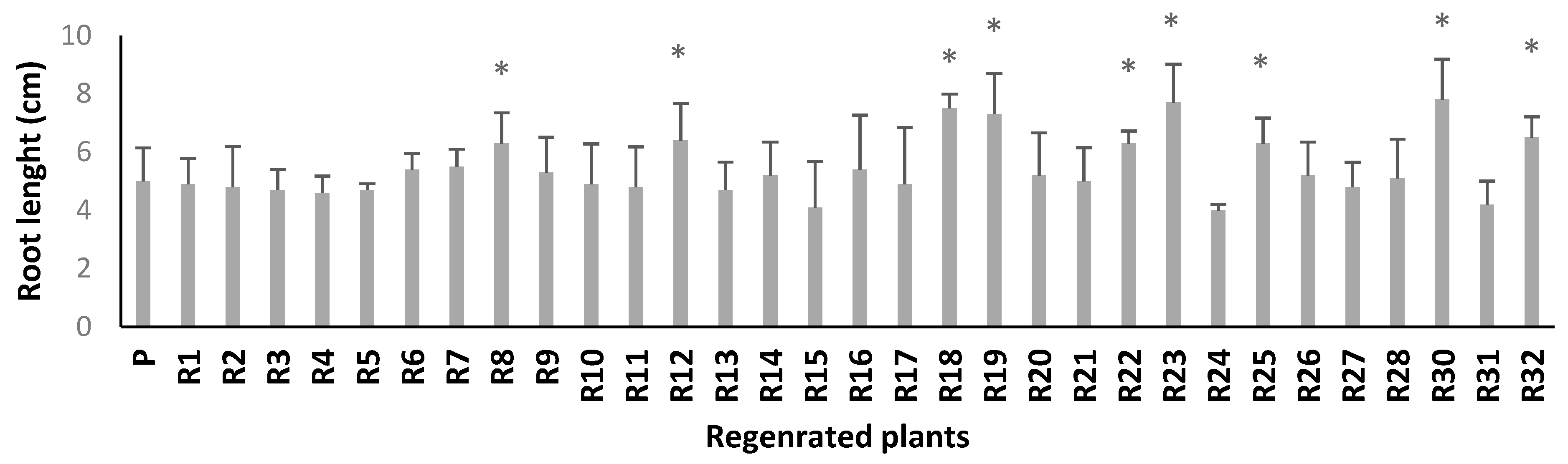
2.3.2. In Vitro Salt Screening of Regenerated Plants
2.4. Greenhouse Growth and Yield Evaluation
3. Discussion
4. Materials and Methods
4.1. Initiation and Stock
4.2. Callus Induction
4.3. Salt Stress Selection and Regeneration
4.4. Acclimatization
4.5. Salt Stress Test
4.5.1. Evaluation of Growth and Biochemical Parameters in Callus Tissue
Growth Rate and Water Content
Lipid Peroxidation
Ascorbic Acid Content
Enzyme Assays
4.6. Evaluation of Regenerated Plants
4.6.1. In Vitro Screening Salinity Test
4.6.2. Greenhouse Growth and Yield Evaluation
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qureshi, A.S.; Mohammed, M.; Daba, A.W.; Hailu, B.; Belay, G.; Tesfaye, A.; Ertebo, A.M. Improving agricultural productivity on salt-affected soils in Ethiopia: Farmers’ perceptions and proposals. Afr. J. Agric. Res. 2019, 14, 897–906. [Google Scholar]
- Rengasamy, P. Soil salinization. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: Oxford, UK, 2016. [Google Scholar] [CrossRef]
- Wicke, B.; Smeets, E.; Dornburg, V.; Vashev, B.; Gaiser, T.; Turkenburg, W.; Faaij, A. The global technical and economic potential of bioenergy from salt-affected soils. Energy Environ. Sci. 2011, 8, 2669–2681. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Arzani, A.; Ashraf, M. Smart engineering of genetic resources for enhanced salinity. CRC Crit. Rev. Plant Sci. 2016, 35, 146–189. [Google Scholar] [CrossRef]
- Vaidyanathan, H.; Sivakumar, P.; Chakrabarty, R.; Thomas, G. Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.) differential response in salt-tolerant and sensitive varieties. Plant Sci. 2003, 165, 1411–1418. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Gu, R.; Liu, Q.; Pei, D.; Jiang, X. Understanding saline and osmotic tolerance of Populus euphratica suspended cells. Plant Cell Tissue Organ Cult. 2004, 78, 261–265. [Google Scholar] [CrossRef]
- Al-Khateeb, S.A.; Al-Khateeb, A.A.; Sattar, M.N.; Mohmand, A.S. Induced in vitro adaptation for salt tolerance in date palm (Phoenix dactylifera L.) cultivar Khalas. Biol. Res. 2020, 53, 37. [Google Scholar] [CrossRef]
- Davenport, S.B.; Gallego, S.M.; Benavides, M.P.; Tomaro, M.L. Behaviour of antioxidant defense system in the adaptive response to salt stress in Helianthus annuus L. cells. Plant Growth Regul. 2003, 40, 81–88. [Google Scholar] [CrossRef]
- Ghane, S.G.; Lokhande, V.H.; Nikam, T.D. Growth, physiological, and biochemical responses in relation to salinity tolerance for In Vitro selection in oil seed crop Guizotia abyssinica Cass. J. Crop. Sci. Biotechnol. 2014, 17, 11–20. [Google Scholar] [CrossRef]
- Lutts, S.; Almansouri, M.; Kinet, J.M. Salinity and water stress have contrasting effects on the relationship between growth and cell viability during and after stress exposure in durum wheat callus. Plant Sci. 2004, 167, 9–18. [Google Scholar] [CrossRef]
- Queirós, F.; Fidalgo, F.; Santos, I.; Salema, R. In vitro selection of salt tolerant cell lines in Solanum tuberosum L. Biol. Plant. 2007, 51, 728–734. [Google Scholar] [CrossRef]
- Anwar, F.; Sharmila, P.; Pardha Saradhi, P. An optimal protocol for in vitro regeneration, efficient rooting and stable transplantation of chickpea. Physiol. Mol. Biol. Plants 2008, 9, 7256–7265. [Google Scholar] [CrossRef]
- Miki, Y.; Hashiba, M.; Hisajima, S. Establishment of salt stress tolerant rice plants through step up NaCl treatment in vitro. Biol. Plant. 2001, 44, 391–395. [Google Scholar] [CrossRef]
- Nikam, A.A.; Devarumath, R.M.; Shitole, M.G. Gamma radiation, in vitro selection for salt (NaCl) tolerance, and characterization of mutants in sugarcane (Saccharum officinarum L.). In Vitro Cell. Dev. Biol. Plant 2014, 50, 766–776. [Google Scholar] [CrossRef]
- Santangeli, M.; Capo, C.; Beninati, S.; Pietrini, F.; Forni, C. Gradual Exposure to Salinity Improves Tolerance to Salt Stress in Rapeseed (Brassica napus L.). Water 2019, 11, 1667. [Google Scholar] [CrossRef]
- Shankhdhar, D.; Shankhdhar, S.C.; Mani, S.C.; Pant, R.C. In vitro selection for salt tolerance in rice. Biol. Plant. 2000, 43, 477–480. [Google Scholar] [CrossRef]
- Evans, D.A.; Sharp, W.R. Somaclonal and gametoclonal variation. In Handbook of Plant Cell Culture; Evans, D.A., Sharp, W.R., Ammirato, P.V., Eds.; Macmillan Publishing Company: New York, NY, USA, 1998; pp. 97–132. [Google Scholar]
- Karp, A. On the current understanding of somaclonal variation. In Surveys of Plant Molecular and Cell Biology; Miflin, B.F., Ed.; Oxford University Press: Oxford, UK, 1991; pp. 1–58. [Google Scholar]
- Khan, M.H.; Panda, S.K. Induction of oxidative stress in roots of Oryza sativa L. in response to salt stress. Biol. Plant. 2002, 45, 625–627. [Google Scholar] [CrossRef]
- Veilleux, R.E.; Johnson, A.T. Somaclonal variation: Molecular analysis, transformation interaction, and utilization. Plant Breed Rev. 1998, 16, 229–268. [Google Scholar]
- Elmaghrabi, A.M.; Dennis, F.; Rogers, H.J.; Ochatt, S.J. Nuclear Migration: An Indicator of Plant Salinity Tolerance in vitro. Front. Plant Sci. 2019, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Hammerschlag, F.A.; Garces, S.; Koch-Dean, M.; Ray, S.; Lewers, K.; Maas, J.; Smith, B.J. In vitro response of strawberry cultivars and regenerants to Colletorichum acutatum. Plant Cell Tissue Organ Cult. 2006, 84, 255–261. [Google Scholar] [CrossRef]
- Karp, A. Somaclonal variation as a tool for crop improvement. Euphytica 1995, 85, 295–302. [Google Scholar] [CrossRef]
- Scowcroft, W.R.; Larkin, P.J. Applicatiolls Olp/Allt Cell Tissue and Organ Culture—Ciba Foundation Symposium/07; Willey Eastern Ltd.: Chichester, UK, 1988; p. 21. [Google Scholar]
- Filipecki, M.; Malepszy, S. Unintended consequences of plant transformation: A molecular insight. J. Appl. Genet. 2006, 47, 277–286. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 5 March 2021).
- Butu, M.; Rodino, S. 11—Fruit and Vegetable—Based Beverages—Nutritional Properties and Health Benefits. In Natural Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 303–338. ISBN 9780128166895. [Google Scholar]
- Ünlükara, A.; Kurunç, A.; Kesmez, G.D.; Yurtseven, E.; Suarez, D.L. Effects of salinity on eggplant (Solanum melongena L.) growth and evapotranspiration. Irrig. Drain. 2010, 59, 203–214. [Google Scholar]
- Granja, M.M.C.; Medeiros, M.J.L.; Silva, M.M.A.; Camara, T.R.; Willadino, L.; Ulisses, C. Response to in vitro salt stress in sugarcane is conditioned by concentration and condition of exposure to NaCl. Acta Boil. Colomb. 2018, 23, 30–38. [Google Scholar] [CrossRef]
- Karan, R.; Subudhi, P.K. Approaches to Increasing Salt Tolerance in Crop Plants. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 63–88. [Google Scholar]
- Rodríguez-Rosales, M.P.; Kerkeb, L.; Bueno, P.; Donaire, J.P. Changes induced by NaCl in lipid content and composition, lipoxygenase, plasma membrane H+-ATPase and antioxidant enzyme activities of tomato (Lycopersicon esculentum. Mill) calli. Plant Sci. 1999, 143, 143–150. [Google Scholar] [CrossRef]
- Shavrukov, Y. Salt stress or salt shock: Which genes are we studying? J. Exp. Bot. 2013, 64, 119–127. [Google Scholar] [CrossRef]
- Magioli, C.; Barrôco, R.M.; Rocha, C.A.B.; Santiago-Fernandes, L.D.; Mansur, E.; Engler, G.; Margis-Pinheiro, M.; Sachetto-Martins, G. Somatic embryo formation in Arabidopsis and eggplant is associated with expression of a glycine-rich protein gene (Atgrp-5). Plant Sci. 2001, 161, 559–567. [Google Scholar] [CrossRef]
- Kantharajah, A.S.; Golegaonkar, P.G. Somatic embryogenesis in eggplant. Sci. Hortic. 2004, 99, 107–117. [Google Scholar] [CrossRef]
- Tarré, E.; Magioli, C.; Margis-Pinheiro, M.; Sachetto-Martins, G.; Mansur, E.; Fernandes, L.D. Somatic embryogenesis and adventitious root initiation have a common origin in eggplant (Solanum melongena L.). Rev. Bras. Bot. 2004, 27, 70–84. [Google Scholar] [CrossRef][Green Version]
- Kaur, M.; Dhatt, A.S.; Sandhu, J.S.; Amrik, S.A.S.; Gosal, S.S. Effect of media composition and explant type on the regeneration of eggplant (Solanum melongena L.). Afr. J. Biotechnol. 2013, 12, 860–866. [Google Scholar]
- Franklin, G.; Sheeba, C.; Sita, L. Regeneration of eggplant (Solanum melongena L.) from root explants. In Vitro Cell. Dev. Biol. Plant 2004, 40, 188–191. [Google Scholar] [CrossRef]
- Zayova, E.; Vassilevvska-Ivanova, R.; Kraptchev, B.; Stoeva, D. Indirect shoot organogenesis of eggplant (Solanum melongena L.). J. Cent. Eur. Agric. 2012, 13, 446–457. [Google Scholar] [CrossRef]
- Shivaraj, G.; Rao, S. Rapid and efficient plant regeneration of eggplant (Solanum melongena L.) from cotyledonary leaf explants. Indian J. Biotechnol. 2011, 10, 125–129. [Google Scholar]
- Plaza-Wüthrich, S.; Blösch, R.; Tadele, Z. Efficiency of In Vitro Regeneration is Dependent on the Genotype and Size of Explant in Tef [Eragrostis tef (Zucc.) Trotter]. Adv. Crop Sci. Technol. 2015, 3, 179. [Google Scholar]
- Leone, A.; Costa, A.; Tucci, M.; Grillo, S. Adaptation versus shock response to polyethylene glycol-induced low water potential in cultured potato cells. Physiol. Plant. 1994, 92, 21–30. [Google Scholar] [CrossRef]
- Hossain, Z.; Mandal, A.K.A.; Datta, S.K.; Biswas, A.K. Development of NaCl tolerant line in Chrysanthemum morifolium Ramat. through shoot organogenesis of selected callus line. J. Biotechnol. 2007, 129, 658–667. [Google Scholar] [CrossRef]
- Jain, S.M.; Ochatt, S.J.; Kulkarni, V.M.; Predieri, S. In vitro culture for mutant development. Acta Hortic. 2010, 865, 59–68. [Google Scholar] [CrossRef]
- Suprasanna, P.; Jain, S.M.; Ochatt, S.J.; Kulkarni, V.M.; Predieri, S. Applications of in vitro techniques in mutation breeding of vegetatively propagated crops. In Plant Mutation Breeding and Biotechnolog; Shu, Q.Y., Forster, B.P., Nakagawa, H., Eds.; CAB International: Wallingford, UK, 2012; pp. 369–383. [Google Scholar]
- Ochatt, S.J.; Marconi, P.L.; Radice, S.; Arnozis, P.A.; Caso, O.H. In vitro recurrent selection of potato: Production and characterization of salt tolerant cell lines and plants. Plant Cell Tissue Organ Cult. 1999, 55, 1–8. [Google Scholar] [CrossRef]
- Olmos, E.; Hellín, E. Mechanisms of salt tolerance in a cell line of Pisum sativum: Biochemical and physiological aspects. Plant Sci. 1996, 120, 37–45. [Google Scholar] [CrossRef]
- Abed Alrahman, N.M.; Shibli, R.A.; Ereifej, K.I.; Hindiyeh, M.Y. Influence of salinity on growth and physiology of in vitro grown cucumber (Cucumis sativus L.). Jordan J. Agric. Sci. 2005, 1, 93–106. [Google Scholar]
- Benavídes, M.P.; Marconi, P.L.; Gallego, S.M.; Comba, M.E.; Tomaro, M.L. Relationship between antioxidant defence systems and salt tolerance in Solanum tuberosum. Aust. J. Plant Physiol. 2000, 27, 273–278. [Google Scholar] [CrossRef]
- Fidalgo, F.; Santos, A.; Santos, I.; Salema, R. Effects of longterm salt stress on antioxidant defence systems, leaf water relations and chloroplast ultrastructure of potato plants. Ann. Appl. Biol. 2004, 145, 185–192. [Google Scholar] [CrossRef]
- Hannachi, S.; Van Labeke, M.C. Salt stress affects germination, seedling growth and physiological responses differentially in eggplant cultivars (Solanum melongena L.). Sci. Hortic. 2018, 228, 56–65. [Google Scholar] [CrossRef]
- Sotiropoulos, T.E.; Dimassi, K.N.; Tsirakoglou, V.; Therios, I.N. Response of two Prunus rootstocks to KCl induced salinity in vitro. Biol. Plant. 2006, 50, 477–480. [Google Scholar] [CrossRef]
- Koc, N.K.; Bas, B.; Koc, M.; Kusek, M. Investigations of in vitro selection for salt tolerant lines in sour orange (Citrus aurantium L.). Biotechnology 2009, 8, 155–159. [Google Scholar] [CrossRef]
- Niknam, V.; Meratan, A.A.; Ghaffari, S.M. The effect of salt stress on lipid peroxidation and antioxidative enzymes in callus of two Acanthophyllum species. In Vitro Cell. Dev. Biol. Plant 2011, 47, 297–308. [Google Scholar] [CrossRef]
- Niknam, V.; Razavi, N.; Ebrahimzadeh, H.; Sharifizadeh, B. Effect of NaCl on biomass, protein and proline contents, and antiioxidant enzyles in seedling and calli of two Trigonella species. Biol. Plant. 2006, 50, 591–596. [Google Scholar] [CrossRef]
- Rout, G.R.; Senapati, S.K.; Panda, J.J. Selection of salt tolerant plants of Nicotiana tabacum L. through in vitro and its biochemical characterization. Acta Biol. Hung. 2008, 59, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Plant Biol. 2005, 59, 651–681. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular response to high salinity. Annu. Rev. Plant Physiol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Dracup, M. Increasing salt tolerance of plants through cell culture requires greater understanding of tolerance mechanisms. Aust. J. Plant Physiol. 1991, 18, 1–15. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Binzel, M.L.; Hasegawa, P.M.; Handa, A.K.; Bressan, R.A. Adaptation of tobacco cells to NaCl. Plant Physiol. 1985, 79, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Demirel, T.; Turkan, I. Comparative lipid peroxidation, antioxidant defense system and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005, 53, 247–257. [Google Scholar] [CrossRef]
- Jain, M.; Mathur, G.; Koul, S.; Sarin, N.B. Ameliorative effects of proline on salt stressed-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.). Plant Cell Rep. 2001, 20, 463–468. [Google Scholar] [CrossRef]
- Hernández, J.A.; Jiménez, A.; Mullineaux, P.; Sevilla, F. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 2000, 23, 853–862. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative damage, lipid peroxidation, and antioxidant protection in chloroplasts. Chem. Phys. Lipids 1987, 44, 327–340. [Google Scholar] [CrossRef]
- Karpe, A.; Nikam, A.A.; Chimote, K.P.; Kalwade, S.B.; Kawar, P.G.; Babu, H.; Devarumath, R.M.; Suprasanna, P. Differential responses to salinity stress of two varieties (CoC 671 and Co 86032) of sugarcane (Saccharum officinarum L.). Afr. J. Biotechnol. 2012, 11, 9028–9035. [Google Scholar]
- Özdemir, F.; Bor, M.; Demiral, T.; Türkan, İ. Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul. 2004, 42, 203–211. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Turkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 2009, 67, 2–9. [Google Scholar] [CrossRef]
- Alhasnawi, A.N.; Che Radziah, C.M.Z.; Kadhimi, A.A. Enhancement of antioxidant enzyme activities in rice callus by ascorbic acid under salinity stress. Biol. Plant. 2016, 60, 783–787. [Google Scholar] [CrossRef]
- Gossett, D.R.; Banks, S.W.; Millhollon, E.P.; Lucas, M.C. Antioxidant response to NaCl stress in a control and a NaCl-tolerant cotton cell line grown in the presence of paraquat, buthionine sulfoximine, and exogenous glutathione. Plant Physiol. 1996, 112, 803–809. [Google Scholar] [CrossRef]
- Abdelgawad, K.F.; El-Mogy, M.M.; Mohamed, M.I.A.; Garchery, C.; Stevens, R.G. Increasing Ascorbic Acid Content and Salinity Tolerance of Cherry Tomato Plants by Suppressed Expression of the Ascorbate Oxidase Gene. Agronomy 2019, 9, 51. [Google Scholar] [CrossRef]
- Gossett, D.R.; Millhollon, E.P.; Lucas, M.C. Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop. Sci. 1994, 34, 706–714. [Google Scholar] [CrossRef]
- Hernández, J.A.; Olmos, E.; Corpas, F.J.; Sevilla, F.; Del Río, L.A. Salt-induced oxidative stress in chloroplasts of pea plants. Plant Sci. 1995, 105, 151–167. [Google Scholar] [CrossRef]
- Formentin, E.; Sudiro, C.; Ronci, M.B.; Locato, V.; Barizza, E.; Stevanato, P.; Ijaz, B.; Zottini, M.; De Gara, L.; Lo Schiavo, F. H2O2 Signature and Innate Antioxidative Profile Make the Difference Between Sensitivity and Tolerance to Salt in Rice Cells. Front. Plant Sci. 2018, 9, 1549. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Plant Sci. 2000, 19, 267–290. [Google Scholar] [CrossRef]
- Qian, H.F.; Peng, X.F.; Han, X.; Ren, J.; Zhan, K.Y.; Zhu, M. The stress factor, exogenous ascorbic acid, affects plant growth and the antioxidant system in Arabidopsis thaliana. Russ. J. Plant. Physiol. 2014, 61, 467–475. [Google Scholar] [CrossRef]
- Azevedo Neto, A.D.; Prisco, J.T.; Eneas-Filho, J.; de Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 5, 87–94. [Google Scholar] [CrossRef]
- Kusvuran, S.; Ellialtioglu, S.; Yasar, F.; Abak, K. Antioxidative enzyme activities in the leaves and callus tissues of salt-tolerant and salt-susceptible melon varieties under salinity. Afr. J. Biotech. 2012, 11, 635–641. [Google Scholar]
- Sevengor, S. Investigations on Antioxydant Enzyme Activities under In Vitro and In Vivo Conditions to Obtain Salt Tolerance in Squash (Cucurbita pepo L.). Ph.D. Thesis, Graduate School of Natural and Applied Sciences, Ankara Universitiy, Ankara, Turkey, 2010. [Google Scholar]
- Wyatt, S.E.; Dolph, A.L.; Avery, A.A.; Carpita, N.C. Plasma membrane-cell-wall adhesion and its role in response to biotic and abiotic stresses. In Current Issues in Plant Molecular and Cellular Biology, Proceedings of the VIIIth International Congress on Plant Tissue and Cell Culture, Florence, Italy, 12–17 June 1994; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA, 1995; pp. 19–29. [Google Scholar]
- Murshige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, F.C.; Prange, R.K. Improving the thiobarbituric acid reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Shukla, V.K.S.; Kokate, C.K.; Srivastava, K.C. Spectrophotometric determination of ascorbic acid. Microchem. J. 1979, 24, 124–126. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and highlight intensity enhance activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1226. [Google Scholar] [CrossRef] [PubMed]


| NaCl (mM) on Which Line Was Selected and Tested | RGR (% of Control) | RWC (%) | MDA (nmol g−1 (FW)) | AsA (μg g−1 (FW)) | SOD (µmol min−1 mg−1 FW) | CAT (µmol min−1 mg−1 FW) |
|---|---|---|---|---|---|---|
| 0 | 100 ± 0.0 a | 99 ± 0.57 a | 1.83 ± 0.35 b | 102.6 ± 1.15 c | 231.6 ± 7.26 c | 126 ± 2.08 c |
| 40 | 75 ± 0.77 b | 95 ± 1.15 a | 2.10 ± 0.11 b | 150.03 ± 0.57 b | 280.3 ± 7.79 b | 136 ± 3.78 c |
| 80 | 65 ± 1.15 b | 87 ± 1.15 b | 4.50 ± 0.57 a | 228.3 ± 0.57 a | 305 ± 7.63 b | 211 ± 4.93 b |
| 120 | 25 ± 0.57 c | 69 ± 1.15 c | 6.20 ± 0.88 a | 260.5 ± 1.73 a | 370 ± 8.66 a | 238 ± 4.40 a |
| Lines | NaCl (mM) | FW (g) | DW (g) | TWC (g H2O/g DW) |
|---|---|---|---|---|
| P | 0 | 140.9 ± 3.2 c | 25.7 ± 1.2 b | 0.81 ± 0.9 b |
| 80 | 80.6 ± 3.2 c | 20.3 ± 4.2 b | 0.74 ± 3.2 c | |
| 120 | 48.3 ± 3.5 c | 15.1 ± 4.5 b | 0.68 ± 2.1 c | |
| R8 | 0 | 170.7 ± 1.6 b | 30.4 ± 2.6 a | 0.82 ± 1.3 b |
| 80 | 150.8 ± 2.3 b | 28.4 ± 3.3 a | 0.81 ± 2.1 b | |
| 120 | 120.3 ±1.7 b | 23.2 ± 2.7 a | 0.80 ± 1.6 b | |
| R12 | 0 | 169.6 ± 1.2 b | 30.1 ± 2.2 a | 0.82 ± 1.1 b |
| 80 | 158. 9 ± 2.4 b | 28.2 ± 1.4 a | 0.82 ± 2.3 b | |
| 120 | 128.3 ± 6.2 b | 23.6 ± 2.2 a | 0.81 ± 2.1 b | |
| R18 | 0 | 215.5 ± 2.7 a | 31 ± 0.7 a | 0.85 ± 2.7 a |
| 80 | 200.4 ± 5.1 a | 29.1 ± 3.1 a | 0.85 ± 1.3 a | |
| 120 | 165.5 ± 2.1 a | 24.2 ± 1.1 a | 0.85 ± 1.1 a | |
| R19 | 0 | 216.5 ± 2.1 a | 31.5 ± 1.1 a | 0.85 ± 2.1 a |
| 80 | 200.3 ± 2.0 a | 29.4 ± 1.0 a | 0.85 ± 0.9 a | |
| 120 | 166.4 ± 2.0 a | 24.3 ± 3.1 a | 0.85 ± 2.3 a | |
| R22 | 0 | 170.6 ± 3.7 b | 30.2 ± 3.7 a | 0.82 ± 0.8 b |
| 80 | 165.3 ± 2.1 b | 28.3 ± 1.1 a | 0.82 ± 2.3 b | |
| 120 | 125.6 ±1.2 b | 23.1 ± 1.2 a | 0.81 ±1.7 b | |
| R23 | 0 | 217.5 ±1.9 a | 31.5 ± 2.9 a | 0.85 ±1.5 a |
| 80 | 202.3 ± 5.1 a | 29.4 ± 3.1 a | 0.85 ± 1.3 a | |
| 120 | 165.3 ± 2.1 a | 24.2 ± 1.1 a | 0.85 ± 0.9 a | |
| R25 | 0 | 167.5 ± 1.2 b | 30.8 ± 2.2 a | 0.81 ± 1.3 b |
| 80 | 157.2 ± 2.0 b | 28.7 ± 2.0 a | 0.81 ± 0.8 b | |
| 120 | 130.2 ± 2.0 b | 24.5 ± 2.3 a | 0.81 ± 1.1 b | |
| R28 | 0 | 165.8 ± 3.7 b | 29.6 ± 2.7 a | 0.82 ±2.1 b |
| 80 | 152.3 ± 2.1 b | 27.5 ± 2.2 a | 0.81 ± 2.1 b | |
| 120 | 122.4 ± 1.2 b | 22.2 ± 1.5 a | 0.81 ± 1.1 b | |
| R30 | 0 | 217.1 ± 4.2 a | 33.2 ± 3.2 a | 0.84 ± 2.2 a |
| 80 | 201.0 ± 3.6 a | 31.1 ± 3.7 a | 0.84 ± 2.5 a | |
| 120 | 168.0 ± 3.9 a | 26 ± 3.9 a | 0.84 ± 2.9 a | |
| R32 | 0 | 160.3 ± 2.9 b | 30.4 ± 1.9 a | 0.81 ± 1.1 b |
| 80 | 249.3 ± 2.5 a | 28.4 ± 2.8 a | 0.80 ± 3.1 b | |
| 120 | 120.3 ± 0.9 b | 23.2 ± 0.8 a | 0.80 ± 1.8 b |
| Parameter | NaCl (mM) | P | R8 | R12 | R18 | R19 | R22 | R23 | R25 | R28 | R30 | R32 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit number | 0 | 6 ± 1.8 b | 6 ± 1.3 b | 7 ± 1.4 b | 10 ± 0.5 a | 10 ± 1.2 a | 6 ± 1.2 b | 12 ± 1.9 a | 6 ± 1.2 b | 8 ± 1.3 b | 10 ± 1.4 a | 7 ± 0.5 b |
| 80 | 3 ± 1.6 c | 8 ± 1.3 b | 10 ± 1.1 b | 12 ± 1.1 a | 12 ± 1.5 a | 8 ± 3.2 b | 13 ± 2.6 a | 8 ± 2.2 b | 9 ± 1.4 b | 12 ± 1.3 a | 8 ± 0.9 b | |
| 120 | 1 ± 1.1 c | 7 ± 1.2 b | 8 ± 1.1 b | 11 ± 2.3 a | 10 ± 1.7 a | 7 ± 2.4 b | 11 ± 2.2 a | 7 ± 1.8 b | 7 ± 1.3 b | 9 ± 1.1 a | 7 ± 1.1 b | |
| Fruit weight | 0 | 180 ± 1.2 b | 200 ± 1.6 b | 210 ± 1.1 b | 250 ± 2.8 a | 235 ±1.3 a | 220 ±3.2 a | 240 ± 2.9 a | 200 ± 2.2 b | 210 ± 1.1 b | 220 ± 2.4 a | 180 ± 2.3 b |
| 80 | 150 ± 3.6 c | 210 ± 2.3 b | 230 ± 2.2 b | 260 ± 3.1 a | 242 ±2.3 a | 230 ±2.3 b | 255 ± 2.1 a | 205 ± 1.2 b | 223 ± 1.3 b | 228 ± 2.5 b | 195 ± 1.8 b | |
| 120 | 80 ± 0.9 c | 150 ± 1.9 b | 160 ± 2.9 b | 180 ± 1.8 a | 185 ± 2.6 a | 145 ±1.9 b | 195 ± 1.8 a | 175 ± 2.5 a | 164 ± 0.7 b | 177 ± 0.9 a | 143 ± 1.5 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannachi, S.; Werbrouck, S.; Bahrini, I.; Abdelgadir, A.; Siddiqui, H.A.; Van Labeke, M.C. Obtaining Salt Stress-Tolerant Eggplant Somaclonal Variants from In Vitro Selection. Plants 2021, 10, 2539. https://doi.org/10.3390/plants10112539
Hannachi S, Werbrouck S, Bahrini I, Abdelgadir A, Siddiqui HA, Van Labeke MC. Obtaining Salt Stress-Tolerant Eggplant Somaclonal Variants from In Vitro Selection. Plants. 2021; 10(11):2539. https://doi.org/10.3390/plants10112539
Chicago/Turabian StyleHannachi, Sami, Stefaan Werbrouck, Insaf Bahrini, Abdelmuhsin Abdelgadir, Hira Affan Siddiqui, and Marie Christine Van Labeke. 2021. "Obtaining Salt Stress-Tolerant Eggplant Somaclonal Variants from In Vitro Selection" Plants 10, no. 11: 2539. https://doi.org/10.3390/plants10112539
APA StyleHannachi, S., Werbrouck, S., Bahrini, I., Abdelgadir, A., Siddiqui, H. A., & Van Labeke, M. C. (2021). Obtaining Salt Stress-Tolerant Eggplant Somaclonal Variants from In Vitro Selection. Plants, 10(11), 2539. https://doi.org/10.3390/plants10112539







