Optimising Artificial Moss Growth for Environmental Studies in the Mediterranean Area
Abstract
:1. Introduction
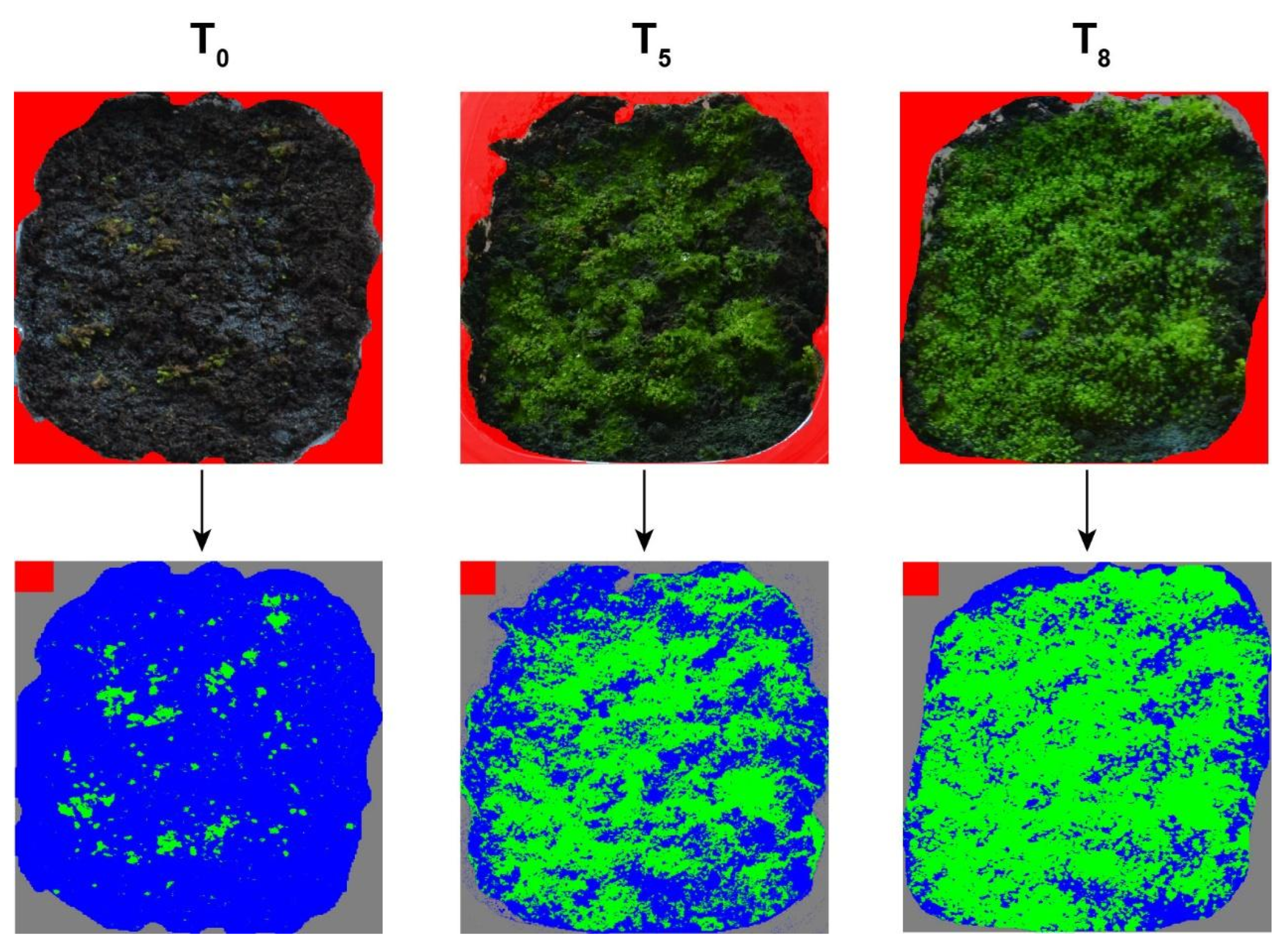
2. Results
3. Discussion
3.1. Moss Species Growth
3.2. Alien Invasive Species
4. Materials and Methods
4.1. Moss Processing and Experimental Design
4.2. Moss Growth Measurement
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeFalco, L.A.; Detling, J.K.; Tracy, C.R.; Warren, S.D. Physiological variation among native and exotic winter annual plants associated with microbiotic crusts in the Mojave Desert. Plant Soil 2001, 234, 1–14. [Google Scholar] [CrossRef]
- Alpert, P.; Oliver, M.J. Drying without dying. In Desiccation and Survival in Plants; Black, M., Pritchard, H.W., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 3–43. [Google Scholar]
- Cruz de Carvalho, R.; Catalá, M.; Branquinho, C.; Marques da Silva, J.; Barreno, E. Dehydration rate determines the degree of membrane damage and desiccation tolerance in bryophytes. Physiol. Plant. 2017, 159, 277–289. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.; Maurício, A.; Pereira, M.F.; Marques da Silva, J.; Branquinho, C. All for One: The Role of Colony Morphology in Bryophyte Desiccation Tolerance. Front. Plant Sci. 2019, 10, 1360. [Google Scholar] [CrossRef]
- Hilty, J.H.; Eldridge, D.J.; Rosentreter, R.; Wicklow-Howard, M.C.; Pellant, M. Recovery of biological soil crusts following wildfire in Idaho. J. Range Manag. 2004, 57, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Memon, M.K.C.; Lal, M. Problems of development in mosses and moss allies. Proc. Natl. Acad. Sci. USA 1981, B47, 115–152. [Google Scholar]
- Varela, Z.; Roiloa, S.R.; Fernández, J.A.; Retuerto, R.; Carballeira, A.; Aboal, J.R. Physiological and growth responses of transplants of the moss Pseudoscleropodium purum to atmospheric pollutants. Water Air Soil Pollut. 2013, 224, 1753. [Google Scholar] [CrossRef]
- Ares, A.; Varela, Z.; Aboal, J.R.; Carballeira, A.; Fernández, J.A. Active biomonitoring with the moss Pseudoscleropodium purum: Comparison between different types of transplants and bulk deposition. Ecotoxicol. Environ. Saf. 2015, 120, 74–79. [Google Scholar] [CrossRef]
- Bowker, M.A.; Belnap, J. A simple classification of soil types as habitats of biological soil crusts on the Colorado Plateau, USA. J. Veg. Sci. 2008, 19, 831–840. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.; dos Santos, P.; Branquinho, C. Production of moss-dominated biocrusts to enhance the stability and function of the margins of artificial water bodies. Restor. Ecol. 2018, 26, 419–421. [Google Scholar] [CrossRef]
- Garabito, D.; Vallejo, R.; Montero, E.; Garabito, J.; Martínez-Abaigar, J. Green buildings envelopes with bryophytes. A review of the state of the art. Boletín Soc. Española Briología 2017, 48–49. Available online: https://docplayer.es/111392867-Boletin-de-la-sociedad-espanola-de-briologia-2017-contenidos.html (accessed on 29 August 2021).
- Tian, G.Q.; Bai, X.L.; Xu, J.; Wang, X.D. Experimental Studies on the Natural Restoration and the Artificial Culture of the Moss Crusts on Fixed Dunes in the Tengger Desert, China. Front. Biol. China 2006, 1, 13. [Google Scholar] [CrossRef]
- Bai, X.L.; Wang, Y.; Xu, J.; Li, X.R.; Zhang, J.G. Characteristics of reproduction and growth of mosses in the soil crust of fixed dunes in Shapotou area. Chin. J. Desert Res. 2003, 23, 172–176. [Google Scholar]
- Xu, S.; Yin, C.; He, M.; Wang, Y. A technology for rapid reconstruction of moss-dominated soil crusts. Environ. Eng. Sci. 2008, 25, 1129–1138. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Zhao, Y.G.; Ran, M.Y. Experimental research on artificial culture method of moss crust in Hilly Loess Plateau region. Acta Bot. Boreali-Occident. Sin. 2009, 29, 586–592. [Google Scholar]
- Xiao, B.; Wang, Q.H.; Zhao, Y.G.; Shao, M.A. Artificial culture of biological soil crusts and its effects on overland flow and infiltration under simulated rainfall. Appl. Soil Ecol. 2011, 48, 11–17. [Google Scholar] [CrossRef]
- Xiao, B.; Zhao, Y.; Wang, Q.; Li, C. Development of artificial moss-dominated biological soil crusts and their effects on runoff and soil water content in a semi-arid environment. J. Arid. Environ. 2015, 117, 75–83. [Google Scholar] [CrossRef]
- Bu, C.F.; Yang, J.Z.; Zhang, X.C. Cultivation experiment of moss plants from biological soil crusts in Mu Us sandy land. Chin. J. Desert Res. 2011, 31, 937–941. [Google Scholar]
- Doherty, K.D.; Antoninka, A.J.; Bowker, M.A.; Ayuso, S.V.; Johnson, N.C. A novel approach to cultivate biocrusts for restoration and experimentation. Ecol. Restor. 2015, 33, 13–16. [Google Scholar] [CrossRef]
- Antoninka, A.; Bowker, M.A.; Reed, S.C.; Doherty, K. Production of greenhouse-grown biocrust mosses and associated cyanobacteria to rehabilitate dryland soil function. Restor. Ecol. 2015, 24, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Bowker, M.A.; Zhang, Y.; Zaady, E. Enhanced recovery of biological soil crusts after disturbance. In Biological Soil Crusts: An Organizing Principle in Drylands; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 499–523. [Google Scholar]
- Martin, A. The Magical World of Moss Gardening; Timber Press: Portland, OR, USA, 2015. [Google Scholar]
- Schenk, G. Moss Gardening: Including Lichens, Liverworts, and Other Miniatures; Timber Press: Portland, OR, USA, 1997. [Google Scholar]
- Proctor, M.C.F. Physiological ecology. Bryophyt. Biol. 2000, 2, 237–268. [Google Scholar]
- Proctor, M.C.F. The physiological basis of bryophyte production. Bot. J. Linn. Soc. 1990, 104, 61–77. [Google Scholar] [CrossRef]
- Zotz, G.; Rottenberger, S. Seasonal changes in diel CO2 exchange of three Central European moss species: A one-year field study. Plant Biol. 2001, 3, 661–669. [Google Scholar] [CrossRef]
- Furness, S.B.; Grime, J.P. Growth rate and temperature responses in bryophytes: II. A comparative study of species of contrasted ecology. J. Ecol. 1982, 70, 525–536. [Google Scholar] [CrossRef]
- Glime, J.M. Ecophysiology of Development: Protonemata. In Bryophyte Ecology. Physiological Ecology; Michigan Technological University; International Association of Bryologists, 2017; Volume 1, Chapter 5-3; Available online: http://digitalcommons.mtu.edu/bryophyte-ecology/ (accessed on 16 April 2021).
- Ros, R.M.; Mazimpaka, V.; Abou-Salama, U.; Aleffi, M.; Blockeel, T.L.; Brugués, M.; Cros, R.M.; Dia, M.G.; Dirkse, G.M.; Draper, I.; et al. Mosses of the Mediterranean, an Annotated Checklist. Cryptogam. Bryol. 2013, 34, 99–283. [Google Scholar] [CrossRef]
- Delivering Alien Invasive Species Inventories for Europe. Available online: http://www.europealiens.org/ (accessed on 20 August 2021).
- Sérgio, C.; Garcia, C.A.; Stow, S.; Martins, A.; Vieira, C.; Hespanhol, H.; Sim-Sim, M. How are anthropogenic pressures facilitating the invasion of Campylopus introflexus (dicranaceae, Bryopsida) in mainland Portugal? Cryptogam. Bryol. 2018, 39, 283–292. [Google Scholar] [CrossRef]
- He, X.; He, K.S.; Hyvönen, J. Will bryophytes survive in a warming world? Perspect. Plant. Ecol. Evol. Syst. 2016, 19, 49–60. [Google Scholar] [CrossRef]
- Proctor, M.C.F.; Oliver, M.J.; Wood, A.J.; Alpert, P.; Stark, L.R.; Cleavitt, N.L.; Mishler, B.D. Desiccation tolerance in bryophytes: A review. Bryologist 2007, 110, 595–621. [Google Scholar] [CrossRef]
- Frahm, J.P. A Preliminary Study of The Infraspecific Taxa of Hypnum Cupressiforme in Europe; Univ.-Bibliothek, 2009; ISSN 0945-3466. Available online: http://www.frahmia.de/downloads/archive_of_bryology/Archive%2040.pdf (accessed on 28 September 2021).
- Bjerke, J.W.; Bokhorst, S.; Zielke, M.; Callaghan, T.V.; Bowles, F.W.; Phoenix, G.K. Contrasting sensitivity to extreme winter warming events of dominant sub-Arctic heathland bryophyte and lichen species. J. Ecol. 2011, 99, 1481–1488. [Google Scholar] [CrossRef]
- Hugonnot, V. Comparative investigations of niche, growth rates and reproduction between the native moss Campylopus pilifer and the invasive C. introflexus. J. Bryol. 2017, 39, 79–84. [Google Scholar] [CrossRef]
- Munzi, S.; Varela, Z.; Paoli, L. Is the length of the drying period critical for photosynthesis reactivation in lichen and moss components of biological soil crusts? J. Arid Environ. 2019, 166, 86–90. [Google Scholar] [CrossRef]
- Essl, F.; Steinbauer, K.; Dullinger, S.; Mang, T.; Moser, D. Little, but increasing evidence of impacts by alien bryophytes. Biol. Invasions 2014, 16, 1175–1184. [Google Scholar] [CrossRef]
- Real, C. The Pixelclasser Package. R Package Version 1.0. 2020. Available online: https://cran.r-project.org/web/packages/pixelclasser/index.html (accessed on 22 October 2021).
- Bennet, K.P.; Campbell, C. Support vector machines: Hype or Hallelujah. ACM SIGKDD Explor. Newsl. 2000, 2, 1–13. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 20 July 2021).
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: London, UK, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]

| Species | T (°C) | Photoperiod (Hours) | Initial Cover (%) | Final Cover (%) | Max. Cover (%) | Time to Max. Cover (Weeks) |
|---|---|---|---|---|---|---|
| Bryum argenteum | 15 | 12 h | 8.63 ± 2.15 | 58.10 ± 10.95 | 58.10 ± 10.95 | 8 |
| 16 h | 23.60 ± 7.81 | 69.01 ± 11.40 | 69.01 ± 11.40 | 8 | ||
| 20 h | 25.77 ± 5.89 | 64.54 ± 13.31 | 64.54 ± 13.31 | 8 | ||
| 20 | 12 h | 21.87 ± 6.73 | 22.78 ± 6.03 | 54.82 ± 12.73 | 5 | |
| 16 h | 16.53 ± 9.05 | 35.43 ± 3.81 | 50.26 ± 18.74 | 5 | ||
| 20 h | 10.37 ± 4.67 | 46.38 ± 9.86 | 60.87 ± 21.07 | 6 | ||
| 25 | 12 h | 22.27± 8.45 | 39.13 ± 10.88 | 55.96 ± 12.69 | 6 | |
| 16 h | 31.63 ± 4.33 | 39.95 ± 6.88 | 49.93 ± 7.13 | 4 | ||
| 20 h | 26.93 ± 0.44 | 40.92 ± 4.24 | 46.17 ± 10.87 | 4 | ||
| Campylopus introflexus | 15 | 12 h | 17.09 ± 2.49 | 56.43 ± 31.62 | 56.43 ± 31.62 | 8 |
| 16 h | 21.24 ± 2.99 | 73.98 ± 3.32 | 73.98 ± 3.32 | 8 | ||
| 20 h | 27.56 ± 2.26 | 62.23 ±13.90 | 62.23 ±13.90 | 8 | ||
| 20 | 12 h | 22.04 ± 5.45 | 41.89 ± 24.65 | 74.12 ± 8.47 | 6 | |
| 16 h | 19.68 ± 4.18 | 44.15 ± 35.21 | 69.33 ± 7.62 | 6 | ||
| 20 h | 22.40 ± 8.70 | 41.40 ± 12.56 | 52.28 ± 2.28 | 7 | ||
| 25 | 12 h | 18.61 ± 7.53 | 49.81 ± 11.68 | 55.47 ± 19.45 | 5 | |
| 16 h | 24.24 ± 5.90 | 56.80 ± 18.46 | 64.48 ± 17.68 | 7 | ||
| 20 h | 21.16 ± 1.08 | 72.00 ± 3.20 | 72.47 ± 0.44 | 7 | ||
| Hypnum cupressiforme | 15 | 12 h | 11.35 ± 2.12 | 49.24 ± 11.87 | 49.24 ± 11.87 | 8 |
| 16 h | 24.53 ± 8.11 | 60.46 ± 8.79 | 60.46 ± 8.79 | 8 | ||
| 20 h | 23.71 ± 7.33 | 52.40 ± 33.10 | 53.33 ± 9.22 | 4 | ||
| 20 | 12 h | 12.03 ± 4.45 | 22.44 ± 5.79 | 41.5 ±23.66 | 5 | |
| 16 h | 6.86 ± 1.23 | 47.92 ± 12.27 | 32.93 ± 17.73 | 5 | ||
| 20 h | 4.49 ± 2.06 | 34.51 ± 20.34 | 64.08 ± 25.55 | 4 | ||
| Tortella nitida | 15 | 12 h | 4.88 ± 0.75 | 77.89 ± 5.61 | 77.89 ± 5.61 | 8 |
| 16 h | 5.32 ± 1.99 | 74.30 ± 11.67 | 70.87 ± 17.74 | 6 | ||
| 20 h | 3.92 ± 0.35 | 61.46 ± 24.99 | 68.53 ± 13.23 | 5 | ||
| 20 | 12 h | 2.71 ± 0.65 | 46.38 ± 20.83 | 54.85 ± 6.26 | 6 | |
| 16 h | 2.69 ± 2.14 | 18.05 ± 8.58 | 61.40 ± 12.93 | 5 | ||
| 20 h | 2.56 ± 0.85 | 50.78 ± 12.54 | 73.26 ± 2.65 | 5 | ||
| 25 | 12 h | 3.10 ± 1.90 | 47.40 ± 18.06 | 56.54 ± 7.57 | 5 | |
| 16 h | 2.00 ± 0.71 | 32.10 ± 19.31 | 33.36 ± 16.23 | 5 | ||
| 20 h | 3.16 ± 0.47 | 43.02 ± 29.56 | 43.02 ± 29.56 | 8 | ||
| Tortella squarrosa | 15 | 12 h | 15.68 ± 3.43 | 40.30 ± 27.24 | 43.52 ± 18.14 | 6 |
| 16 h | 15.88 ± 2.36 | 67.09 ± 16.27 | 67.09 ± 16.27 | 8 | ||
| 20 h | 14.99 ± 4.90 | 69.15 ± 5.06 | 69.15 ± 5.06 | 8 | ||
| 20 | 12 h | 17.21 ± 6.19 | 34.65 ± 20.59 | 64.21 ± 25.26 | 5 | |
| 16 h | 18.03 ± 10.46 | 35.67 ± 17.83 | 49. 63 ± 26.86 | 5 | ||
| 20 h | 19.29 ± 5.13 | 36.49 ±10.86 | 47.48 ± 28.05 | 5 | ||
| 25 | 12 h | 20.87 ± 2.53 | 43.20 ± 2.82 | 43.20 ± 2.82 | 8 | |
| 16 h | 15.54 ± 3.65 | 52.76 ± 3.00 | 52.76 ± 3.00 | 8 | ||
| 20 h | 20.14 ± 6.30 | 44.64 ± 6.66 | 48.15 ± 3.56 | 5 |
| Species | Plant Clade | Growth Form | Location | A.I. * | N/U | Coordinates |
|---|---|---|---|---|---|---|
| Bryum argenteum Hedw. | Bryophyta (mosses) | Acrocarpous | Zebreira | Semi-arid | U | 39°51′06.9′′ N 7°04′22.9′′ W |
| Campylopus introflexus (Hedw.) Brid. | Bryophyta (mosses) | Acrocarpous | Barreiro | Dry sub-humid | N | 38°36′50.5′′ N 9°02′31.9′′ W |
| Hypnum cupressiforme Hedw. | Bryophyta (mosses) | Pleurocarpous | Alegrete (Parque Natural de São Mamede) | Dry sub-humid | N | 39°15′14.6′′ N 7°18′05.0′′ W |
| Tortella nitida (Lindb.) Broth. | Bryophyta (mosses) | Acrocarpous | Estremoz | Semi-arid | U | 38°48′01.8′′ N 7°39′41.9′′ W |
| Tortella squarrosa (Brid.) Lindb. | Bryophyta (mosses) | Acrocarpous | Zebreira | Semi-arid | U | 39°51′06.9′′ N 7°04′22.9′′ W |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela, Z.; Real, C.; Branquinho, C.; do Paço, T.A.; Cruz de Carvalho, R. Optimising Artificial Moss Growth for Environmental Studies in the Mediterranean Area. Plants 2021, 10, 2523. https://doi.org/10.3390/plants10112523
Varela Z, Real C, Branquinho C, do Paço TA, Cruz de Carvalho R. Optimising Artificial Moss Growth for Environmental Studies in the Mediterranean Area. Plants. 2021; 10(11):2523. https://doi.org/10.3390/plants10112523
Chicago/Turabian StyleVarela, Zulema, Carlos Real, Cristina Branquinho, Teresa Afonso do Paço, and Ricardo Cruz de Carvalho. 2021. "Optimising Artificial Moss Growth for Environmental Studies in the Mediterranean Area" Plants 10, no. 11: 2523. https://doi.org/10.3390/plants10112523
APA StyleVarela, Z., Real, C., Branquinho, C., do Paço, T. A., & Cruz de Carvalho, R. (2021). Optimising Artificial Moss Growth for Environmental Studies in the Mediterranean Area. Plants, 10(11), 2523. https://doi.org/10.3390/plants10112523










