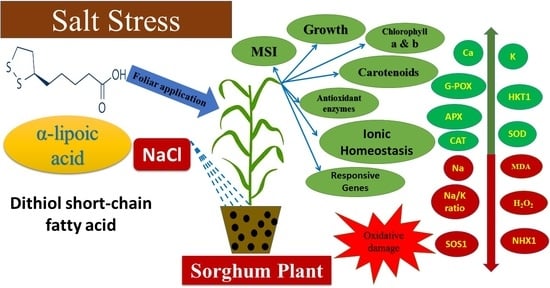
Exogenous Application of Alpha-Lipoic Acid Mitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes
Abstract
1. Introduction
2. Materials and Method
2.1. Growth Conditions and Experimental Design
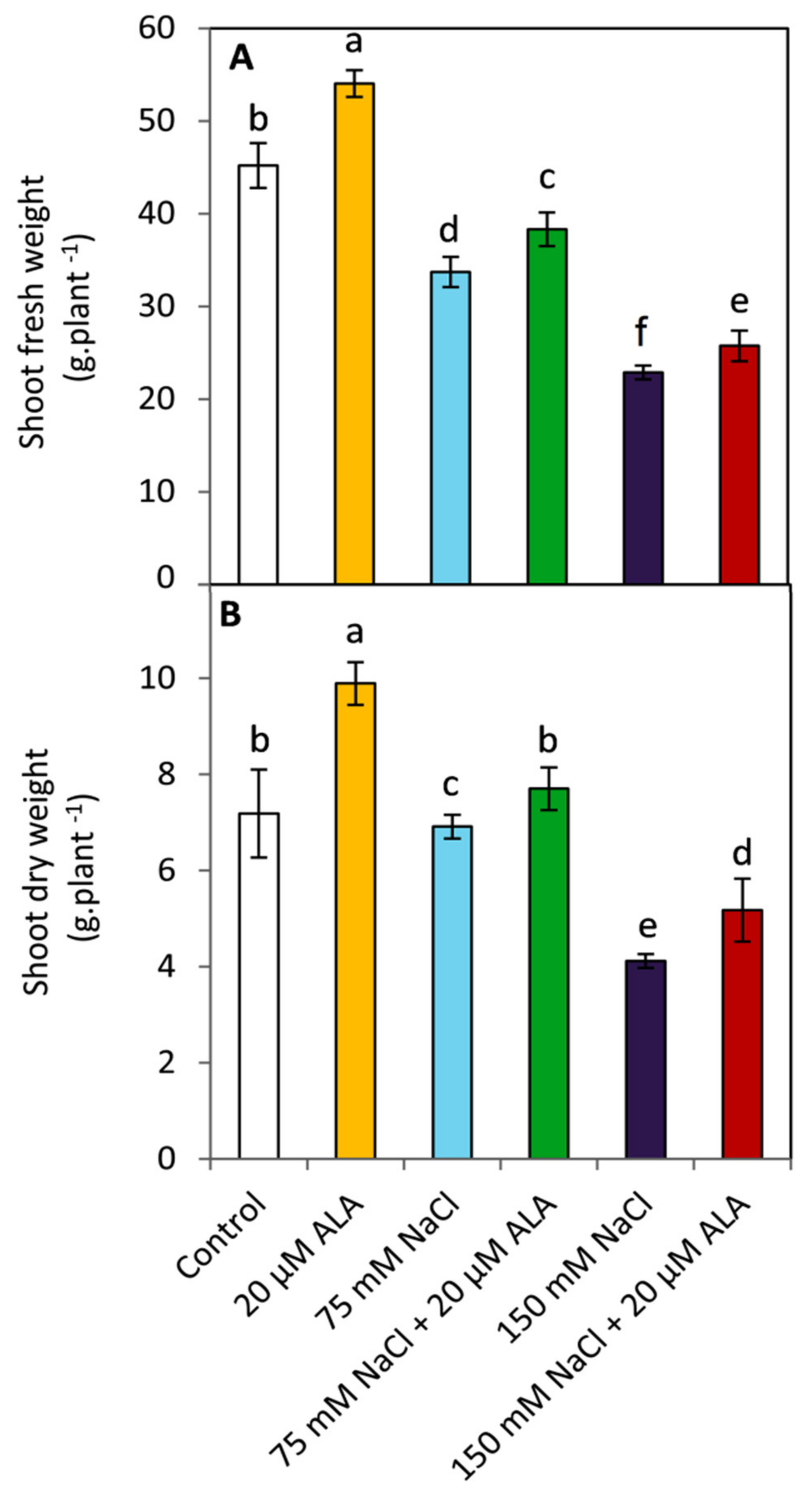
2.2. Determination of Growth Parameters
2.3. Membrane Stability Index (MSI), Hydrogen Peroxide and Lipid Peroxidation
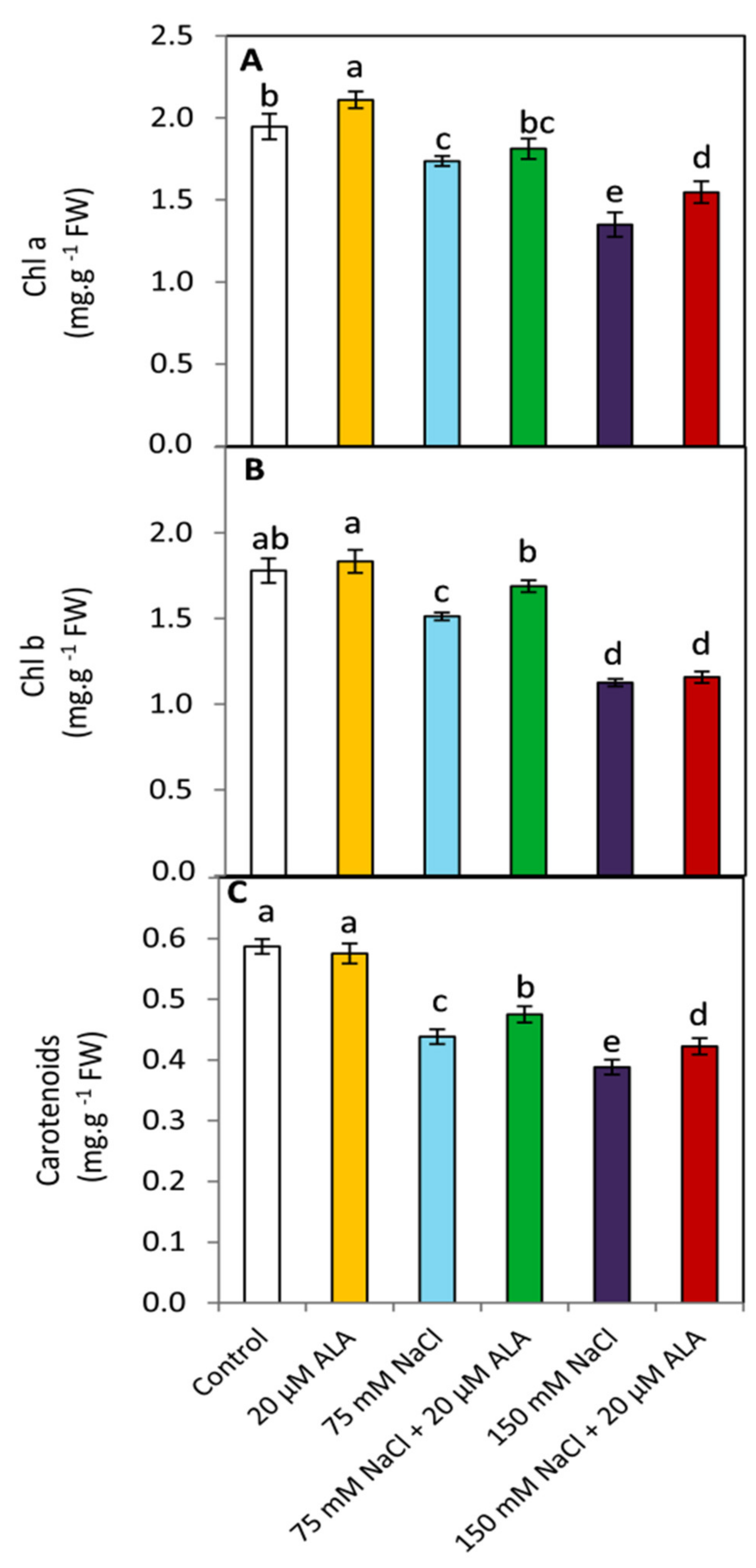
2.4. Determination of Leaf Pigments
2.5. Assay of Antioxidant Enzymes
2.6. Gene Expression
2.7. Statistics
3. Results
3.1. Effect of ALA on Growth Parameters
3.2. Effect of ALA on the Membranes’ Stability and Leaf Oxidative Damage
3.3. Effect of ALA on the Photosynthetic Pigments
3.4. Effect of ALA on the Activities of Antioxidant Enzymes
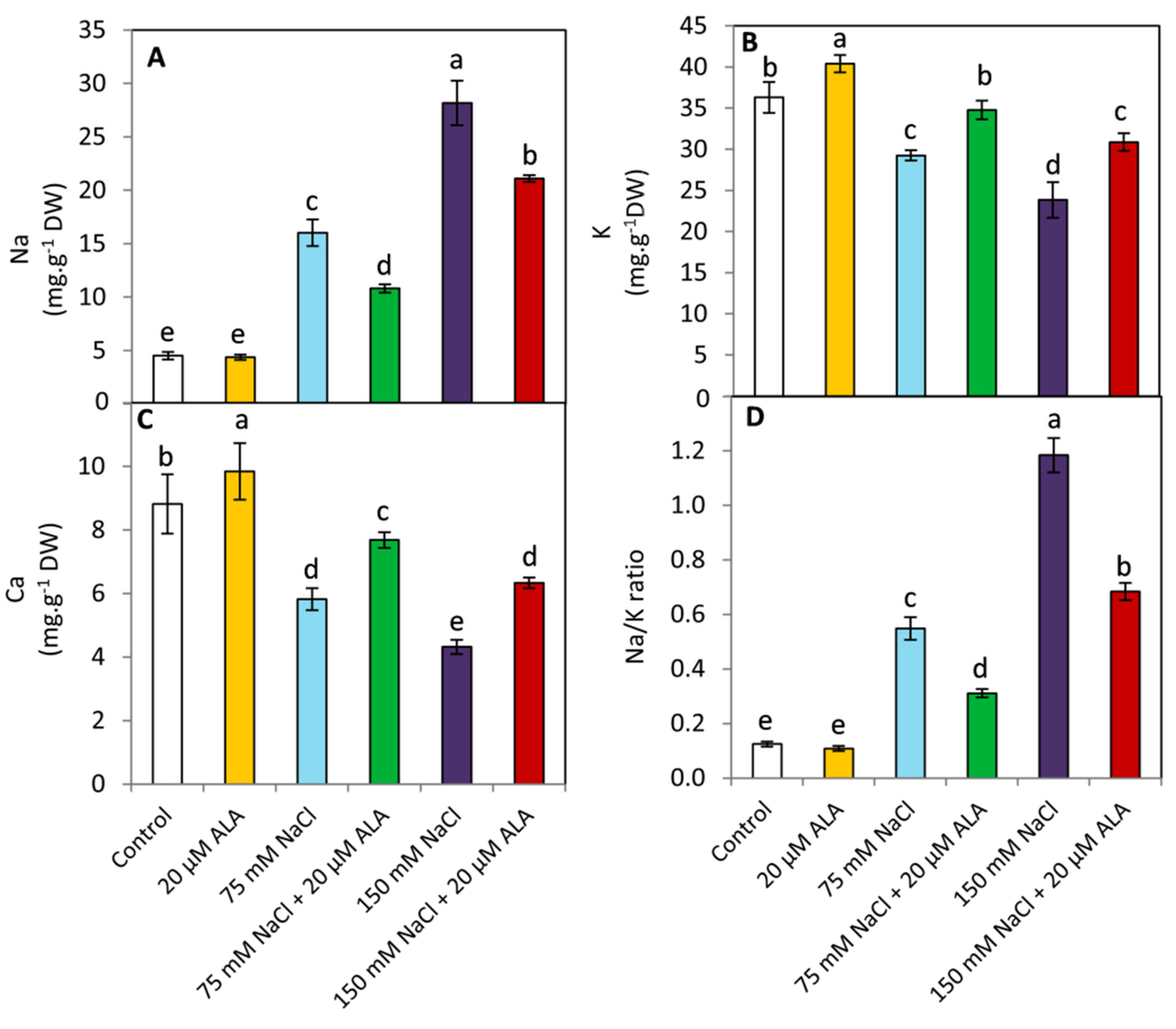
3.5. Effect of ALA on Na, K, Ca and Na/K Ratio
3.6. Effect of ALA on the Expression of SOS1, NHX1 and HKT1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomaz, A.; Palma, P.; Alvarenga, P.; Gonçalves, M.C. Soil salinity risk in a climate change scenario and its effect on crop yield. In Climate Change and Soil Interactions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 351–396. [Google Scholar] [CrossRef]
- Payen, S.; Basset-Mens, C.; Núñez, M.; Follain, S.; Grünberger, O.; Marlet, S.; Perret, S.; Roux, P. Salinisation impacts in life cycle assessment: A review of challenges and options towards their consistent integration. Int. J. Life Cycle Assess. 2016, 21, 577–594. [Google Scholar] [CrossRef]
- Iglesias, M.C.-A. A review of recent advances and future challenges in freshwater salinization. Limnetica 2020, 39, 185–211. [Google Scholar]
- Hossain, M.S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 2019, 1, 1–3. [Google Scholar]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Alnusairi, G.S.; Mazrou, Y.S.; Qari, S.H.; Elkelish, A.A.; Soliman, M.H.; Eweis, M.; Abdelaal, K.; El-Samad, G.A.; Ibrahim, M.F.; ElNahhas, N. Exogenous Nitric Oxide Reinforces Photosynthetic Efficiency, Osmolyte, Mineral Uptake, Antioxidant, Expression of Stress-Responsive Genes and Ameliorates the Effects of Salinity Stress in Wheat. Plants 2021, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- El Nahhas, N.; AlKahtani, M.D.; Abdelaal, K.A.; Al Husnain, L.; AlGwaiz, H.I.; Hafez, Y.M.; Attia, K.A.; El-Esawi, M.A.; Ibrahim, M.F.; Elkelish, A. Biochar and jasmonic acid application attenuates antioxidative systems and improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water. Plant Physiol. Biochem. 2021, 166, 807–817. [Google Scholar] [CrossRef]
- Calone, R.; Sanoubar, R.; Lambertini, C.; Speranza, M.; Vittori Antisari, L.; Vianello, G.; Barbanti, L. Salt tolerance and Na allocation in Sorghum bicolor under variable soil and water salinity. Plants 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Abd Elhady, S.A.; El-Gawad, H.G.A.; Ibrahim, M.F.; Mukherjee, S.; Elkelish, A.; Azab, E.; Gobouri, A.A.; Farag, R.; Ibrahim, H.A.; El-Azm, N.A. Hydrogen peroxide supplementation in irrigation water alleviates drought stress and boosts growth and productivity of potato plants. Sustainability 2021, 13, 899. [Google Scholar] [CrossRef]
- Hoque, T.S.; Hossain, M.A.; Mostofa, M.G.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Methylglyoxal: An emerging signaling molecule in plant abiotic stress responses and tolerance. Front. Plant Sci. 2016, 7, 1341. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, R.; Corpas, F.J.; Carreras, A.; Fernández-Ocaña, A.; Chaki, M.; Luque, F.; Gómez-Rodríguez, M.V.; Colmenero-Varea, P.; Luis, A.; Barroso, J.B. Nitrosative stress in plants. FEBS Lett. 2007, 581, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Singla-Pareek, S.L.; Ray, M.; Reddy, M.; Sopory, S. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem. Biophys. Res. Commun. 2005, 337, 61–67. [Google Scholar] [CrossRef]
- Shohan, M.U.S.; Sinha, S.; Nabila, F.H.; Dastidar, S.G.; Seraj, Z.I. HKT1;5 transporter gene expression and association of amino acid substitutions with salt tolerance across rice genotypes. Front. Plant Sci. 2019, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Djanaguiraman, M.; Stewart, Z.; Ciampitti, I. Agroclimatology of Maize, Sorghum, and Pearl Millet. In Agroclimatology: Linking Agriculture to Climate; Hatfield, J.L., Sivakumar, M.V.K., Prueger, J.H., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2019; pp. 201–241. [Google Scholar] [CrossRef]
- Anglani, C. Sorghum for human food—A review. Plant Foods Hum. Nutr. 1998, 52, 85–95. [Google Scholar] [CrossRef]
- Ayub, M.; Nadeem, M.A.; Tanveer, A.; Husnain, A. Effect of different levels of nitrogen and harvesting times on the growth, yield and quality of sorghum fodder. Asian J. Plant Sci. 2002, 1, 304–307. [Google Scholar] [CrossRef][Green Version]
- Joardar, J.; Razir, S.; Islam, M.; Kobir, M. Salinity impacts on experimental fodder sorghum production. SAARC J. Agric. 2018, 16, 145–155. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.E.; Ullah, F.; Ben-Hur, A.; Reddy, A.S. Transcriptome analysis of drought-resistant and drought-sensitive sorghum (Sorghum bicolor) genotypes in response to PEG-induced drought stress. Int. J. Mol. Sci. 2020, 21, 772. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Chen, P.; Shao, H.; Zhao, S.; Zhang, L.; Zhang, L.; Xu, G.; Sun, J. Responses of photosynthesis and photosystem II to higher temperature and salt stress in Sorghum. J. Agron. Crop. Sci. 2012, 198, 218–225. [Google Scholar] [CrossRef]
- Terzi, R.; Saruhan, G.N.; Güven, F.G.; Kadioglu, A. Alpha lipoic acid treatment induces the antioxidant system and ameliorates lipid peroxidation in maize seedlings under osmotic stress. Arch. Biol. Sci. 2018, 70, 503–511. [Google Scholar] [CrossRef]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Sgherri, C.; Quartacci, M.F.; Izzo, R.; Navari-Izzo, F. Relation between lipoic acid and cell redox status in wheat grown in excess copper. Plant Physiol. Biochem. 2002, 40, 591–597. [Google Scholar] [CrossRef]
- Javeed, H.M.R.; Ali, M.; Skalicky, M.; Nawaz, F.; Qamar, R.; Faheem, M.; Mubeen, M.; Iqbal, M.M.; Vachova, P.; Brestic, M. Lipoic Acid Combined with Melatonin Mitigates Oxidative Stress and Promotes Root Formation and Growth in Salt-Stressed Canola Seedlings (Brassica napus L.). Molecules 2021, 26, 3147. [Google Scholar] [CrossRef]
- Sezgin, A.; Altuntaş, C.; Demiralay, M.; Cinemre, S.; Terzi, R. Exogenous alpha lipoic acid can stimulate photosystem II activity and the gene expressions of carbon fixation and chlorophyll metabolism enzymes in maize seedlings under drought. J. Plant Physiol. 2019, 232, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Turk, H.; Erdal, S.; Karayel, U.; Dumlupinar, R. Attenuation of lead toxicity by promotion of tolerance mechanism in wheat roots by lipoic acid. Cereal Res. Commun. 2018, 46, 424–435. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, J.; Kumar, P. Effects of plant growth regulators and sucrose on post harvest physiology, membrane stability and vase life of cut spikes of gladiolus. Plant Growth Regul. 2008, 55, 221. [Google Scholar] [CrossRef]
- Abd El-Gawad, H.G.; Mukherjee, S.; Farag, R.; Abd Elbar, O.H.; Hikal, M.; Abou El-Yazied, A.; Abd Elhady, S.A.; Helal, N.; ElKelish, A.; El Nahhas, N. Exogenous γ-aminobutyric acid (GABA)-induced signaling events and field performance associated with mitigation of drought stress in Phaseolus vulgaris L. Plant Signal. Behav. 2021, 16, 1853384. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Costache, M.A.; Campeanu, G.; Neata, G. Studies concerning the extraction of chlorophyll and total carotenoids from vegetables. Rom. Biotechnol. Lett. 2012, 17, 7702–7708. [Google Scholar]
- De Carvalho, L.M.J.; Gomes, P.B.; de Oliveira Godoy, R.L.; Pacheco, S.; do Monte, P.H.F.; de Carvalho, J.L.V.; Nutti, M.R.; Neves, A.C.L.; Vieira, A.C.R.A.; Ramos, S.R.R. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): A preliminary study. Food Res. Int. 2012, 47, 337–340. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Dias, M.A.; Costa, M.M. Effect of low salt concentrations on nitrate reductase and peroxidase of sugar beet leaves. J. Exp. Bot. 1983, 34, 537–543. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Havre, G.N. The flame photometric determination of sodium, potassium and calcium in plant extracts with special reference to interference effects. Anal. Chim. Acta 1961, 25, 557–566. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- SAS. SAS/STAT User’s Guide, 3rd ed.; Release 6.0; SAS Inst. Inc.: Cary, NC, USA, 1988. [Google Scholar]
- Kamal, K.Y.; Khodaeiaminjan, M.; Yahya, G.; El-Tantawy, A.A.; Abdel El-Moneim, D.; El-Esawi, M.A.; Abd-Elaziz, M.A.; Nassrallah, A.A. Modulation of cell cycle progression and chromatin dynamic as tolerance mechanisms to salinity and drought stress in maize. Physiol. Plant. 2021, 172, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Netondo, G.W.; Onyango, J.C.; Beck, E. Sorghum and salinity: I. Response of growth, water relations, and ion accumulation to NaCl salinity. Crop Sci. 2004, 44, 797–805. [Google Scholar] [CrossRef]
- Skaggs, T.H.; van Genuchten, M.T.; Shouse, P.J.; Poss, J.A. Macroscopic approaches to root water uptake as a function of water and salinity stress. Agric. Water Manag. 2006, 86, 140–149. [Google Scholar] [CrossRef]
- Navari-Izzo, F.; Quartacci, M.F.; Sgherri, C. Lipoic acid: A unique antioxidant in the detoxification of activated oxygen species. Plant Physiol. Biochem. 2002, 40, 463–470. [Google Scholar] [CrossRef]
- Hasan, M.; Alabdallah, N.M.; Alharbi, B.M.; Waseem, M.; Yao, G.; Liu, X.-D.; El-Gawad, A.; Hany, G.; El-Yazied, A.A.; Ibrahim, M.F. GABA: A Key Player in Drought Stress Resistance in Plants. Int. J. Mol. Sci. 2021, 22, 10136. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ibrahim, H.A.; Abd El-Gawad, H. Folic acid as a protective agent in snap bean plants under water deficit conditions. J. Hortic. Sci. Biotechnol. 2021, 96, 94–109. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; El-Samad, A.; Ashour, H.; El-Sawy, A.M.; Hikal, M.; Elkelish, A.; El-Gawad, H.A.; El-Yazied, A.A.; Hozzein, W.N.; Farag, R. Regulation of agronomic traits, nutrient uptake, osmolytes and antioxidants of maize as influenced by exogenous potassium silicate under deficit irrigation and semiarid conditions. Agronomy 2020, 10, 1212. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; Abou El-Yazied, A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.; Ibrahim, M.F. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins—Major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Melkozernov, A.N.; Blankenship, R.E. Photosynthetic functions of chlorophylls. In Chlorophylls and Bacteriochlorophylls; Springer: Berlin/Heidelberg, Germany, 2006; pp. 397–412. [Google Scholar] [CrossRef]
- Parry, A.D.; Horgan, R. Carotenoids and abscisic acid (ABA) biosynthesis in higher plants. Physiol. Plant. 1991, 82, 320–326. [Google Scholar] [CrossRef]
- Seo, M.; Koshiba, T. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002, 7, 41–48. [Google Scholar] [CrossRef]
- Hasan, M.; Rahman, M.A.; Skalicky, M.; Alabdallah, N.M.; Waseem, M.; Jahan, M.S.; Ahammed, G.J.; El-Mogy, M.M.; El-Yazied, A.A.; Ibrahim, M.F. Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int. J. Mol. Sci. 2021, 22, 6304. [Google Scholar] [CrossRef]
- Ibrahim, M. Induced drought resistance in common bean (Phaseolus vulgaris L.) by exogenous application with active yeast suspension. Middle East J. Appl. Sci. 2014, 4, 806–815. [Google Scholar]
- Ibrahim, M.F.; Elbar, O.H.A.; Farag, R.; Hikal, M.; El-Kelish, A.; El-Yazied, A.A.; Alkahtani, J.; El-Gawad, H.G.A. Melatonin counteracts drought induced oxidative damage and stimulates growth, productivity and fruit quality properties of tomato plants. Plants 2020, 9, 1276. [Google Scholar] [CrossRef]
- Jing, X.; Hou, P.; Lu, Y.; Deng, S.; Li, N.; Zhao, R.; Sun, J.; Wang, Y.; Han, Y.; Lang, T. Overexpression of copper/zinc superoxide dismutase from mangrove Kandelia candel in tobacco enhances salinity tolerance by the reduction of reactive oxygen species in chloroplast. Front. Plant Sci. 2015, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Data, P. Alpha-lipoic acid. Arzneimittelforschung 1995, 45, 872–874. [Google Scholar]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Gorcek, Z.; Erdal, S. Lipoic acid mitigates oxidative stress and recovers metabolic distortions in salt-stressed wheat seedlings by modulating ion homeostasis, the osmo-regulator level and antioxidant system. J. Sci. Food Agric. 2015, 95, 2811–2817. [Google Scholar] [CrossRef]
- Elkelish, A.; El-Mogy, M.M.; Niedbała, G.; Piekutowska, M.; Atia, M.A.M.; Hamada, M.M.A.; Shahin, M.; Mukherjee, S.; El-Yazied, A.A.; Shebl, M.; et al. Roles of Exogenous α-Lipoic Acid and Cysteine in Mitigation of Drought Stress and Restoration of Grain Quality in Wheat. Plants 2021, 10, 2318. [Google Scholar] [CrossRef]



| Gene Name | Sequence | NCBI Accession | |
|---|---|---|---|
| SOS1 | F | 5′-ACTTGCAGGAGGAATACAAC-3′ | NM001176582 |
| R | 5′- CGAGAAGAGAAGACCACATC-3′, | ||
| HKT1 | F | 5′-TGCTAATGTTTATCGTGCTG-3′ | HQ845286 |
| R | 5′-AGGCTGATCCTCTTCCTAAC-3′ | ||
| NHX1 | F | 5′-CGTGATGTCGCATTACACCT-3′ | AY270040 |
| R | 5′- CTGGCAAACTCCCACTTCTC-3′ | ||
| GAPDH | F | 5′-TGACGACATCAAGAAGGTGGTG-3′ | NM_001082253 |
| R | 5′-:GAAGGTGGAGGAGTGGGTGTC-3′ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, M.H.M.; Raafat, A.; El-Yazied, A.A.; Selim, S.; Azab, E.; Khojah, E.; El Nahhas, N.; Ibrahim, M.F.M. Exogenous Application of Alpha-Lipoic Acid Mitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes. Plants 2021, 10, 2519. https://doi.org/10.3390/plants10112519
Youssef MHM, Raafat A, El-Yazied AA, Selim S, Azab E, Khojah E, El Nahhas N, Ibrahim MFM. Exogenous Application of Alpha-Lipoic Acid Mitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes. Plants. 2021; 10(11):2519. https://doi.org/10.3390/plants10112519
Chicago/Turabian StyleYoussef, Montaser H. M., Aly Raafat, Ahmed Abou El-Yazied, Samy Selim, Ehab Azab, Ebtihal Khojah, Nihal El Nahhas, and Mohamed F. M. Ibrahim. 2021. "Exogenous Application of Alpha-Lipoic Acid Mitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes" Plants 10, no. 11: 2519. https://doi.org/10.3390/plants10112519
APA StyleYoussef, M. H. M., Raafat, A., El-Yazied, A. A., Selim, S., Azab, E., Khojah, E., El Nahhas, N., & Ibrahim, M. F. M. (2021). Exogenous Application of Alpha-Lipoic Acid Mitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes. Plants, 10(11), 2519. https://doi.org/10.3390/plants10112519