The Variability for the Biochemical Indicators at the Winter Wheat Assortment and Identifying the Sources with a High Antioxidant Activity
Abstract
:1. Introduction
2. Results
- -
- Ratio between peroxidase content after 25% PEG treatment and peroxidase content in the control-significant correlation.
- -
- Ratio between catalase content at 25% PEG treatment and control catalase content—distinctly significant correlation;
- -
- Ratio between catalase content at 40% PEG treatment and control catalase content—significant correlation.
- The ratio between the stem length at 15% PEG treatment and the length of the stem at the control after 24 days from sowing (T2);
- The ratio of stem length to 15% PEG treatment and control stem length on average on determinations made at 15, 24, and 35 days;
- The ratio of stem length to 20% PEG treatment and control stem length on average on determinations made at 15, 24, and 35 days;
- The ratio of stem weight to the 15% PEG treatment and the weight of the stem to the control.
- -
- Distinctly significant positive with the ratio of peroxidase content to 20% PEG treatment and peroxidase content to control.
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Laboratory Researche Methods
- -
- The activity of peroxidase (guaiacol-peroxidase type E.C.1.11.1.7) was determined colorimetrically at λ = 470 nm and was expressed as the variation in absorbance per minute due to the oxidation of guaiacol from the extract of one gram of fresh substance [51].
- -
- The activity of catalase (E.C.1.11.1.6) was determined by the Sinha method by colorimetrically determining the amount of H2O2 decomposed for 1 min by the enzyme in 1 g fresh substance. The method is based on the fact that potassium chromate in acidic medium is reduced by hydrogen peroxide to chromic acetate, which can be colorimetric at 570 nm [52,53].
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okarter, N.; Liu, C.S.; Sorrells, M.E.; Liu, R.H. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010, 119, 249–257. [Google Scholar] [CrossRef]
- Mughal, I.; Shah, Y.; Tahir, S.; Haider, W.; Fayyaz, M.; Yasmin, T.; Ilyas, M.; Farrakh, S. Protein quantification and enzyme activity estimation of Pakistani wheat landraces. PLoS ONE 2020, 15, e0239375. [Google Scholar] [CrossRef]
- Narwal, S.; Thakur, V.; Sheoran, S.; Dahiya, S.; Jaswal, S.; Gupta, R.K. Antioxidant activity and phenolic content of the Indian wheat varieties. J. Plant Biochem. Biotechnol. 2014, 23, 11–17. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef]
- Tricker, P.J.; ElHabti, A.; Schmidt, J.; Fleury, D. The physiological and genetic basis of combined drought and heat tolerance in wheat. J. Exp. Bot. 2018, 69, 3195–3210. [Google Scholar] [CrossRef] [Green Version]
- Khanna-Chopra, R.; Selote, D.S. Acclimation to drought stress generates oxidative stress tolerance in drought-resistant than-susceptible wheat cultivar under field conditions. Environ. Expt. Bot. 2007, 60, 276–283. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhang, M.; Zhao, Z.; Ren, Y.; Li, Q.; Wang, W. Wheat TaPUB1 modulates plant drought stress resistance by improving antioxidant capability. Sci. Rep. 2017, 7, 7549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, K.J. Thiol-based peroxidases and ascorbate peroxidases: Why plants rely on multiple peroxidase systems in the photosynthesizing chloroplast? Mol. Cells 2016, 39, 20–25. [Google Scholar]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought Stress Tolerance in Wheat and Barley: Advances in Physiology, Breeding and Genetics Research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.N.; Kirkha, M.B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Saxena, D.C. Role of antioxidant systems in wheat genotypes tolerance to water stress. Biol. Plant. 1998, 41, 384–394. [Google Scholar] [CrossRef]
- Kido, É.A.; Ferreira-Neto, J.R.C.; Pandolfi, V.; de Melo Souza, A.C.; Benko-Iseppon, A.M. Drought Stress Tolerance in Plants: Insights from Transcriptomic Studies. In Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2016; Volume 2, pp. 153–185. [Google Scholar]
- Khazayi, H.; Kafi, M.; Masumi, A. Physiological effects of stress induced by polyethylene glycol on germination of chickpea genotypes. J. Agron. Res. Iran 2008, 2, 453. [Google Scholar]
- Chakraborty, U.; Pradhan, B. Oxidative stress in five wheat varieties (Triticum aestivum L.) exposed to water stress and study of their antioxidant enzyme defense system, water stress responsive metabolites and H2O2 accumulation. Braz. J. Plant Physiol. 2012, 24, 17–130. [Google Scholar] [CrossRef] [Green Version]
- Păunescu, A.; Dodocioiu, A.M.; Băbeanu, C.; Păunescu, G.; Buzatu, G.D. Total phenols content and antioxidant activity of whole grain flours from some wheat lines tested in the south-west of Romania. SGEM 2016, 1, 845–852. [Google Scholar]
- Zhang, Y.; Shih, D.S. Isolation of an osmotion like protein genefron strawerry and analysis of the response of this gene to abiotic stresses. J. Plant Physiol. 2007, 164, 68–77. [Google Scholar] [CrossRef]
- Tașgin, E.; Okkeș, A.; Nalbantoglu, B.; Petrova Popova, L. Effects of salicylic acid and cold treatments on protein levels and on the activities of antioxidant enzymes in the apoplast of winter wheat leaves. Phytochemistry 2006, 67, 71–75. [Google Scholar] [CrossRef]
- Nikolaeva, M.K.; Maevskaya, S.N.; Shugaev, A.G.; Bukhov, N.G. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of the wheat cultivars varying in productivity. Russ. J. Plant Physiol. 2010, 57, 87–95. [Google Scholar] [CrossRef]
- Hameed, A.; Goher, M.; Iqbal, N. Biochemicals Indices of Drought Tolerance in Wheat (Triticum aestivum L.) at Early Seedling Stage. Philipp. Agric. Sci. 2014, 97, 236–242. [Google Scholar]
- Sallam, A.; Amro, A.; EL-Akhdar, A.; Dawood, M.F.A.; Kumamaru, T.; Stephen Baenziger, P. Genetic diversity and genetic variation in morpho-physiological traits to improve heat tolerance in Spring barley. Mol. Biol. Rep. 2018, 45, 2441–2453. [Google Scholar] [CrossRef] [PubMed]
- Al Abdallat, A.M.; Ayad, J.Y.; Abu Elenein, J.M.; Al Ajlouni, Z.; Harwood, W.A. Overexpression of the transcription factor HvSNAC1 improves drought tolerance in barley (Hordeum vulgare L.). Mol. Breed. 2014, 33, 401–414. [Google Scholar] [CrossRef]
- Păunescu, G.; Păunescu, A.R. Identification of wheat varieties tolerant to water stress based on ratio between the stem growth measured in seedlings after 20% PEG treatment and the stem growth measured after water treatment 15 days after sowing. EWAC 2018, 17, 91–97. [Google Scholar]
- Păunescu, R.A. The stem growth measured in seedlings after 20% PEG treatment 15 days after sowing is significantly correled with field response to drought in the field. Rom. Agric. Res. 2018, 35, 29–37. [Google Scholar]
- Zahoor, A.; Waraich, E.A.; Barutçular, C.; Hossain, A.; Erman, M.; Çiğ, F.; Gharib, H. El Sabagh, A. Enhancing drought tolerance in wheat through improving morphophysiological and antioxidants activities of plants by the supplementation of foliar silicon. Phyton 2020, 89, 529–539. [Google Scholar]
- Wang, J.Y.; Xiong, Y.C.; Li, F.M.; Siddique, K.H.M.; Turner, N.C. Effects of drought stress on morphophysiological traits, biochemical characteristics, yield and yield components in different ploidy wheat: A meta-analysis. Adv. Agron. 2017, 143, 139–173. [Google Scholar]
- El Sabagh, A.; Hossain, A.; Barutcular, C.; Islam, M.S.; Awan, S.I.; Galal, A.; Iqbal, M.A.; Sytar, O.; Yildirim, M.; Meena, R.S.; et al. Wheat (Triticum aestivum L.) production under drought and heat stress-adverse effects, mechanisms and mitigation: A review. Appl. Ecol. Environ. Res. 2019, 17, 8307–8332. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, G.K.; Jwa, N.S.; Iwahashi, H.; Rakwal, R. Importance of ascorbate peroxidases OsAPX1 and OsAPX2 in the rice pathogen response pathways and growth and reproduction revealed by their transcriptional profiling. Gene 2003, 322, 93–103. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Passaia, G.; Barcellos Rosa, R.; Werner Ribeiro, C.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Cai, J.; Yang, F.X.; Zhou, B.; Zhou, L.R. Ascorbate peroxidase from Jatropha curcas enhances salt tolerance in transgenic Arabidopsis. Genet. Mol. Res. 2015, 14, 4879–4889. [Google Scholar] [CrossRef]
- Fryer, M.J.; Ball, L.; Oxborough, K.; Karpinski, S.; Mullineaux, P.M.; Baker, N.R. Control of Ascorbate Peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. 2003, 33, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.A.; Omara, I.R.; Hafez, M.Y.; Samar, M.E.; El Sabagh, A. Anatomical, biochemical and physiological changes in some Egyptian wheat cultivars inoculated with Puccinia gramini f. sp. tritici. Fresenius Environ. Bull. 2018, 27, 296–305. [Google Scholar]
- Shen, X.; Zhou, Y.; Duan, L.; Li, Z.; Eneji, A.E.; Li, J. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J. Plant Physiol. 2010, 167, 1248–1252. [Google Scholar] [CrossRef]
- Yu, Q.; Rengel, Z. Drought and salinity differentially influence activities of superoxide dismutase in narrow-leafed lupins. Plant Sci. 1999, 142, 1–11. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Prasad, T.K. Mechanisms of chilling-induced oxidative stress injury and tolerance in developing maize seedlings: Changes in antioxidant system, oxidation of proteins and lipids, and protease activities. Plant J. 1996, 10, 1017–1026. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sharma, S.K. Plants as natural antioxidants. Nat. Prod. Radiance 2006, 5, 326–334. [Google Scholar]
- Jallouli, S.; Ayadi, S.; Landi, S.; Capasso, G.; Santini, G.; Chamekh, Z.; Zouari, I.; Ben Azaiez, F.E.; Trifa, Y.; Esposito, S. Physiological and Molecular Osmotic Stress Responses in Three Durum Wheat (Triticum Turgidum ssp Durum) Genotypes. Agronomy 2019, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Kirova, E.; Pecheva, D.; Simova-Stoilova, L. Drought response in winter wheat: Protection from oxidative stress and mutagenesis effect. Acta Physiol. Plant. 2021, 43, 8. [Google Scholar] [CrossRef]
- Simova-Stoilova, L.; Kirova, E.; Pecheva, D. Drought stress response in winter wheat varieties–changes in leaf proteins and proteolytic activities. Acta Bot. Croat. 2020, 79, 121–130. [Google Scholar] [CrossRef]
- Kartseva, T.; Dobrikova, A.; Kocheva, K.; Alexandrov, V.; Georgiev, G.; Brestič, M.; Misheva, S. Optimal Nitrogen Supply Ameliorates the Performance of Wheat Seedlings under Osmotic Stress in Genotype-Specific Manner. Plants 2021, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.B.; Liang, Z.S.; Shao, M.A.; Sun, Q. Dynamic changes of antioxidative enzymes of 10 wheat genotypes at soil water deficits. Colloids Surf. B Biointerfaces 2005, 42, 187–195. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal. Curr. Sci. 2005, 89, 1113–1121. [Google Scholar]
- Farnese, F.S.; Menezes-Silva, P.E.; Gusman, G.S.; Oliveira, J.A. When bad guys become good ones: The key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front. Plant Sci. 2016, 7, 471. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [Green Version]
- Todorova, D.; Sergiev, I.; Katerova, Z.; Shopova, E.; Dimitrova, L.; Brankova, L. Assessment of the Biochemical Responses of Wheat Seedlings to Soil Drought after Application of Selective Herbicide. Plants 2021, 10, 733. [Google Scholar] [CrossRef]
- Putter, J. Peroxidase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinhan, Germany, 1974; pp. 685–690. [Google Scholar]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–391. [Google Scholar] [CrossRef]
- Paunescu, R.A. Identification of Resistant Phenotypes in order to Improve the Behaviour of Wheat in Drought Conditions on the Luvisoil from Simnic. Ph.D. Thesis, University of Agriculture and Veterinary Medicine, Bucharest, Romania, 2017. [Google Scholar]
- Gavuzzi, P.; Rizza, F.; Palumbo, M.; Campaline, R.G.; Ricciardi, G.L.; Borghi, B. Evaluation of field and laboratory predictors of drought and heat tolerance in winter cereals. Can. J. Plant Sci. 1997, 77, 523–553. [Google Scholar] [CrossRef]
- Hawkins, D. Biomeasurement: A Student’s Guide to Biological Statistics, 2rd ed.; Oxford University Press: Oxford, UK, 2009; pp. 10–360. [Google Scholar]
| No. | Cultivar | PEROXIDASE (ΔA/1 min/1 gsp) | ||||
|---|---|---|---|---|---|---|
| In Normal Conditions (Water–Control) | In Water-Stress-Induced Conditions (25% PEG 10,000) | In Water-Stress-Induced Conditions (40% PEG 4000) | Ratio PEG (25%/Control) | Ratio PEG (40%/Control) | ||
| 35. | KRISTINA | 57.60 | 211.43 | 319.02 | 3.671 | 5.539 |
| 39. | MOLDAU | 91.69 | 308.8 | 0 | 3.368 | 0.000 |
| 40. | MV PALMA | 66.21 | 220.78 | 0 | 3.335 | 0.000 |
| 50. | TRIVALE | 23.27 | 71.31 | 0 | 3.064 | 0.000 |
| 31. | GK HATTYU | 104.88 | 293 | 431.14 | 2.794 | 4.111 |
| 36. | LADA | 72.94 | 188.09 | 141.2 | 2.579 | 1.936 |
| 48. | SHOHAM | 71.46 | 177.44 | 0 | 2.483 | 0.000 |
| 30. | GRUIA | 75.75 | 176.8 | 200.6 | 2.334 | 2.648 |
| 47. | ROMULUS | 75.16 | 168.75 | 0 | 2.245 | 0.000 |
| 34. | KARLYGASH | 72.01 | 158.66 | 275.62 | 2.203 | 3.828 |
| 41. | NIKIFOR | 85.89 | 180.38 | 100.54 | 2.100 | 1.171 |
| 29. | GK GOBE | 119.08 | 238.71 | 174.45 | 2.005 | 1.465 |
| 43. | ORQUAL | 69.33 | 134.46 | 0 | 1.939 | 0.000 |
| 25. | GIAVA | 111.83 | 216 | 321.96 | 1.932 | 2.879 |
| 33. | IZVOR | 114.15 | 216.16 | 190.17 | 1.894 | 1.666 |
| 27. | GK ELET | 105.89 | 199.7 | 387 | 1.886 | 3.655 |
| 21. | EXOTIC | 76.19 | 141 | 332 | 1.851 | 4.358 |
| 42. | ORATORIO | 72.12 | 128.97 | 0 | 1.788 | 0.000 |
| 18. | ELIANA | 85.96 | 125.55 | 87.59 | 1.461 | 1.019 |
| 26. | GK DAVID | 207.61 | 301 | 543.27 | 1.450 | 2.617 |
| 46. | ROMANSA | 111.45 | 157.97 | 76.76 | 1.417 | 0.689 |
| 11. | CUBUS | 108.57 | 151.5 | 219.64 | 1.395 | 2.023 |
| 1. | AGRON | 134.4 | 176 | 218.3 | 1.310 | 1.624 |
| 5. | BITOP | 140.21 | 176.25 | 162.7 | 1.257 | 1.160 |
| 38. | LOVRIN 34 | 126.92 | 144.34 | 0 | 1.137 | 0.000 |
| 6. | BOEMA | 151.28 | 171.53 | 165 | 1.134 | 1.091 |
| 13. | DELABRAD | 122.7 | 137.88 | 166.65 | 1.124 | 1.358 |
| 28. | GLOSA | 137.53 | 151.6 | 372 | 1.102 | 2.705 |
| 2. | ALEX | 130.9 | 141.28 | 191.75 | 1.079 | 1.465 |
| 23. | FLAMURA 85 | 171 | 176 | 316 | 1.029 | 1.848 |
| 3. | AZTEC | 138 | 140.77 | 161 | 1.020 | 1.167 |
| 32. | MIRANDA | 90.6 | 92.25 | 170.25 | 1.018 | 1.879 |
| 22. | FAUR | 107.55 | 108 | 184.48 | 1.004 | 1.715 |
| 14. | DEMETRA | 129.66 | 128.76 | 215.12 | 0.993 | 1.659 |
| 7. | SIMNIC 50 | 121.1 | 113.92 | 255.1 | 0.941 | 2.107 |
| 16. | DROPIA | 120.4 | 111.57 | 140.68 | 0.927 | 1.168 |
| 19. | ENESCO | 132.89 | 121.96 | 321.83 | 0.918 | 2.422 |
| 24. | GABRIELA | 149.67 | 135.62 | 265 | 0.906 | 1.771 |
| 20. | ESQUISIT | 199.74 | 168.06 | 219.21 | 0.841 | 1.097 |
| 12. | DARIEL | 132.97 | 110.44 | 163.74 | 0.831 | 1.231 |
| 4. | BEZOSTAIA | 162.83 | 130.92 | 175 | 0.804 | 1.075 |
| 15. | DOR | 160.66 | 126.42 | 166.11 | 0.787 | 1.034 |
| 17. | DUNAI | 164.75 | 125.78 | 211.99 | 0.763 | 1.287 |
| 49. | SIMNIC 30 | 59.58 | 43.96 | 0 | 0.738 | 0.000 |
| 10. | CRINA | 133.22 | 84.7 | 138.87 | 0.636 | 1.042 |
| 44. | JULIUS | 207.74 | 121.65 | 0 | 0.586 | 0.000 |
| 8. | CAPO | 179.5 | 103.76 | 182.28 | 0.578 | 1.015 |
| 37. | LITERA | 139.04 | 77.74 | 0 | 0.559 | 0.000 |
| 45. | NATHAN | 238.33 | 113.04 | 0 | 0.474 | 0.000 |
| 9. | CAROLINA | 126.33 | 56.63 | 163.25 | 0.448 | 1.292 |
| No. | Cultivar | ASCORBAT PEROXIDAZE (μgAsA/1 min/1 gsp) | ||||
|---|---|---|---|---|---|---|
| In Normal Conditions—Water–Control | In Water-Stress-Induced Conditions—25% PEG 10,000 | In Water-Stress-Induced Conditions—40% PEG 4000 | Ratio PEG 25%/Control | Ratio PEG 40%/Control | ||
| 50. | TRIVALE | 4693 | 40,522 | 0 | 8.635 | 0.000 |
| 49. | SIMNIC 30 | 4754 | 35,528 | 0 | 7.473 | 0.000 |
| 29. | GK GOBE | 2990 | 21,994 | 3661 | 7.356 | 1.224 |
| 8. | CAPO | 16,313 | 57,206 | 4550 | 3.507 | 0.279 |
| 9. | CAROLINA | 5834 | 17,921 | 11,101 | 3.072 | 1.903 |
| 7. | SIMNIC 50 | 9829 | 29,888 | 14,349 | 3.041 | 1.460 |
| 11. | CUBUS | 4606 | 11,865 | 13,154 | 2.576 | 2.856 |
| 40. | MV PALMA | 52,089 | 129,907 | 0 | 2.494 | 0.000 |
| 10. | CRINA | 13,795 | 33,881 | 30,699 | 2.456 | 2.225 |
| 41. | NIKIFOR | 75,225 | 179,104 | 41,893 | 2.381 | 0.557 |
| 37. | LITERA | 26,825 | 62,474 | 0 | 2.329 | 0.000 |
| 14. | DEMETRA | 9350 | 20,147 | 6711 | 2.155 | 0.718 |
| 48. | SHOHAM | 34,285 | 70,888 | 0 | 2.068 | 0.000 |
| 23. | FLAMURA 85 | 31,788 | 60,423 | 80,790 | 1.901 | 2.542 |
| 5. | BITOP | 13,523 | 24,456 | 20,302 | 1.808 | 1.501 |
| 6. | BOEMA | 11,930 | 20,898 | 0 | 1.752 | 0.000 |
| 22. | FAUR | 29,801 | 51,107 | 34,830 | 1.715 | 1.169 |
| 24. | GABRIELA | 33,376 | 51,546 | 43,200 | 1.544 | 1.294 |
| 30. | GRUIA | 18,476 | 28,177 | 13,396 | 1.525 | 0.725 |
| 21. | EXOTIC | 20,906 | 31,348 | 52,677 | 1.499 | 2.520 |
| 3. | AZTEC | 12,420 | 17,802 | 4565 | 1.433 | 0.368 |
| 28. | GLOSA | 28,813 | 37,094 | 14,970 | 1.287 | 0.520 |
| 39. | MOLDAU | 63,595 | 70,754 | 0 | 1.113 | 0.000 |
| 43. | ORQUAL | 35,074 | 38,866 | 0 | 1.108 | 0.000 |
| 16. | DROPIA | 23,299 | 25,689 | 22,446 | 1.103 | 0.963 |
| 17. | DUNAI | 28,531 | 31,389 | 18,868 | 1.100 | 0.661 |
| 36. | LADA | 13,827 | 13,470 | 16,152 | 0.974 | 1.168 |
| 12. | DARIEL | 7299 | 6995 | 12,564 | 0.958 | 1.721 |
| 27. | GK ELET | 25,027 | 23,328 | 7278 | 0.932 | 0.291 |
| 2. | ALEX | 9663 | 9001 | 5758 | 0.931 | 0.596 |
| 25. | GIAVA | 56,518 | 50,899 | 61,391 | 0.901 | 1.086 |
| 45. | NATHAN | 34,463 | 29,747 | 0 | 0.863 | 0.000 |
| 13. | DELABRAD | 20,398 | 17,253 | 20,925 | 0.846 | 1.026 |
| 15. | DOR | 20,618 | 17,412 | 39,525 | 0.845 | 1.917 |
| 35. | KRISTINA | 37,092 | 29,585 | 0 | 0.798 | 0.000 |
| 47. | ROMULUS | 46,875 | 35,744 | 0 | 0.763 | 0.000 |
| 38. | LOVRIN 34 | 29,747 | 21,739 | 0 | 0.731 | 0.000 |
| 33. | IZVOR | 43,547 | 29,441 | 26,106 | 0.676 | 0.599 |
| 1. | AGRON | 10,847 | 6428 | 6007 | 0.593 | 0.554 |
| 46. | PKB ROMANSA | 70,721 | 39,318 | 60,942 | 0.556 | 0.862 |
| 32. | MIRANDA | 51,011 | 27,675 | 15,796 | 0543 | 0.310 |
| 26. | GK DAVID | 35,090 | 18,654 | 0 | 0.532 | 0.000 |
| 4. | BEZOSTAIA | 9221 | 45,85 | 5098 | 0.497 | 0.553 |
| 42. | ORATORIO | 73,625 | 31,888 | 0 | 0.433 | 0.000 |
| 44. | JULIUS | 80,042 | 30,550 | 0 | 0.382 | 0.000 |
| 19. | ENESCO | 65,789 | 24,314 | 12,227 | 0.370 | 0.186 |
| 18. | ELIANA | 36,772 | 13,413 | 29,207 | 0.365 | 0.794 |
| 31. | GK HATTYU | 17,537 | 6002 | 12,975 | 0.342 | 0.740 |
| 34. | KARLYGASH | 34,110 | 11,376 | 6852 | 0.334 | 0.201 |
| 20. | ESQUISIT | 10,981 | 2838 | 20,284 | 0.258 | 1.847 |
| No. | Cultivar | CATALASE | ||||
|---|---|---|---|---|---|---|
| In Normal Conditions—Water–Control | In Water-Stress-Induced Conditions—25% PEG 10,000 | In Water-Stress-Induced Conditions—40% PEG 4000 | Ratio PEG 25%/Control | Ratio PEG 40%/Control | ||
| 48. | SHOHAM | 656 | 3318 | - | 5.058 | - |
| 16. | DROPIA | 298 | 1407 | 1492 | 4.721 | 5.007 |
| 35. | KRISTINA | 896.8 | 3155.81 | 5534.08 | 3.519 | 6.171 |
| 4. | BEZOSTAIA | 1536.98 | 4471 | 1087.7 | 2.909 | 0.708 |
| 50. | TRIVALE | 987 | 2765 | - | 2.801 | - |
| 42. | ORATORIO | 1652 | 4578 | - | 2.771 | - |
| 31. | GK HATTYU | 1945.44 | 5378.13 | 5536.33 | 2.764 | 2.846 |
| 18. | ELIANA | 1046 | 2862 | 290 | 2.736 | 0.277 |
| 41. | NIKIFOR | 1288 | 3422 | 3422 | 2.657 | 2.657 |
| 9. | CAROLINA | 435.73 | 1142.05 | - | 2.621 | - |
| 34. | KARLYGASH | 1819.21 | 3519.15 | 2557.91 | 1.934 | 1.406 |
| 49. | SIMNIC 30 | 982 | 1876 | - | 1.910 | - |
| 20. | ESQUISIT | 1749 | 3309 | 3137 | 1.892 | 1.794 |
| 36. | LADA | 1482.57 | 2753.87 | 4020.1 | 1.857 | 2.712 |
| 7. | SIMNIC 50 | 366.97 | 680.13 | - | 1.853 | - |
| 33. | IZVOR | 929 | 1685.36 | 1021.05 | 1.814 | 1.099 |
| 22. | FAUR | 763 | 1362.8 | - | 1.786 | - |
| 46. | PKB ROMANSA | 1128 | 1864 | - | 1.652 | - |
| 19. | ENESCO | 701 | 1141 | 4565 | 1.628 | 6.512 |
| 28. | GLOSA | 1782.55 | 2868.62 | 3832.3 | 1.609 | 2.150 |
| 27. | GK ELET | 1993.32 | 3110.42 | 4885.5 | 1.560 | 2.451 |
| 43. | ORQUAL | 1808 | 2784 | - | 1.540 | - |
| 30. | GRUIA | 1839.45 | 2604 | 2703.61 | 1.416 | 1.470 |
| 11. | CUBUS | 1253 | 1654 | 1497 | 1.320 | 1.195 |
| 45. | NATHAN | 1387 | 1829 | - | 1.319 | - |
| 32. | MIRANDA | 1432.37 | 1668.02 | 1904.12 | 1.165 | 1.329 |
| 26. | GK DAVID | 3992.5 | 4158.68 | 1923.07 | 1.042 | 0.482 |
| 29. | GK GOBE | 3472.56 | 3501.76 | 3645.2 | 1.008 | 1.050 |
| 12. | DARIEL | 1370 | 1380 | 965 | 1.007 | 0.704 |
| 39. | MOLDAU | 1208 | 1211 | - | 1.002 | - |
| 23. | FLAMURA 85 | 5086 | 5086 | 5086 | 1.000 | 1.000 |
| 24. | GABRIELA | 5086 | 5086 | 5086 | 1.000 | 1.000 |
| 21. | EXOTIC | 1115 | 1103.4 | 1274 | 0.990 | 1.143 |
| 2. | ALEX | 1020.46 | 921.75 | 3071.01 | 0.903 | 3.009 |
| 40. | MV PALMA | 2200 | 1877 | - | 0.853 | - |
| 15. | DOR | 1173 | 990 | 1265 | 0.844 | 1.078 |
| 8. | CAPO | 1020 | 854.3 | - | 0.838 | - |
| 44. | JULIUS | 2100 | 1685 | - | 0.802 | - |
| 38. | LOVRIN 34 | 1625 | 1265 | - | 0.778 | - |
| 1. | AGRON | 3010 | 2285.71 | 4605.66 | 0.759 | 1.530 |
| 3. | AZTEC | 2700 | 1898.88 | 2207.8 | 0.703 | 0.818 |
| 37. | LITERA | 987 | 674 | - | 0.683 | - |
| 10. | CRINA | 820.55 | 542.1 | 382.04 | 0.661 | 0.466 |
| 13. | DELABRAD | 1450 | 938 | - | 0.647 | - |
| 5. | BITOP | 3300.23 | 2086.96 | 1082.78 | 0.632 | 0.328 |
| 47. | ROMULUS | 4562 | 2647 | - | 0.580 | - |
| 25. | GIAVA | 3014.31 | 1266.82 | 3274.2 | 0.420 | 1.086 |
| 14. | DEMETRA | 1662 | 286 | 1577 | 0.172 | 0.949 |
| 6. | BOEMA | 3800 | 334.38 | - | 0.088 | - |
| 17. | DUNAI | 3570 | 312 | 1342 | 0.087 | 0.376 |
| Cultivar | YI | Ratio PEROX PEG 25%/ct | Ratio PEROX PEG 40%/ct | Ratio ASC PEG 25%/ct | Ratio ASC PEG 40%/ct | Ratio CAT PEG 25%/ct | Ratio CAT PEG 40%/ct |
|---|---|---|---|---|---|---|---|
| GLOSA | 1.252 | 1.102 | 2.705 | 1.287 | 0.520 | 1.609 | 2.150 |
| GRUIA | 1.220 | 2.334 | 2.648 | 1.525 | 0.725 | 1.416 | 1.470 |
| IZVOR | 1.219 | 1.894 | 1.666 | 0.676 | 0.599 | 1.814 | 10.991 |
| FAUR | 1.153 | 1.004 | 1.715 | 1.715 | 1.169 | 1.786 | 0.000 |
| DELABRAD | 1.116 | 1.124 | 1.358 | 0.846 | 1.026 | 0.647 | 0.000 |
| CRINA | 0.996 | 0.636 | 1.042 | 2.456 | 2.225 | 0.000 | 0.000 |
| ALEX | 0.955 | 1.079 | 1.465 | 0.931 | 0.596 | 0.903 | 3.009 |
| DROPIA | 0.941 | 0.927 | 1.168 | 1.103 | 0.963 | 4.721 | 5.007 |
| SIMNIC 30 | 0.895 | 0.738 | 1.029 | 7.473 | 5.972 | 1.910 | - |
| BEZOSTAIA | 0.854 | 0.804 | 1.075 | 0.497 | 0.553 | 2.909 | 0.708 |
| BOEMA | 0.847 | 1.134 | 1.091 | 1.752 | 1.400 | 0.000 | 0.000 |
| ROMULUS | 0.800 | 2.245 | 3.130 | 0.763 | 0.609 | 0.580 | 0.000 |
| LOVRIN 34 | 0.753 | 1.137 | 1.585 | 0.731 | 0.584 | 0.000 | 0.000 |
| Corelation with YI | 0.25 | 0.31 | −0.11 | −0.18 | 0.14 | 0.42 |
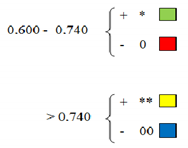 | Ratio Length Stem PEG 15%/ct T1 | Ratio Length Stem PEG 15%/ct T2 | Ratio Length Stem PEG 15%/ct T3 | Ratio Length Stem PEG 15%/ct aver. | Ratio Length Stem PEG 20%/ct T1 | Ratio Length Stem PEG 20%/ct T2 | Ratio Length Stem PEG 20%/ct T3 | Ratio Length Stem PEG 20%/ct Aver. | Ratio Weight Root PEG 15%/ct | Ratio Weight Root PEG 20%/ct | Ratio Weight Stem PEG 15%/ct | Ratio Weight Stem PEG 20%/ct | Ratio Root/Stem PEG 15%/Root/Stem/ct | Ratio Root/Stem PEG 20%/Root/Stem/ct | Seed Germ. Stress/Normal Var (%) | |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
| Peroxidase PEG 25%/per ct | 23 | 0.491 | 0.727 | 0.380 | 0.710 | 0.469 | 0.589 | 0.296 | 0.636 | 0.540 | 0.033 | 0.702 | 0.150 | 0.095 | −0.131 | 0.224 |
| Peroxidase PEG 40%/per ct | 24 | 0.463 | 0.668 | 0.412 | 0.684 | 0.321 | 0.479 | 0.322 | 0.515 | 0.436 | −0.114 | 0.689 | −0.081 | 0.109 | −0.001 | 0.269 |
| Asc PEG 25%/asc ct | 25 | −0.090 | −0.383 | 0.388 | −0.062 | −0.108 | −0.055 | 0.208 | 0.002 | −0.186 | 0.076 | −0.223 | −0.248 | −0.007 | 0.402 | −0.407 |
| Asc PEG 40%/asc ct | 26 | −0.231 | −0.440 | 0.379 | −0.173 | −0.205 | −0.095 | 0.219 | −0.066 | −0.220 | 0.091 | −0.242 | −0.231 | −0.042 | 0.384 | −0.467 |
| Cat PEG 25%/cat ct | 27 | 0.169 | 0.352 | 0.127 | 0.280 | 0.183 | 0.740 | 0.357 | 0.561 | 0.250 | 0.796 | 0.223 | 0.762 | −0.105 | 0.204 | −0.406 |
| Cat PEG 40%/cat ct | 28 | 0.279 | 0.326 | 0.003 | 0.294 | 0.236 | 0.656 | 0.170 | 0.479 | 0.218 | 0.734 | 0.181 | 0.700 | −0.084 | 0.189 | −0.259 |
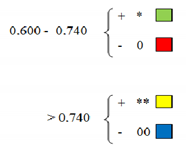 | Red. Coleoptil Length (%) | Ratio Length pl PEG 25%/Length pl. ct | Ratio Length pl PEG 40%/Length pl. ct | Initial Water Cont. (%) | Water Loss after 4 h (%) | Water Loss after 20 h (%) | Water Loss after 24 h (%) | Peroxidase PEG 25%/per ct | Peroxidase PEG 40%/per ct | Asc per PEG 25%/Asc per ct | Asc per PEG 40%/Asc per ct | Catalase PEG 25%/Cat ct | Catalase PEG 40%/Cat ct | |||
| 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | ||||
| Peroxidase PEG 25%/per ct | 23 | −0.393 | 0.045 | 0.111 | 0.204 | 0.590 | −0.295 | 0.114 | 1 | |||||||
| Peroxidase PEG 40%/per ct | 24 | −0.781 | 0.128 | 0.115 | 0.067 | 0.156 | −0.351 | −0.194 | 0.771 | 1 | ||||||
| Ascorbat peroxidase PEG 25%/asc ct | 25 | 0.235 | −0.468 | −0.863 | −0.055 | 0.156 | −0.200 | −0.131 | −0.336 | −0.298 | 1 | |||||
| Ascorbat peroxidase PEG 40%/asc ct | 26 | 0.338 | −0.450 | −0.843 | −0.043 | 0.027 | −0.127 | −0.088 | −0.397 | −0.404 | 0.985 | 1 | ||||
| Catalase PEG 25%/cat ct | 27 | 0.202 | −0.255 | 0.049 | 0.242 | 0.410 | 0.009 | 0.255 | 0.290 | −0.042 | −0.147 | −0.130 | 1 | |||
| Catalase PEG 40%/cat ct | 28 | 0.109 | −0.115 | 0.160 | 0.189 | 0.446 | 0.006 | 0.274 | 0.289 | 0.005 | −0.258 | −0.260 | 0.932 | 1 | ||
| Parameters | Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | 0:00 | 3:00 | 6:00 | 9:00 | 11:00 | 13:00 | 15:00 | 17:00 | 19:00 | 22:00 |
| Temperature (°C) | 15.0 | 14.0 | 15.0 | 18.0 | 20.0 | 25.0 | 25.0 | 20.0 | 18.0 | 16.0 |
| Light (lux) | 0 | 0 | 1 | 2 | 4 | 5 | 4 | 3 | 1 | 0 |
| Humidity (%) | 60 | 65 | 65 | 60 | 55 | 55 | 55 | 55 | 55 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paunescu, R.A.; Bonciu, E.; Rosculete, E.; Paunescu, G.; Rosculete, C.A.; Babeanu, C. The Variability for the Biochemical Indicators at the Winter Wheat Assortment and Identifying the Sources with a High Antioxidant Activity. Plants 2021, 10, 2443. https://doi.org/10.3390/plants10112443
Paunescu RA, Bonciu E, Rosculete E, Paunescu G, Rosculete CA, Babeanu C. The Variability for the Biochemical Indicators at the Winter Wheat Assortment and Identifying the Sources with a High Antioxidant Activity. Plants. 2021; 10(11):2443. https://doi.org/10.3390/plants10112443
Chicago/Turabian StylePaunescu, Ramona Aida, Elena Bonciu, Elena Rosculete, Gabriela Paunescu, Catalin Aurelian Rosculete, and Cristina Babeanu. 2021. "The Variability for the Biochemical Indicators at the Winter Wheat Assortment and Identifying the Sources with a High Antioxidant Activity" Plants 10, no. 11: 2443. https://doi.org/10.3390/plants10112443
APA StylePaunescu, R. A., Bonciu, E., Rosculete, E., Paunescu, G., Rosculete, C. A., & Babeanu, C. (2021). The Variability for the Biochemical Indicators at the Winter Wheat Assortment and Identifying the Sources with a High Antioxidant Activity. Plants, 10(11), 2443. https://doi.org/10.3390/plants10112443







