Epiphytic PGPB Bacillus megaterium AFI1 and Paenibacillus nicotianae AFI2 Improve Wheat Growth and Antioxidant Status under Ni Stress
Abstract
:1. Introduction
2. Results
2.1. Bacterial Identification
2.2. 16 S rRNA Sequencing of PGPB
2.3. Effect of Ni and PGPB on Wheat Growth
2.4. Ni Accumulation
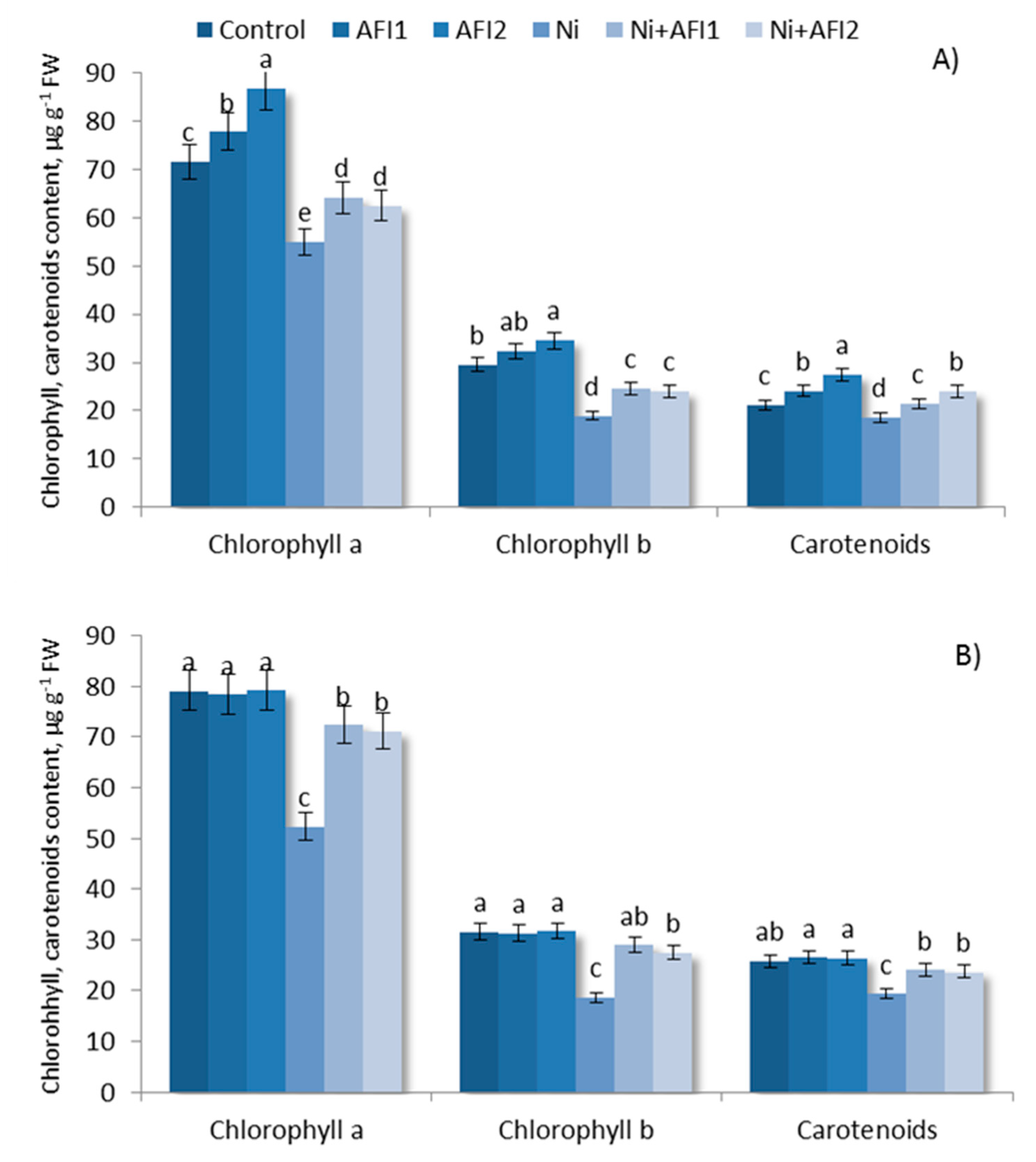
2.5. Chlorophyll and Carotenoid Content
2.6. Antioxidant Enzymes Responses
2.7. LPO
2.8. Proline
3. Discussion
4. Materials and Methods
4.1. Screening and Isolation of Ni-Resistant PGPB
4.2. Bacterial Physiological and Biochemical Characteristics
4.3. Bacterial Phytohormones Assay
4.4. 16 S rRNA Sequencing of PGPB
4.5. Experimental Design
4.6. Determination of Ni
4.7. Chlorophyll and Carotenoids Analysis
4.8. Extraction of Proteins and Enzymes
4.9. Lipid Peroxidation (LPO)
4.10. Superoxide Dismutase (SOD)
4.11. Ascorbate Peroxidase (APX)
4.12. Catalase (CAT)
4.13. Guaiacol Peroxidase (POX)
4.14. Proline
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vodyanitskii, Y.N.; Plekhanova, I.O.; Prokopovich, E.V.; Savichev, A.T. Soil contamination with emissions of non-ferrous metallurgical plants. Eur. Soil Sci. 2011, 44, 217–226. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Zaidi, A.; Khan, M.S. Heavy metal mediated phytotoxic impact on winter wheat: Oxidative stress and microbial management of toxicity by Bacillus subtilis BM2. RSC Adv. 2019, 9, 6125–6142. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, M.; Fariduddin, Q.; Hayat, S.; Ahmad, A. Nickel: An overview of uptake, essentiality and toxicity in plants. Bull. Environ. Contam. Toxicol. 2011, 86, 1–17. [Google Scholar] [CrossRef]
- Sreekanth, T.; Nagajyothi, P.; Lee, K.; Prasad, T. Occurrence, physiological responses and toxicity of nickel in plants. Int. J. Environ. Sci. Technol. 2013, 10, 1129–1140. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Imtiaz, M.; Dai, Z.; Mehmood, S.; Adeel, M.; Liu, J.; Tu, S. Nickel stressed responses of rice in Ni subcellular distribution, antioxidant production, and osmolyte accumulation. Environ. Sci. Pollut. Res. 2017, 24, 20587–20598. [Google Scholar] [CrossRef] [PubMed]
- Rajindiran, S.; Dotaniya, M.L.; Coumar, M.V.; Panwar, N.R.; Saha, J.K. Heavy metal polluted soils in India: Status and countermeasures. JNKVV Res. J. 2015, 49, 320–337. Available online: https://www.researchgate.net/publication/296514347_Heavy_metal_polluted_Soils_in_India_status_and_countermeasures (accessed on 26 October 2021).
- Wang, C.J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.D.; Liu, N.X.; Wang, U.P.; Guo, J.H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE 2012, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Huang, D.; Liu, J. Functions and toxicity of nickel in plants: Recent advances and future prospects. Clean 2009, 37, 304–313. [Google Scholar] [CrossRef]
- Ivanishchev, V.V.; Abramova, E.A. Accumulation of nickel ions in seedlings of Vicia sativa L. and manifestations of oxidative stress. Environ. Sci. Pollut Res. 2015, 22, 7897–7905. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, N.P.; Kalimova, I.B. Dynamics of growth, proliferation and differentiation of wheat root cells exposed to a high nickel concentration. Rus. J. Plant. Physiol. 2008, 55, 787–798. [Google Scholar] [CrossRef]
- Amari, T.; Ghnaya, T.; Abdelly, C. Nickel, cadmium and lead phytotoxicity and potential of halophytic plants in heavy metal extraction. S. Afr. J. Bot. 2017, 111, 99–110. [Google Scholar] [CrossRef]
- Llamas, A.; Sanz, A. Organ-distinctive changes in respiration rates of rice plants under nickel stress. Plant. Growth Regul. 2008, 54, 63–69. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Physiological role of nickel and its toxic effects on higher plants. Rus J. Plant. Physiol. 2006, 53, 257–277. [Google Scholar] [CrossRef]
- Prasad, S.M.; Dwivedi, R.; Zeeshan, M. Growth, photosynthetic electron transport, and antioxidant responses of young soybean seedlings to simultaneous exposure of nickel and UV-B stress. Photosynthetica 2005, 43, 177–185. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Bahmanziari, H. Stimulating and toxicity effects of nickel on growth, yield, and fruit quality of cucumber supplied with different nitrogen sources. J. Plant Nutr. Soil Sci. 2012, 175, 474–481. [Google Scholar] [CrossRef]
- Amari, T.; Ghnaya, T.; Debez, A.; Taamalli, M.; Ben Youssef, N.; Lucchini, G.; Sacchi, G.; Abdelly, C. Comparative Ni tolerance and accumulation potentials between Mesembryanthemum crystallinum (halophyte) and Brassica juncea: Metal accumulation, nutrient status and photosynthetic activity. J. Plant Physiol. 2014, 171, 1634–1644. [Google Scholar] [CrossRef]
- Aziz, H.; Sabir, M.; Ahmad, H.R.; Aziz, T.; Zia-ur-Rehman, M.; Hakeem, K.R.; Ozturk, M. Alleviating effect of calcium on nickel toxicity in Rice. Clean Soil Air Water. 2015, 43, 901–909. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Alscher, R.G.; Erturk, N.; Heatrh, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef]
- Baccouch, S.; Chaoui, A.; El Ferjani, E. Nickel toxicity induces oxidative damage in Zea mays roots. J. Plant. Nutr. 2001, 24, 1085–1097. [Google Scholar] [CrossRef]
- Pietrini, F.; Iori, V.; Ivanova, A.; Shevyakova, N.; Radyukina, N.; Kuznetsov, V.; Zacchini, M. Evaluation of nickel tolerance in Amaranthus paniculatus L. plants by measuring photosynthesis, oxidative status, antioxidative response and metal-binding molecule content. Environ. Sci. Pollut Res. 2015, 22, 482–494. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, C.; Tariq, M.; Xiao, Q.; Zhang, W.; Huang, K.; Lu, Q.; Lin, K.; Liu, Z. The response and tolerance mechanisms of lettuce (Lactuca sativa L.) exposed to nickel in a spiked soil system. Chemosphere 2019, 222, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Najafi Kakavand, S.; Karimi, N.; Ghasempour, H.-R. Salicylic acid and jasmonic acid restrains nickel toxicity by ameliorating antioxidant defense system in shoots of metallicolous and non-metallicolous Alyssum inflatum Náyr. Populations. Plant Physiol. Biochem. 2019, 135, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Skłodowska, M. Antioxidative responses and proline level in leaves and roots of pea plants subjected to nickel stress. Acta Physiol. Plant. 2005, 27, 329–340. [Google Scholar] [CrossRef]
- Boominathan, R.; Doran, P.M. Ni-induced oxidative stress in roots of the Ni hyperaccumulator, Alyssum bertolonii. New Phytol. 2002, 156, 205–215. [Google Scholar] [CrossRef]
- Parlak, K.U. Effect of nickel on growth and biochemical characteristics of wheat (Triticum aestivum L.) seedlings. NJAS Wageningen J. Life Sci. 2016, 76, 1–5. [Google Scholar] [CrossRef]
- Zahra, S.; Mahmood, S.; Noreen, S.; Akrem, A. Independent and combined nickel and cadmium induced lipid peroxidation of biological membranes and its mitigation through antioxidant enzymes in Grewia asiatica L. Pakistan J. Life Soc. Sci. 2018, 16, 48–54. [Google Scholar]
- Spence, C.; Bais, H. The role of plant growth regulators as chemical signals in plant–microbe interactions: A double edged sword. Curr. Opin. Plant Biol. 2015, 27, 52–58. [Google Scholar] [CrossRef]
- Khan, W.U.; Ahmad, S.R.; Yasin, N.A.; Ali, A.; Ahmad, A.; Akram, W. Application of Bacillus megaterium MCR-8 improved phytoextraction and stress alleviation of nickel in Vinca rosea. Int J. Phytoremediat. 2017, 19, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.; Svatoš, A.; Merten, D.; Büchel, G.; Kothe, E. Hydroxamate siderophores produced by Streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Can. J. Microbiol. 2008, 54, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Lata, C. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef] [Green Version]
- Aktan, Y.; Tan, S.; Icgen, B. Characterization of lead-resistant river isolate Enterococcus faecalis and assessment of its multiple metal and antibiotic resistance. Environ. Monit. Assess. 2013, 185, 5285–5293. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.; Srivastava, S. Zinc resistance mechanisms in bacteria. Curr. Sci. 2001, 81, 768–775. Available online: https://www.jstor.org/stable/24106396 (accessed on 26 October 2021).
- Pishchik, V.N.; Vorob’ev, N.I.; Provorov, N.A.; Khomyakov, Y.V. Mechanisms of plant and microbial adaptation to heavy metals in plant–microbial systems. Microbiology 2016, 85, 257–271. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, B.R.; Raghuwanshi, R. Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.). Biocatal. Agric. Biotech. 2014, 3, 121–128. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.-C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-M.; Tang, Y.-Q.; Mori, K.; Wu, X.-L. Distribution of culturable endophytic bacteria in aquatic plants and their potential for bioremediation in polluted waters. Aquat. Biol. 2012, 15, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Perez, M.H.A.; Garcıa-Ribera, R.; Quesada, T.; Aguilera, M.; Ramos-Cormenzana, A.; Monteoliva-Sanchez, M. Biosorption of heavy metals by the exopolysaccharide produced by Paenibacillus Jamilae. World J. Microbiol. Biotechnol. 2008, 24, 2699–2704. [Google Scholar] [CrossRef]
- Awan, S.A.; Ilyas, N.; Khan, I.; Ali Raza, M.A.; Rehman, A.U.; Rizwan, M.; Rastogi, A.; Tariq, R.; Brestic, M. Bacillus siamensis reduces cadmium accumulation and improves growth and antioxidant defense system in two wheat (Triticum aestivum L.) varieties. Plants 2020, 9, 878. [Google Scholar] [CrossRef]
- Saif, S.; Khan, M.S. Assessment of toxic impact of metals on proline, antioxidant enzymes, and biological characteristics of Pseudomonas aeruginosa inoculated Cicer arietinum grown in chromium and nickel-stressed sandy clay loam soils. Environ. Monit. Assess. 2018, 190, 5. [Google Scholar] [CrossRef]
- Pandey, S.; Ghosh, P.K.; Ghosh, S.; De, T.K.; Maiti, T.K. Role of Heavy Metal Resistant Ochrobactrum sp. and Bacillus spp. strains in Bioremediation of a Rice Cultivar and Their PGPR Like Activities. J. Microbiol. 2013, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.; Ameen, N.; Murtaza, B.; Imran, M.; Shahid, M.; Abbas, G.; Naeem, M.A.; Jacobsen, S.-E. Comparative Physiological and Biochemical Evaluation of Salt and Nickel Tolerance Mechanisms in Two Contrasting Tomato Genotypes. Physiol. Plantarum. 2020, 168, 27–37. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.J.; Kim, K.M. Metal resistant endophytic bacteria reduces cadmium, nickel toxicity, and enhances expression of metal stress relatd genes with improved growth of Oryza sativa, via regulating its antioxidant machinery and endogenous hormones. Plants 2019, 8, 363. [Google Scholar] [CrossRef] [Green Version]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Pishchik, V.; Mirskaya, G.; Chizhevskaya, E.; Chebotar, V.; Chakrabarty, D. Nickel-tolerance in plant-bacterial associations. PeerJ 2021, 9, e12230. [Google Scholar] [CrossRef] [PubMed]
- Taubel, M.; Kampfer, P.; Sandra Buczolits, S.; Lubitz, W.; Busse, H.-J. Bacillus barbaricus sp. nov., isolated from an experimental wall painting. Int. J. Syst. Evol. Microbiol. 2003, 53, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.Z.; Siddiqi, M.H.; Im, W.T.; Kim, Y.-J.; Yang, D.-C. Paenibacillus kyungheensis sp. nov., isolated from flowers of magnolia. Int. J. Syst. Evol. Microbiol. 2015, 65, 3959–3964. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Q.; Zhou, X.-K.; Dang, L.-Z.; Cheng, J.; Hozzein, W.N.; Liu, M.-J.; Hu, Q.; Li, W.-J.; Duan, Y.-Q. Paenibacillus nicotianae sp. nov., isolated from a tobacco sample. Antonie Van Leeuwenhoek 2014, 106, 1199–1205. [Google Scholar] [CrossRef]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020, 11, 5753–5798. [Google Scholar] [CrossRef]
- Fierros-Romero, G.; Gómez-Ramírez, M.; Sharma, A.; Pless, R.C.; Rojas-Avelizapa, N.G. czcD gene from Bacillus megaterium and Microbacterium liquefaciens as a potential nickel–vanadium soil pollution biomarker. J. Basic Microbiol. 2020, 60, 22–26. [Google Scholar] [CrossRef]
- Pandey, N.; Pathak, G.C. Nickel alters antioxidative defense and water status in green gram. Indian J. Plant Physiol. 2006, 11, 113–118. [Google Scholar]
- Gajewska, E.; Skłodowska, M. Differential biochemical responses of wheat shoots and roots to nickel stress: Antioxidative reactions and proline accumulation. Plant Growth Regul. 2007, 54, 179–188. [Google Scholar] [CrossRef]
- Bhalerao, S.A.; Amit, S.S.; Anukthi, C.P. Toxicity of nickel in plants. Int. J. Pure App. Biosci. 2015, 32, 345–355. [Google Scholar]
- Ahmad, M.S.; Ashraf, M.; Hussain, M. Phytotoxic effects of nickel on yield and concentration of macro- and micro-nutrients in sunflower (Helianthus annuus L.) achenes. J. Hazard. Mater. 2011, 185, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Hasheminasab, H.; Taghi Assad, M.; Aliakbari, A.; Rasoul Sahhafi, S. Influence of Drought Stress on Oxidative damage and antioxidant defense systems in tolerant and susceptible wheat genotypes. J. Agric. Sci. 2012, 4, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Jamali, S.S.; Borzouei, A.; Aghamirzaei, M.; Khosronejad, H.R.; Fathi, M. Cell membrane stability and biochemical response of seven wheat cultivars under salinity stress. Braz. J. Bot. 2015, 38, 63–69. [Google Scholar] [CrossRef]
- Sairam, R.K.; Saxena, D.C. Oxidative Stress and Antioxidants in Wheat Genotypes: Possible Mechanism of Water Stress Tolerance. J. Agron. Crop. Sci. 2000, 184, 55–61. [Google Scholar] [CrossRef]
- Amjad, M. Nickel toxicity induced changes in nutrient dynamics and antioxidant profiling in two maize (Zea mays L.) hybrids. Plants 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lastochkina, O.; Pusenkova, L.; Yuldashev, R.; Babaev, M.; Garipova, S.; Blagova, D.; Khairullin, R.; Aliniaeifard, S. Effects of Bacillus subtilis on some physiological and biochemical parameters of Triticum aestivum L. (wheat) under salinity. Plant Physiol. Biochem. 2017, 121, 80–88. [Google Scholar] [CrossRef]
- Kuramshina, Z.M.; Smirnova, Y.V.; Khairullin, R.M. Increasing Triticum aestivum tolerance to cadmium stress through endophytic strains of Bacillus subtilis. Rus. J. Plant Physiol. 2016, 63, 636–644. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, L.; He, Y.; Zhang, H.; Li, W.; Chen, H.; Ma, D.; Yin, J. Genome-wide identification and transcriptional expression analysis of superoxide dismutase (SOD) family in wheat (Triticum aestivum). PeerJ 2019, 7, e8062. [Google Scholar] [CrossRef] [Green Version]
- Lakhdar, A.; Iannelli, M.A.; Debez, A.; Massacci, A.; Jedidi, N.; Abdelly, C. Effect of municipal solid waste compost and sewage sludge use on wheat (Triticum durum): Growth, heavy metal accumulation, and antioxidant activity. J. Sci. Food Agric. 2010, 90, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.B.; Ali, S.; Azam, A.; Hina, S.; Ahsan, M.; Farooq, B.A.; Bharwana, S.A.; Gill, M.B. Morphological, physiological and biochemical responses of plants to nickel stress: A review. Afr. J. Agric. Res. 2013, 8, 1596–1602. [Google Scholar]
- Singh, K.; Pandey, S.N. Effect of nickel-stresses on uptake, pigments and antioxidative responses of water lettuce, Pistia stratiotes L. J. Environ. Biol. 2011, 32, 391–394. [Google Scholar]
- Gajewska, E.; Skłodowska, M. Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. BioMetals 2006, 20, 27–36. [Google Scholar] [CrossRef]
- Danna, C.H.; Bartoli, C.G.; Sacco, F.; Ingala, L.R.; Santa-Maria, G.E.; Guiamet, J.J.; Ugalde, R.A. Thylakoid-bound ascorbateperoxidase mutant exhibits impaired electron transport and photosynthetic activity. Plant Physiol. 2003, 132, 2116–2125. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ma, H.; Jia, P.; Wang, J.; Jia, L.; Zhang, T.; Yang, Y.; Chen, H.; Wei, X. Responses of seedling growth and antioxidant activity to excess iron and copper in Triticum aestivum L. Ecotoxicol. Environ. Saf. 2012, 86, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajewska, E.; Wielanek, M.; Bergier, K.; Skłodowska, M. Nickel-induced depression of nitrogen assimilation in wheat roots. Acta Physiol. Plant 2015, 31, 1291–1300. [Google Scholar] [CrossRef]
- Gajewska, E.; Głowacki, R.; Mazur, J.; Skłodowska, M. Differential response of wheat roots to Cu, Ni and Cd treatment: Oxidative stress and defense reactions. Plant Growth Regul. 2013, 71, 13–20. [Google Scholar] [CrossRef]
- Santos, S.; Neto, I.F.F.; Machado, M.D.; Soares, H.M. Siderophore Production by Bacillus megaterium: Effect of Growth Phase and Cultural Conditions. Appl. Biochem. Biotechnol. 2014, 172, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.M.H.; Vilas-Boas, Â.; Sousa, C.A.; Soares, H.M.V.M.; Soares, E.V. Comparison of five bacterial strains producing siderophores with ability to chelate iron under alkaline conditions. AMB Expr. 2019, 9, 78. [Google Scholar] [CrossRef]
- Bhatt, K.; Maheshwari, D.K. Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3Biotech 2020, 10, 36. [Google Scholar] [CrossRef]
- Ma, Y.; Prasad, M.N.V.; Rajkumar, M.; Freitas, H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 2011, 29, 248–258. [Google Scholar] [CrossRef]
- Cabello-Conejo, M.I.; Prieto-Fernández, Á.; Kidd, P.S. Exogenous treatments with phytohormones can improve growth and nickel yield of hyperaccumulating plants. Sci. Total Environ. 2014, 494–495, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xing, L.; Wang, X.; Hou, Y.-J.; Gao, J.; Wang, P.; Duan, C.G.; Zhu, X.; Zhu, J.K. The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes. Sci. Signal. 2014, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Kudoyarova, G.R.; Kholodova, V.P.; Veselov, D.S. Current state of the problem of water relations in plants under water deficit. Russ. J. Plant Physiol. 2013, 60, 165–175. [Google Scholar] [CrossRef]
- Wang, H.; Feng, T.; Peng, X.; Yan, M.; Tang, X. Up-regulation of chloroplasticantioxidant capacity is involved in alleviation of nickel toxicity of Zea mays L. by exogenous salicylic acid. Ecotoxicol. Environ. Saf. 2009, 72, 1354–1362. [Google Scholar] [CrossRef]
- Ghasemi, R.; Ghaderian, S.M.; Krämer, U. Interference of nickel with copper and iron homeostasis contributes to metal toxicity symptoms in the nickel hyperaccumulator plant Alyssum inflatum. New Phytol. 2009, 184, 566–580. [Google Scholar] [CrossRef]
- El-Meihy, R.M.; Abou-Aly, H.E.; Youssef, A.M.; Tewfike, T.A.; El-Alkshar, E.A. Efficiency of heavy metals-tolerant plant growth promoting bacteria for alleviating heavy metals toxicity on sorghum. Environ. Exp. Bot. 2019, 162, 295–301. [Google Scholar] [CrossRef]
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Garshina, D.; Ivanov, S.; Yuldashev, R.; Khafizova, R.; Allagulova, C.; Fedorova, K.; Avalbaev, A.; Maslennikova, D.; Bosacchi, M. Seed Priming with Endophytic Bacillus subtilis Modulates Physiological Responses of Two Different Triticum aestivum L. Cultivars under Drought Stress. Plants 2020, 9, 1810. [Google Scholar] [CrossRef]
- Kitajima, K.; Hogan, K.P. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, N.; Yu, G. Opposing shifts in distributions of chlorophyll concentration and composition in grassland under warming. Sci. Rep. 2021, 11, 15736. [Google Scholar] [CrossRef]
- Smith, D.L.; Gravel, V.; Yergeau, E. Editorial: Signaling in the phytomicrobiome. Front. Plant Sci. 2017, 8, 611. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.J.; Ullah, S.; Ahmad, I.; Rauf, A.; Nadeem, S.M.; Khan, M.Y.; Hussain, S.; Bulgariue, L. Nickel phytoextraction through bacterial inoculation in Raphanus sativus. Chemosphere 2018, 190, 234–242. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Rana, A.; Saharan, B.; Joshi, M.; Prasanna, R.; Kumar, K.; Nain, L. Identification of multi-trait PGPR isolates and evaluating their potential as inoculants for wheat. Ann. Microbiol. 2011, 61, 893–900. [Google Scholar] [CrossRef]
- Hugh, R.; Leifson, F. The taxonomic significance of fermentative oxydation metabolism of carbohydrates by various Gram-negative bacteria. J. Bacteriol. 1953, 66, 24–26. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.H.; Lyne, P.M. (Eds.) Microbiological Methods, 5th ed.; Butterworth & Co, Ltd.: London, UK, 1985; p. 450. [Google Scholar]
- Pérez-Miranda, S.; Cabirol, N.; George-Téllez, R.; Zamudio-Rivera, L.S.; Fernández, F.J. O-CAS, a fast and universal method for siderophore detection. J. Microbiol. Methods 2007, 70, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Pishchik, V.N.; Vorobyev, N.I.; Ostankova, Y.V.; Semenov, A.V.; Totolian, A.A.; Popov, A.A.; Khomyakov, Y.V.; Udalova, O.R.; Shibanov, D.V.; Vertebny, V.E.; et al. Impact of Bacillus subtilis on Tomato Plants Growth and Some Biochemical Characteristics under Combined Application with Humic Fertilizer. Int. J. Plant Soil Sci. 2018, 22, 1–12. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 287–297. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, L. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. Catalases. Methods Enzym. Anal. 1971, 3, 273–286. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay catalase and peroxidase. In Methods in Enzymology; Academic Press: New York, NY, USA, 1955; pp. 764–775. [Google Scholar]
- Bates, L.S. Rapid determination of free proline for water stress studies. Plant. Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]





| IAA, µg/L | ABA, µg/L | GAS3 µg/L | Siderophores | |
|---|---|---|---|---|
| AFI1 | 33.6 ± 0.36 | 14.4 ± 0.41 | 2.90 ± 0.28 | + |
| AFI2 | 61.7 ± 0.35 | 40.4 ± 0.28 | 40.0 ± 0.82 | − |
| Shoots | Roots | Seedlings | |||
|---|---|---|---|---|---|
| Length, mm | FW, mg | Length, mm | FW, mg | FW, mg | |
| Chinese spring S | |||||
| Control | 282.9 ± 29.50 b | 26.9 ± 4.18 c | 90.7 ± 5.61 ab | 12.6 ± 1.97 c | 39.5 ± 5.74 c |
| AFI1 | 347.3 ± 15.20 a | 38.5 ± 2.02 a | 99.1 ± 5.88 a | 21.5 ± 2.41 a | 59.9 ± 4.00 a |
| AFI2 | 342.7 ± 13.43 b | 35.0 ± 2.58 b | 83.2 ± 3.56 b | 18.7 ± 2.13 b | 53.7 ± 3.84 b |
| Ni | 157.9 ± 8.20 d | 12.3 ± 0.99 e | 56.4 ± 6.20 c | 6.3 ± 1.08 d | 18.6 ± 1.90 e |
| Ni + AFI1 | 168.9 ± 11.45 cd | 12.8 ± 0.88 e | 62.1 ± 4.07 c | 7.0 ± 0.74 d | 19.8 ± 1.37 de |
| Ni + AFI2 | 179.6 ± 8.18 c | 15.1 ± 1.41 d | 59.3 ± 5.52 c | 7.6 ± 0.79 d | 22.8 ± 2.01 d |
| Leningradskaya 6 T | |||||
| Control | 268.8 ± 4.66 b | 23.6 ± 1.50 b | 99.5 ± 4.09 c | 8.4 ± 0.62 b | 32.0 ± 1.72 b |
| AFI1 | 295.8 ± 4.96 a | 30.0 ± 1.16 a | 154.7 ± 11.70 a | 11.4 ± 1.61 a | 41.3 ± 2.39 a |
| AFI2 | 285.3 ± 4.64 a | 31.8 ± 0.81 a | 133.2 ± 6.05 b | 11.1 ± 1.05 a | 43.0 ± 1.46 a |
| Ni | 223.1 ± 7.03 d | 12.0 ± 0.83 d | 49.8 ± 2.55 d | 4.7 ± 0.29 c | 16.6 ± 0.92 d |
| Ni + AFI1 | 242.3 ± 6.14 c | 15.2 ± 0.58 c | 56.7 ± 3.59 d | 5.0 ± 0.30 c | 20.3 ± 0.66 e |
| Ni + AFI2 | 255.9 ± 6.99 bc | 15.2 ± 0.64 c | 60.9 ± 3.09 d | 4.9 ± 0.42 c | 20.1 ± 0.76 c |
| Treatment | Chlorophyll a/b Ratio | Total Chlorophyll, µg g−1 FW |
|---|---|---|
| Chinese spring S | ||
| Control | 2.4 | 101.31 ± 1.94 c |
| AFI1 | 2.4 | 110.16 ± 3.23 b |
| AFI2 | 2.5 | 121.35 ± 2.66 a |
| Ni | 2.9 | 74.07 ± 2.88 e |
| Ni + AFI1 | 2.6 | 88.78 ± 2.00 d |
| Ni + AFI2 | 2.6 | 86.53 ± 3.15 d |
| Leningradskaya 6 T | ||
| Control | 2.5 | 110.79 ± 3.01 a |
| AFI1 | 2.5 | 109.81 ± 3.19 a |
| AFI2 | 2.5 | 110.90 ± 2.89 a |
| Ni | 2.8 | 71.05 ± 2.65 c |
| Ni + AFI1 | 2.5 | 101.37 ± 2.79 b |
| Ni + AFI2 | 2.6 | 98.61 ± 2.00 b |
| Treatment | CAT in Roots µM H2O2 mg−1 Protein min−1 | CAT in Shoots µM H2O2 mg−1 Protein min−1 | SOD in Roots U mg−1 Protein min−1 | SOD in Shoots U mg−1 Protein min−1 |
|---|---|---|---|---|
| Chinese spring S | ||||
| Control | 299.9 ± 16.9 a | 566.2 ± 25.6 a | 0.285 ± 0.013 d | 0.353 ± 0.018 a |
| AFI1 | 255.1 ± 15.4 b | 572.7 ± 29.4 a | 0.298 ± 0.014 cd | 0.256 ± 0.013 d |
| AFI2 | 223.3 ± 9.4 c | 570.2 ± 24.2 a | 0.313 ± 0.013 bc | 0.313 ± 0.014 b |
| Ni | 307.7 ± 14.9 a | 540.5 ± 26.0 a | 0.339 ± 0.014 a | 0.295 ± 0.014 c |
| Ni + AFI1 | 250.4 ± 12.7 b | 570.8 ± 34.1 a | 0.331 ± 0.016 a | 0.238 ± 0.014 e |
| Ni + AFI2 | 222.8 ± 8.9 c | 563.4 ± 20.0 a | 0.324 ± 0.016 ab | 0.244 ± 0.015 de |
| Leningradskaya6 T | ||||
| Control | 550.5 ± 18.7 a | 501.1 ± 18.6 a | 0.137 ± 0.005 c | 0.140 ± 0.005 a |
| AFI1 | 490.7 ± 14.7 b | 499.6 ± 13.1 a | 0.157 ± 0.007 ab | 0.135 ± 0.005 a |
| AFI2 | 432.0 ± 13.4 c | 509.2 ± 18.2 a | 0.164 ± 0.007 ab | 0.146 ± 0.006 a |
| Ni | 285.9 ± 15.3 d | 491.5 ± 15.5 a | 0.173 ± 0.006 a | 0.115 ± 0.004 b |
| Ni + AFI1 | 257.6 ± 16.9 e | 490.0 ± 19.9 a | 0.149 ± 0.005 bc | 0.097 ± 0.006 c |
| Ni + AFI2 | 220.8 ± 15.7 f | 501.0 ± 25.9 a | 0.159 ± 0.011 ab | 0.102 ± 0.005 b |
| Treatment | POX in Roots (U s−1 g−1 FW) | POX in Shoots (U s−1 g−1 FW) | APX in Roots (nM Ascorbate mg−1 Protein min−1) | APX in Shoots (nM Ascorbate mg−1 Protein min−1) |
|---|---|---|---|---|
| Chinese spring S | ||||
| Control | 61.9 ± 2.8 c | 32.5 ± 1.3 d | 5.60 ± 0.37 d | 2.09 ± 0.07 d |
| AFI1 | 70.6 ± 2.1 b | 42.1 ± 1.8 c | 6.51 ± 0.23 c | 2.41 ± 0.13 bc |
| AFI2 | 75.0 ± 3.4 b | 42.3 ± 1.6 c | 6.79 ± 0.44 c | 2.30 ± 0.15 bc |
| Ni | 116.8 ± 4.9 a | 74.0 ± 3.2 b | 9.05 ± 0.32 a | 5.24 ± 0.17 a |
| Ni + AFI1 | 118.8 ± 8.2 a | 82.3 ± 4.3 a | 7.75 ± 0.37 b | 2.48 ± 0.09 b |
| Ni + AFI2 | 119.2 ± 3.7 a | 83.3 ± 4.3 a | 6.58 ± 0.28 c | 2.27 ± 0.07 c |
| Leningradskaya 6 T | ||||
| Control | 65.9 ± 3.7 c | 36.1 ± 2.3 d | 4.30 ± 0.24 d | 1.34 ± 0.06 e |
| AFI1 | 72.2 ± 4.8 c | 38.4 ± 2.1 d | 4.95 ± 0.19 c | 1.54 ± 0.09 d |
| AFI2 | 72.4 ± 3.4 c | 37.6 ± 2.1 d | 4.76 ± 0.25 c | 1.86 ± 0.09 c |
| Ni | 101.7 ± 5.5 b | 55.3 ± 2.4 c | 7.16 ± 0.40 a | 3.75 ± 0.18 a |
| Ni + AFI1 | 109.3 ± 5.5 a | 64.3 ± 2.2 b | 6.50 ± 0.30 b | 2.39 ± 0.13 b |
| Ni + AFI2 | 105.7 ± 4.1 ab | 68.9 ± 3.2 a | 6.18 ± 0.29 b | 2.33 ± 0.16 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pishchik, V.N.; Filippova, P.S.; Mirskaya, G.V.; Khomyakov, Y.V.; Vertebny, V.E.; Dubovitskaya, V.I.; Ostankova, Y.V.; Semenov, A.V.; Chakrabarty, D.; Zuev, E.V.; et al. Epiphytic PGPB Bacillus megaterium AFI1 and Paenibacillus nicotianae AFI2 Improve Wheat Growth and Antioxidant Status under Ni Stress. Plants 2021, 10, 2334. https://doi.org/10.3390/plants10112334
Pishchik VN, Filippova PS, Mirskaya GV, Khomyakov YV, Vertebny VE, Dubovitskaya VI, Ostankova YV, Semenov AV, Chakrabarty D, Zuev EV, et al. Epiphytic PGPB Bacillus megaterium AFI1 and Paenibacillus nicotianae AFI2 Improve Wheat Growth and Antioxidant Status under Ni Stress. Plants. 2021; 10(11):2334. https://doi.org/10.3390/plants10112334
Chicago/Turabian StylePishchik, Veronika N., Polina S. Filippova, Galina V. Mirskaya, Yuriy V. Khomyakov, Vitaliy E. Vertebny, Viktoriya I. Dubovitskaya, Yuliya V. Ostankova, Aleksandr V. Semenov, Debasis Chakrabarty, Evgeny V. Zuev, and et al. 2021. "Epiphytic PGPB Bacillus megaterium AFI1 and Paenibacillus nicotianae AFI2 Improve Wheat Growth and Antioxidant Status under Ni Stress" Plants 10, no. 11: 2334. https://doi.org/10.3390/plants10112334
APA StylePishchik, V. N., Filippova, P. S., Mirskaya, G. V., Khomyakov, Y. V., Vertebny, V. E., Dubovitskaya, V. I., Ostankova, Y. V., Semenov, A. V., Chakrabarty, D., Zuev, E. V., & Chebotar, V. K. (2021). Epiphytic PGPB Bacillus megaterium AFI1 and Paenibacillus nicotianae AFI2 Improve Wheat Growth and Antioxidant Status under Ni Stress. Plants, 10(11), 2334. https://doi.org/10.3390/plants10112334










