Production of Genistein in Amaranthus tricolor var. tristis and Spinacia oleracea by Expression of Glycine max Isoflavone Synthase
Abstract
1. Introduction
2. Results
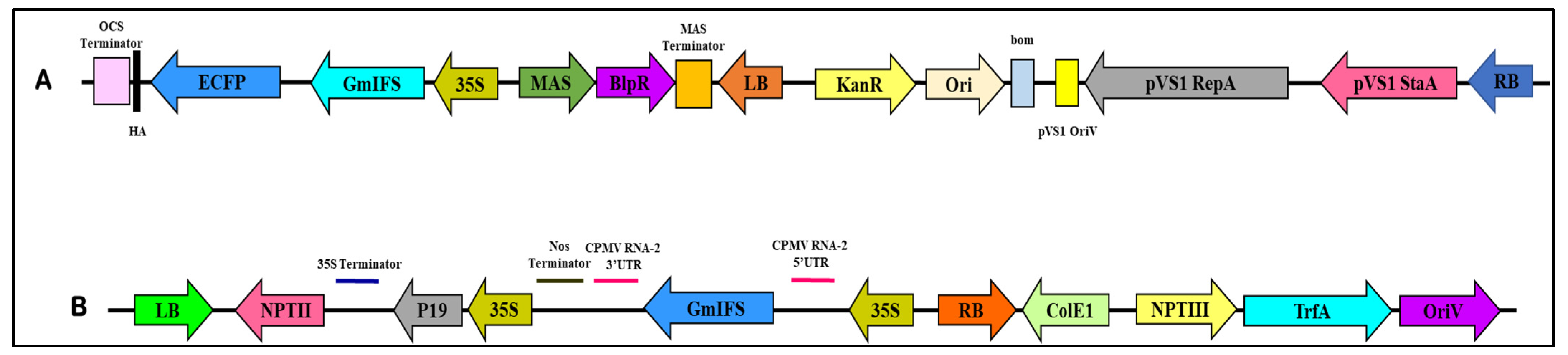
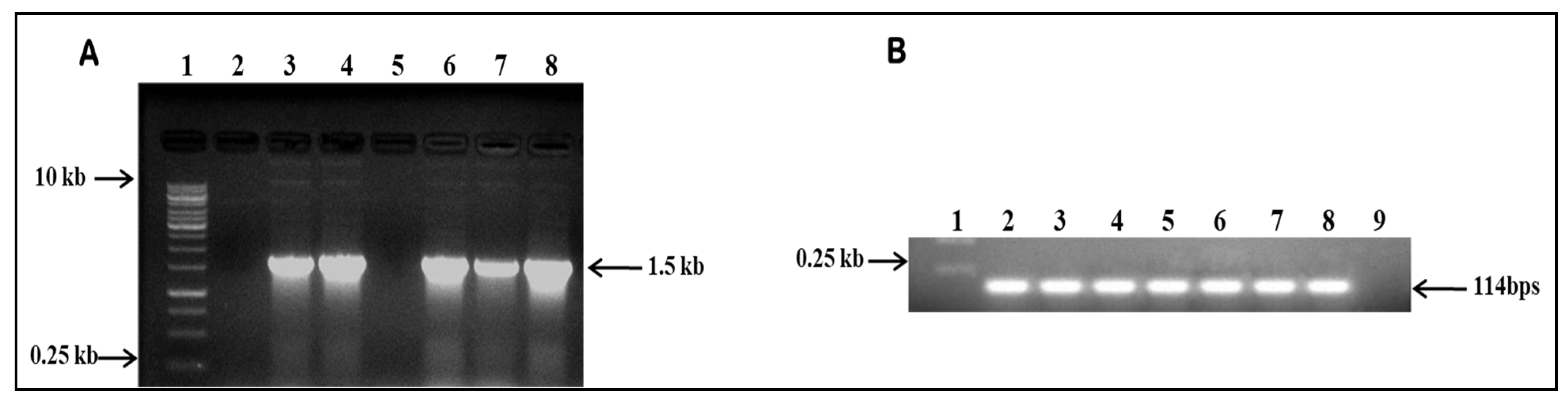
2.1. Confirmation of Cloned IFS in Plant Expression Vector
2.2. Transformation of Agrobacterium tumefaciens with GmIFS and Plant Transformation
2.3. HPLC Detection of Naringenin and Genistein in Infiltrated Samples
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Construction of Plant Expression Vector
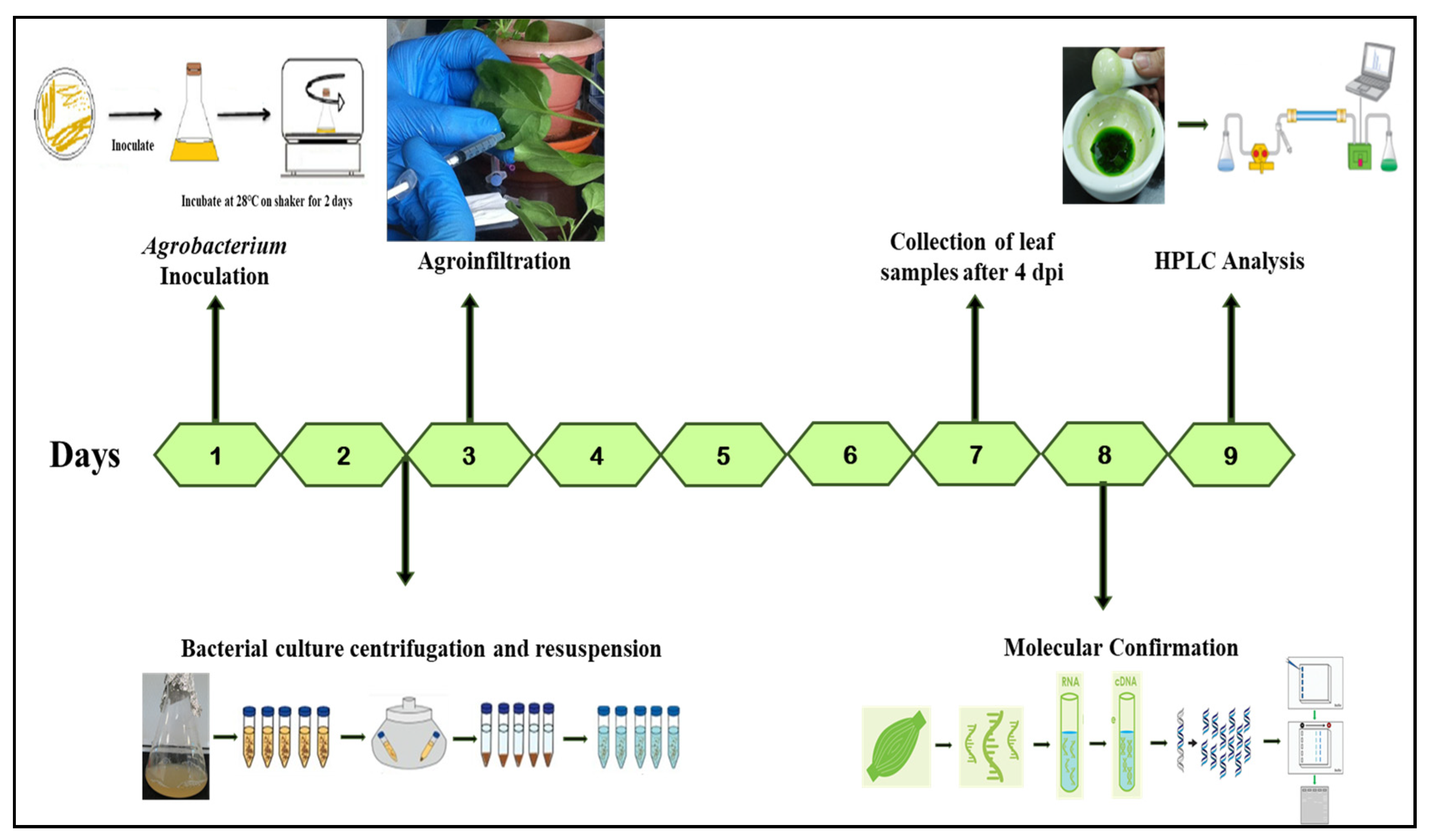
4.3. Agroinfiltration
4.4. RNA Isolation and cDNA Synthesis
4.5. HPLC Detection
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CaMV | Cauliflower mosaic virus |
| cDNA | Complementary deoxyribonucleic acid |
| DNA | Deoxyribonucleic acid |
| dNTP’s | Deoxynucleotide triphosphates |
| °C | Degrees Celsius |
| FW | Fresh weight |
| HPLC | High-performance liquid chromatography |
| mg L−1 | Milligram per liter |
| mL | Milliliter |
| nm | Nanometer |
| RNA | Ribonucleic acid |
| rpm | Revolutions per minute |
| µL | Microliter |
| µM | Micromolar |
| µ | Micron |
| U | Unit |
References
- Yoon, G.-A.; Park, S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014, 8, 618–624. [Google Scholar] [CrossRef]
- Miadoková, E.; Masterová, I.; Vlcková, V.; Dúhová, V.; Tóth, J. Antimutagenic potential of homoisoflavonoids from Muscari racemosum. J. Ethnopharmacol. 2002, 81, 381–386. [Google Scholar] [CrossRef]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Iwasaki, M.; Inoue, M.; Otani, T.; Sasazuki, S.; Kurahashi, N.; Miura, T.; Yamamoto, S.; Tsugane, S. Plasma isoflavone level and subsequent risk of breast cancer among Japanese women: A nested case-control study from the Japan Public Health Center-based prospective study group. J. Clin. Oncol. 2008, 26, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Scarpato, R.; Paganucci, L.; Bertoli, A.; Fiore, L.; Pistelli, L.; Federico, G. Licoflavone C attenuates the genotoxicity of cancer drugs in human peripheral lymphocytes. Phytother. Res. 2008, 22, 1650–1654. [Google Scholar] [CrossRef]
- Popova, P.; Zarev, Y.; Mihaylova, R.; Momekov, G.; Ionkova, I. Antiproliferative activity of extract from in vitro callus cultures of Astragalus vesicarius ssp. carniolicus (A. Kern.) Chater. Pharmacia 2021, 68, 217. [Google Scholar] [CrossRef]
- Kohen, F.; Gayer, B.; Kulik, T.; Frydman, V.; Nevo, N.; Katzburg, S.; Limor, R.; Sharon, O.; Stern, N.; Somjen, D. Synthesis and Evaluation of the Antiproliferative Activities of Derivatives of Carboxyalkyl Isoflavones linked to N-t-Boc-hexylenediamine. J. Med. Chem. 2007, 50, 6405–6410. [Google Scholar] [CrossRef]
- Foudah, A.; Abdel-Kader, A. Isoflavonoids, Flavonoids—From Biosynthesis to Human Health; Justino, G.C., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef][Green Version]
- Ryan-Borchers, T.A.; Park, J.S.; Chew, B.P.; McGuire, M.K.; Fournier, L.R.; Beerman, K.A. Soy isoflavones modulate immune function in healthy postmenopausal women. Am. J. Clin. Nutr. 2006, 83, 1118–1125. [Google Scholar] [CrossRef]
- Reiter, R.J.; Paredes, S.D.; Korkmaz, A.; Jou, M.-J.; Tan, D.-X. Melatonin combats molecular terrorism at the mitochondrial level. Interdiscip. Toxicol. 2008, 1, 137–149. [Google Scholar] [CrossRef]
- Dixon, R.A.; Sumner, L.W. Legume natural products: Understanding and manipulating complex pathways for human and animal health. Plant Physiol. 2003, 131, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Malla, A.; Ramalingam, S. Health Perspectives of an Isoflavonoid Genistein and its Quantification in Economically Important Plants. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 11; pp. 353–379. [Google Scholar] [CrossRef]
- Bezek, S.; Ujházy, E.; Mach, M.; Navarová, J.; Dubovický, M. Developmental origin of chronic diseases: Toxicological implication. Interdiscip. Toxicol. 2008, 1, 29–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miadoková, E. Isoflavonoids—an overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Ramawat, K.G. Isoflavonoids. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1849–1865. [Google Scholar] [CrossRef]
- Sabudak, T.; Guler, N. Trifolium L.—A review on its phytochemical and pharmacological profile. Phytother. Res. 2009, 23, 439–446. [Google Scholar] [CrossRef]
- Ørgaard, A.; Jensen, L. The effects of soy isoflavones on obesity. Exp. Biol. Med. 2008, 233, 1066–1080. [Google Scholar] [CrossRef]
- Jung, W.; Yu, O.; Lau, S.-M.C.; O’Keefe, D.P.; Odell, J.; Fader, G.; McGonigle, B. Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat. Biotechnol. 2000, 18, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Sreevidya, V.; Srinivasa Rao, C.; Sullia, S.; Ladha, J.K.; Reddy, P.M. Metabolic engineering of rice with soybean isoflavone synthase for promoting nodulation gene expression in rhizobia. J. Exp. Bot. 2006, 57, 1957–1969. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, J.; Chu, S.; Yan, H.-L.; Yu, D. Diversifying Selection on Flavanone 3-Hydroxylase and Isoflavone Synthase Genes in Cultivated Soybean and Its Wild Progenitors. PLoS ONE 2013, 8, e54154. [Google Scholar] [CrossRef][Green Version]
- Malla, A.; Shanmugaraj, B.; Srinivasan, B.; Sharma, A.; Ramalingam, S. Metabolic Engineering of Isoflavonoid Biosynthesis by Expressing Glycine max Isoflavone Synthase in Allium cepa L. for Genistein Production. Plants 2021, 10, 52. [Google Scholar] [CrossRef]
- Bevan, M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984, 12, 8711–8721. [Google Scholar] [CrossRef] [PubMed]
- Gelvin, S.B. Agrobacterium-Mediated Plant Transformation: The Biology behind the “Gene-Jockeying” Tool. Microbiol. Mol. Biol. Rev. 2003, 67, 16–37. [Google Scholar] [CrossRef]
- Sainsbury, F.; Lomonossoff, G.P. Transient expressions of synthetic biology in plants. Curr. Opin. Plant Biol. 2014, 19, 1–7. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hoshikawa, K.; Ezura, K.; Okazawa, R.; Fujita, S.; Takaoka, M.; Mason, H.S.; Ezura, H.; Miura, K. Improvement of the transient expression system for production of recombinant proteins in plants. Sci. Rep. 2018, 8, 4755. [Google Scholar] [CrossRef]
- Tyurin, A.A.; Suhorukova, A.V.; Kabardaeva, K.V.; Goldenkova-Pavlova, I.V. Transient Gene Expression is an Effective Experimental Tool for the Research into the Fine Mechanisms of Plant Gene Function: Advantages, Limitations, and Solutions. Plants 2020, 9, 1187. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Bulaon, C.J.I.; Phoolcharoen, W. Plant Molecular Farming: A Viable Platform for Recombinant Biopharmaceutical Production. Plants 2020, 9, 842. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Ramalingam, S. Plant Expression Platform for the Production of Recombinant Pharmaceutical Proteins. Austin J. Biotechnol. Bioeng. 2014, 1, 4. [Google Scholar]
- Mardanova, E.S.; Blokhina, E.A.; Tsybalova, L.M.; Peyret, H.; Lomonossoff, G.P.; Ravin, N.V. Efficient Transient Expression of Recombinant Proteins in Plants by the Novel pEff Vector Based on the Genome of Potato Virus X. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Voinnet, O.; Rivas, S.; Mestre, P.; Baulcombe, D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003, 33, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, M.J.; Reed, J.; Brouwer, B.; Osbourn, A. Transient Expression in Nicotiana Benthamiana Leaves for Triterpene Production at a Preparative Scale. J. Vis. Exp. 2018, 58169. [Google Scholar] [CrossRef] [PubMed]
- Burnett, M.J.B.; Burnett, A.C. Therapeutic recombinant protein production in plants: Challenges and opportunities. Plants People Planet 2020, 2, 121–132. [Google Scholar] [CrossRef]
- Sathish, S.; Preethy, K.S.; Venkatesh, R.; Sathishkumar, R. Rapid enhancement of α-tocopherol content in Nicotiana benthamiana by transient expression of Arabidopsis thaliana Tocopherol cyclase and Homogentisate phytyl transferase genes. 3 Biotech 2018, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.A.; Khan, A.A.; Javed, M.S.; Sajid, M.W. Green Leafy Vegetables: A Health Promoting Source. In Handbook of Fertility; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2015; Chapter 18; pp. 205–220. [Google Scholar] [CrossRef]
- Sharma, H.; Rawal, A. Health Security in Ethnic Communities through Nutraceutical Leafy Vegetables. J. Environ. Res. Dev. 2013, 7, 1423–1429. [Google Scholar]
- Tarwadi, K.; Agte, V. Potential of commonly consumed green leafy vegetables for their antioxidant capacity and its linkage with the micronutrient profile. Int. J. Food Sci. Nutr. 2003, 54, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Prakash, J. Studies on Indian Green Leafy Vegetables for Their Antioxidant Activity. Plant Foods Hum. Nutr. 2009, 64, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.M.; Ramamoorthy, D.; Aziz, R.; Malla, A.; Ramalingam, S. Assessment of free radical scavenging activities of leaves and stem fractions of green leafy vegetables. Afr. J. Pharm. Pharmacol. 2014, 8, 1138–1145. [Google Scholar]
- Bhat, R.S.; Al-Daihan, S. Phytochemical constituents and antibacterial activity of some green leafy vegetables. Asian Pac. J. Trop. Biomed. 2014, 4, 189–193. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Sarker, U.; Hossain, M.M.; Oba, S. Nutritional and antioxidant components and antioxidant capacity in green morph Amaranthus leafy vegetable. Sci. Rep. 2020, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Murugan, S.B.; Reshma, A.; Deepika, R.; Balamurugan, S.; Sathishkumar, R. Antioxidant capacities of Amaranthus tristis and Alternanthera sessilis: A comparative study. J. Med. Plants Res. 2013, 7, 2230–2235. [Google Scholar]
- Murugan, S.B.; Sathishkumar, R. Establishment of High Frequency Callus induction and Genetic Transformation in Neglected Leafy Vegetable Amaranthus trisis. Austin J. Biotechnol. Bioeng. 2016, 3, 1058. [Google Scholar]
- Chin, D.P.; Bao, J.H.; Mii, M. Transgenic spinach plants produced by Agrobacterium-mediated method based on the low temperature-dependent high plant regeneration ability of leaf explants. Plant Biotechnol. 2009, 26, 243–248. [Google Scholar] [CrossRef]
- Earley, K.W.; Haag, J.R.; Pontes, O.; Opper, K.; Juehne, T.; Song, K.; Pikaard, C.S. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 2006, 45, 616–629. [Google Scholar] [CrossRef]
- Sainsbury, F.; Thuenemann, E.C.; Lomonossoff, G.P. pEAQ: Versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol. J. 2009, 7, 682–693. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Ng, M.-S.; Cheng, S.-S.; Lo, A.W.-Y.; Xiao, Z.; Shin, T.-S.; Chung, G.; Lam, H.-M. Understanding the Composition, Biosynthesis, Accumulation and Transport of Flavonoids in Crops for the Promotion of Crops as Healthy Sources of Flavonoids for Human Consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef]
- Biała, W.; Jasiński, M. The Phenylpropanoid Case—It Is Transport That Matters. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- García-Calderón, M.; Pérez-Delgado, C.M.; Palove-Balang, P.; Betti, M.; Márquez, A.J. Flavonoids and Isoflavonoids Biosynthesis in the Model Legume Lotus japonicus; Connections to Nitrogen Metabolism and Photorespiration. Plants 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Özçelik, B.; Kartal, M.; Aslan, S.; Sener, B.; Özgüven, M. Quantification of daidzein, genistein and fatty acids in soybeans and soy sprouts, and some bioactivity studies. Acta Biol. Crac. Ser. Bot. 2007, 49, 61–68. [Google Scholar]
- Kumar, A. Metabolic Engineering in Plants. In Plant Biology and Biotechnology: Plant Genomics and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K.V., Eds.; Springer: New Delhi, India, 2015; Volume II, pp. 517–526. [Google Scholar] [CrossRef]
- Lu, X.; Tang, K.; Li, P. Plant Metabolic Engineering Strategies for the Production of Pharmaceutical Terpenoids. Front. Plant Sci. 2016, 7, 1647. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Jadaun, J.S.; Tripathi, S.; Mishra, B.; Narnoliya, L.K.; Sangwan, R.S. Plant Metabolic Engineering. In Omics Technologies and Bio-Engineering; Barh, D., Azevedo, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 9; pp. 143–175. [Google Scholar] [CrossRef]
- Chownk, M.; Thakur, K.; Yadav, S.K. Retrospect and prospects of plant metabolic engineering. J. Plant Biochem. Biotechnol. 2019, 28, 1–13. [Google Scholar] [CrossRef]
- Kapila, J.; De Rycke, R.; Van Montagu, M.; Angenon, G. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci. 1997, 122, 101–108. [Google Scholar] [CrossRef]
- Fischer, R.; Emans, N. Molecular farming of pharmaceutical proteins. Transgenic Res. 2000, 9, 279–299. [Google Scholar] [CrossRef]
- Joh, L.D.; Wroblewski, T.; Ewing, N.N.; VanderGheynst, J.S. High-level transient expression of recombinant protein in lettuce. Biotechnol. Bioeng. 2005, 91, 861–871. [Google Scholar] [CrossRef]
- Bhaskar, P.B.; Venkateshwaran, M.; Wu, L.; Ané, J.-M.; Jiang, J. Agrobacterium-Mediated Transient Gene Expression and Silencing: A Rapid Tool for Functional Gene Assay in Potato. PLoS ONE 2009, 4, e5812. [Google Scholar] [CrossRef]
- Mooney, B.C.; Graciet, E. A simple and efficient Agrobacterium-mediated transient expression system to dissect molecular processes in Brassica rapa and Brassica napus. Plant Direct 2020, 4, e00237. [Google Scholar] [CrossRef] [PubMed]
- Ashwini, M.; Murugan, S.B.; Balamurugan, S.; Sathishkumar, R. Advances in molecular cloning. Mol. Biol. 2016, 50, 1–6. [Google Scholar] [CrossRef]
- Liu, C.-J.; Blount, J.W.; Steele, C.L.; Dixon, R.A. Bottlenecks for metabolic engineering of isoflavone glycoconjugates in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 14578–14583. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hu, Y.; Li, J.; Lin, Z. Production of soybean isoflavone genistein in non-legume plants via genetically modified secondary metabolism pathway. Metab. Eng. 2007, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-H.; Chen, Y.; Wang, M.; Chu, I.K.; Lo, C. Accumulation of Isoflavone Genistin in Transgenic Tomato Plants Overexpressing a Soybean Isoflavone Synthase Gene. J. Agric. Food Chem. 2008, 56, 5655–5661. [Google Scholar] [CrossRef]
- D’Aoust, M.A.; Lavoie, P.O.; Couture, M.M.; Trépanier, S.; Guay, J.M.; Dargis, M.; Mongrand, S.; Landry, N.; Ward, B.J.; Vézina, L.P. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol. J. 2008, 6, 930–940. [Google Scholar] [CrossRef]





| Reaction Mix | Program | ||
|---|---|---|---|
| Components | Volume (µL) | 1. Initial Denaturation | 94 °C for 5 min |
| Emerald AmpR PCR master mix | 10 | 2. Denaturation | 94 °C for 30 s |
| Forward primer (10 pM) | 1 | 3. Annealing | 56 °C for 45 s |
| Reverse primer (10 pM) | 1 | 4. Extension | 72 °C for 45 s |
| Nuclease-free water | 6 | Step 2–4 repeated 25 cycles | |
| DNA template | 2 | Final Extension | 72 °C for 10 min |
| Total | 20 | Final Hold | 4 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malla, A.; Shanmugaraj, B.; Sharma, A.; Ramalingam, S. Production of Genistein in Amaranthus tricolor var. tristis and Spinacia oleracea by Expression of Glycine max Isoflavone Synthase. Plants 2021, 10, 2311. https://doi.org/10.3390/plants10112311
Malla A, Shanmugaraj B, Sharma A, Ramalingam S. Production of Genistein in Amaranthus tricolor var. tristis and Spinacia oleracea by Expression of Glycine max Isoflavone Synthase. Plants. 2021; 10(11):2311. https://doi.org/10.3390/plants10112311
Chicago/Turabian StyleMalla, Ashwini, Balamurugan Shanmugaraj, Ashutosh Sharma, and Sathishkumar Ramalingam. 2021. "Production of Genistein in Amaranthus tricolor var. tristis and Spinacia oleracea by Expression of Glycine max Isoflavone Synthase" Plants 10, no. 11: 2311. https://doi.org/10.3390/plants10112311
APA StyleMalla, A., Shanmugaraj, B., Sharma, A., & Ramalingam, S. (2021). Production of Genistein in Amaranthus tricolor var. tristis and Spinacia oleracea by Expression of Glycine max Isoflavone Synthase. Plants, 10(11), 2311. https://doi.org/10.3390/plants10112311







