Effect of Manure and Compost on the Phytostabilization Potential of Heavy Metals by the Halophytic Plant Wavy-Leaved Saltbush
Abstract
1. Introduction
2. Material and Methods
2.1. Soil Characterization
2.2. Pot Experiments
2.3. Characterization of Compost and Manure
2.4. Plant Analysis
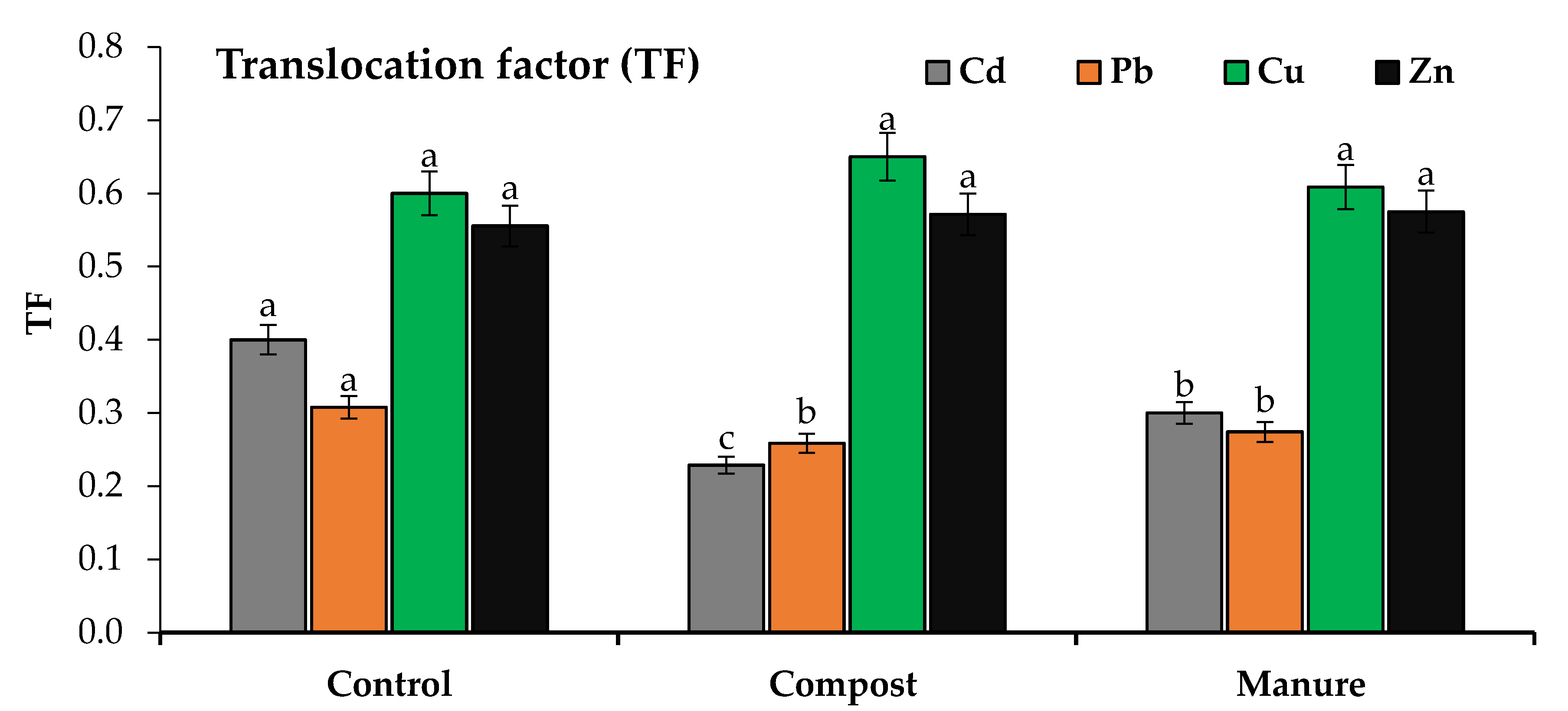
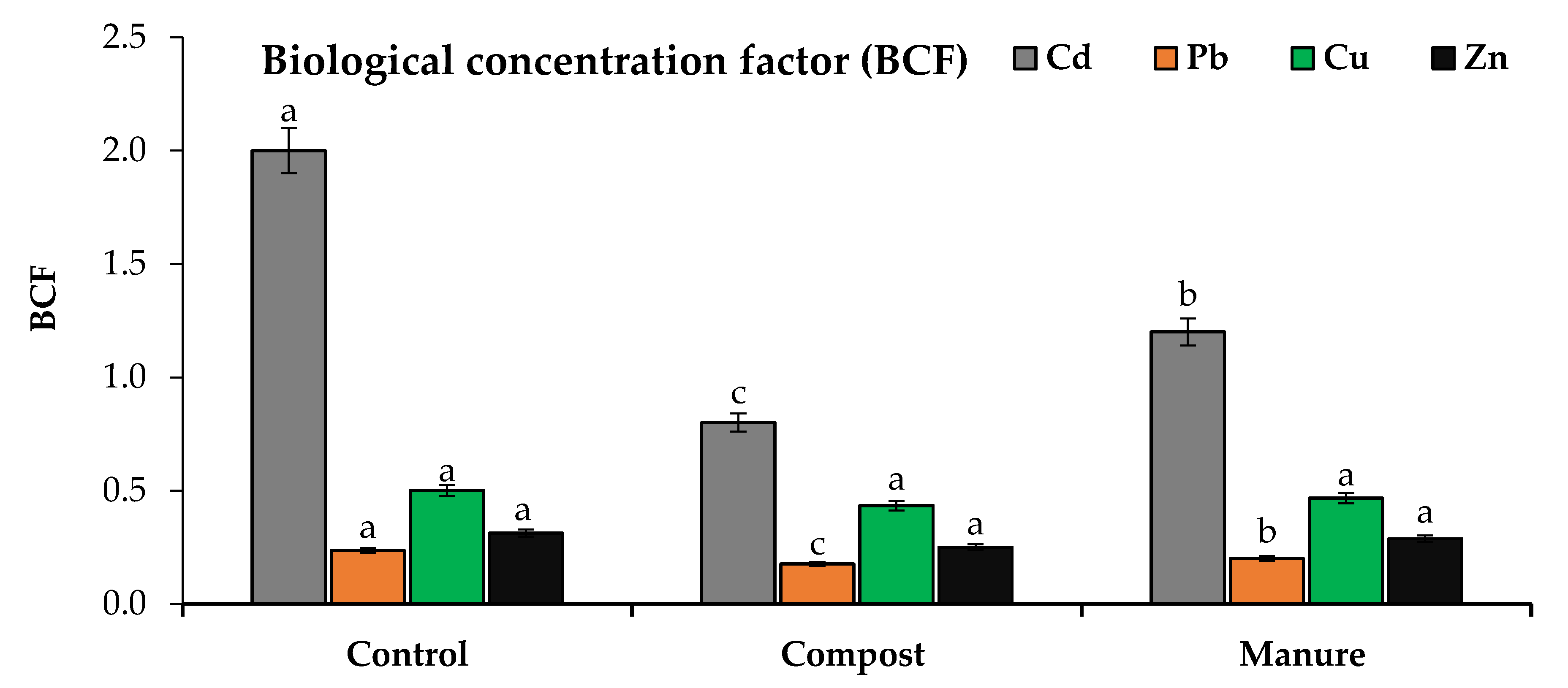
2.5. Bioconcentration and Translocation Factors
2.6. Statistical Analysis
3. Results
3.1. Effect of Cultivation and Organic Fertilization on the Characteristics of Polluted Soil
3.2. Effect of Compost and Manure on the Growth of Wavy-Leaved Saltbush Plants
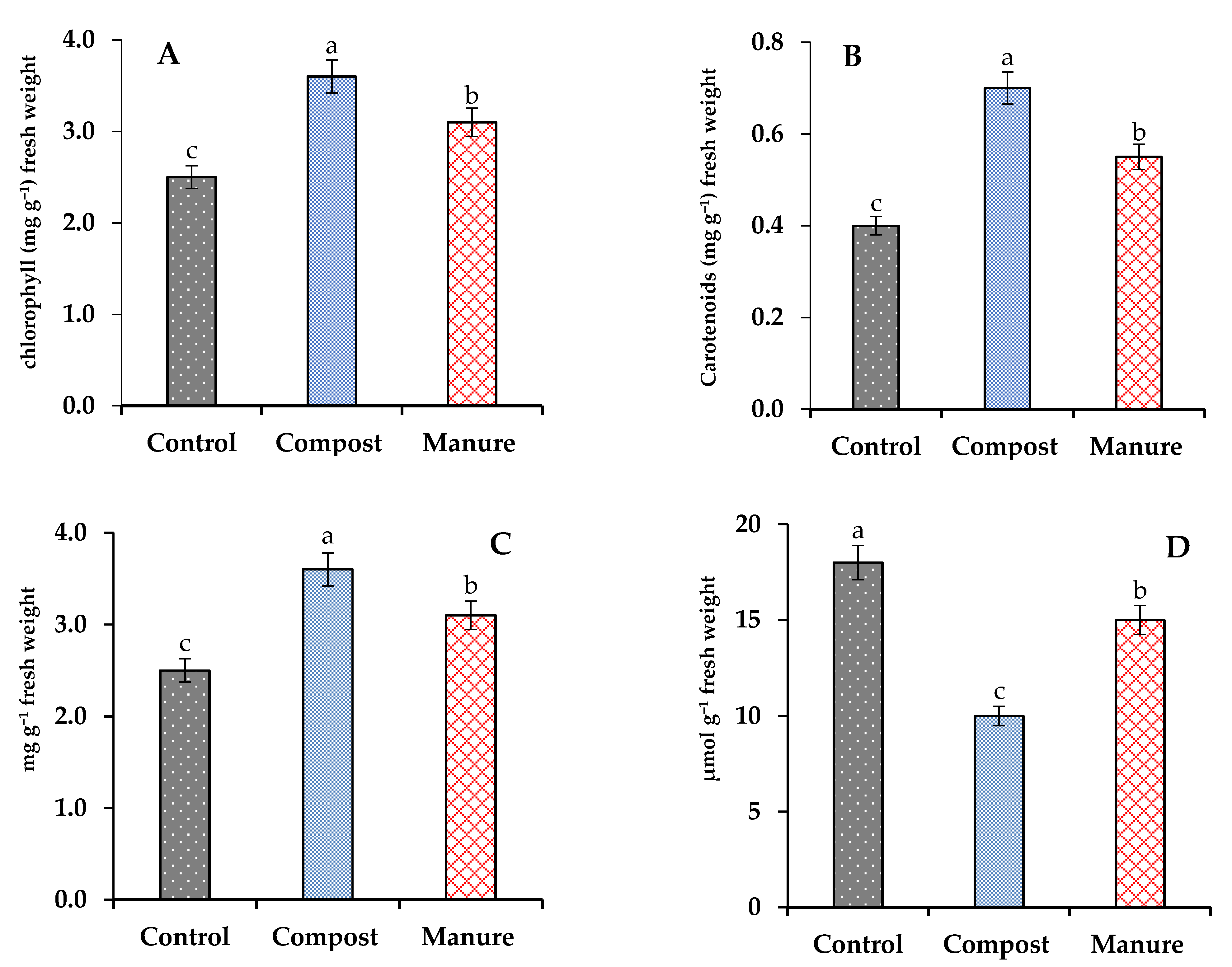
3.3. Effect of Compost and Manure on Photosynthesis Pigments and Malondialdehyde (MDA) and Proline Content
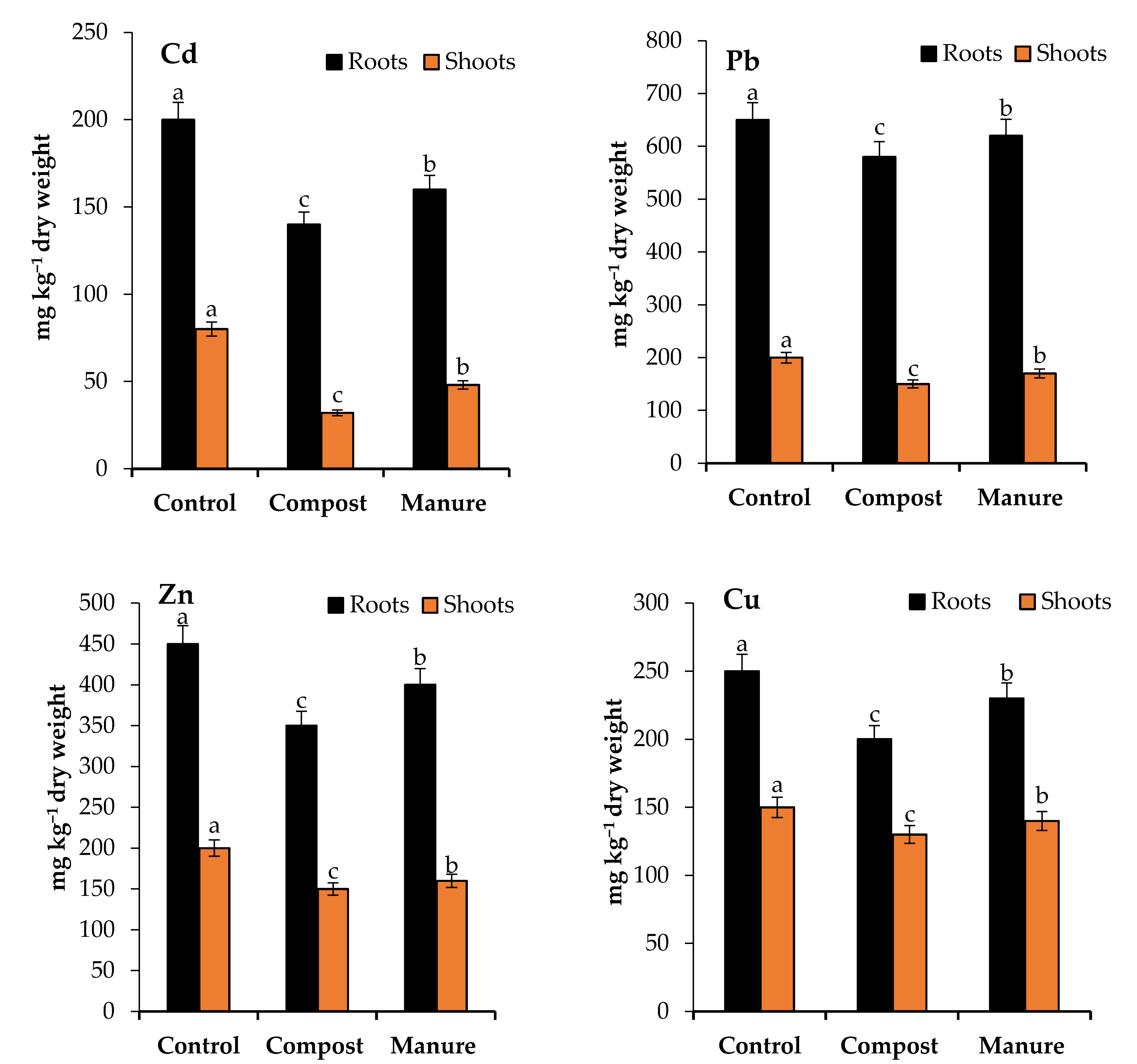
3.4. Cd and Pb Uptake and Translocation as Affected by Compost and Manure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lajayer, B.A.; Moghadam, N.K.; Maghsoodi, M.R.; Ghorbanpour, M.; Kariman, K. Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: Mechanisms and efficiency improvement strategies. Environ. Sci. Pollut. Res. 2019, 26, 8468–8484. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Y.; Nawab, J.; Rahman, Z.; Khan, S.; Idress, M.; Ali, A.; Ahmad, R.; Khan, S.A.; Khan, A.; et al. Contamination features, geo-accumulation, enrichments and human health risks of toxic heavy metal (loids) from fish consumption collected along Swat river, Pakistan. Environ. Technol. Innov. 2020, 17, 100554. [Google Scholar] [CrossRef]
- Ding, Z.; Alharbi, S.; Almaroai, Y.A.; Eissa, M.A. Improving quality of metal-contaminated soils by some halophyte and non-halophyte forage plants. Sci. Total Environ. 2021, 794, 142885. [Google Scholar] [CrossRef]
- Eissa, M.A.; Negim, O.E. Heavy metals uptake and translocation by lettuce and spinach grown on a metal-contaminated soil. J. Soil Sci. Plant Nutr. 2018, 18, 1097–1107. [Google Scholar] [CrossRef][Green Version]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Nedjimi, B. Phytoremediation: A sustainable environmental technology for heavy metals decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Eissa, M.A.; Ahmed, E.M.; Reichman, S.M. Production of the forage halophyte Atriplex amnicola in metal-contaminated soils. Soil Use Manag. 2016, 32, 350–356. [Google Scholar] [CrossRef]
- Eissa, M.A.; Almaroai, Y.A. Phytoremediation capacity of some forage plants grown on a metals-contaminated soil. Soil Sediment Contam. 2019, 28, 569–581. [Google Scholar] [CrossRef]
- Pearce, K.L. Carcass and Eating Quality of Sheep Grazing Saltbush Based Saline Pasture Systems. Doctoral Thesis, Murdoch University, Murdoch, WA, Australia, 2006. [Google Scholar]
- Eissa, M.A. Effect of compost and biochar on heavy metals phytostabilization by the halophytic plant old man saltbush [Atriplex nummularia Lindl]. Soil Sediment Contam. 2019, 28, 135–147. [Google Scholar] [CrossRef]
- Paradelo, R.; Villada, A.; Barral, M.T. Reduction of the short-term availability of copper, lead and zinc in a contaminated soil amended with municipal solid waste compost. J. Hazard. Mater. 2011, 188, 98–104. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Haynes, R.J.; Naidu, R. Use of inorganic and organic wastes for in situ immobilisation of Pb and Zn in a contaminated alkaline soil. Environ. Sci. Pollut. Res. 2012, 19, 1260–1270. [Google Scholar] [CrossRef]
- Rosen, V.; Chen, Y. The influence of compost addition on heavy metal distribution between operationally defined geochemical fractions and on metal accumulation in plant. J. Soils Sediment. 2014, 14, 713–720. [Google Scholar] [CrossRef]
- Achieng, J.O.; Ouma, G.O.; Dhiambo, G.; Muyekho, F. Effect of farmyard manure and inorganic fertilizers on maize production on Alfisols and Ultisols in Kakamega, western Kenya. Agric. Biol. J. N. Am. 2010, 1, 430–439. [Google Scholar] [CrossRef]
- Aytenew, M.; Bore, G. Effects of organic amendments on soil fertility and environmental quality: A review. J. Plant Sci. 2020, 8, 112–119. [Google Scholar] [CrossRef]
- Bernal, M.P.; Clemente, R.; Walker, D.J. The role of organic amendments in the bioremediation of heavy metal-polluted soils. In Environmental Research at the Leading Edge; Gore, R.W., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2007; pp. 1–57. [Google Scholar]
- Achiba, W.B.; Gabteni, N.; Lakhdar, A.; Du Laing, G.; Verloo, M.; Jedidi, N.; Gallali, T. Effects of 5-year application of municipal solid waste compost on the distribution and mobility of heavy metals in a Tunisian calcareous soil. Agric. Ecosyst. Environ. 2009, 130, 156–163. [Google Scholar] [CrossRef]
- Madrid, F.; López, R.; Cabrera, F. Metal accumulation in soil after application of municipal solid waste compost under intensive farming conditions. Agric. Ecosyst. Environ. 2007, 119, 249–256. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, H.; Guo, Y.; Liu, Z.; Jiao, W. Immobilization of heavy metals in contaminated soils by modified hydrochar: Efficiency, risk assessment and potential mechanisms. Sci. Total Environ. 2019, 685, 1201–1208. [Google Scholar] [CrossRef]
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.Q.; Wang, D.F. Immobilization of heavy metals in sewage sludge during land application process in China: A review. Sustainability 2017, 9, 2020. [Google Scholar] [CrossRef]
- Burt, R. Soil Survey Laboratory Methods Manual; Soil Survey Investigations Report, No. 42, Version 4.0; Natural Resources Conservation Service, United States Department of Agriculture: Washington, DC, USA, 2004.
- Lindsay, W.L.; Norvell, W.A. Equilibrium relationship of Zn+2, Fe+3, Ca+2 and H+ with EDTA and DTPA in soils. Soil Sci. Soc. Am. Proc. 1969, 33, 62–68. [Google Scholar] [CrossRef]
- Madhava Rao, K.V.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1. [Google Scholar] [CrossRef]
- Mishra, T.; Pandey, V.C. Phytoremediation of red mud deposits through natural succession. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 409–424. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, S.; Liu, S.; Wang, X.; Zhang, Y.; Liu, T.; Zhou, L.; Zhang, W.; Fu, S. Reforestation makes a minor contribution to soil carbon accumulation in the short term: Evidence from four subtropical plantations. For. Ecol. Manag. 2017, 384, 400–405. [Google Scholar] [CrossRef]
- Qin, S.; Liu, H.; Nie, Z.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: A review. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Zorpas, A.A.; Karagiannidis, A.; Samaras, P.; Voukkali, I.; Sklari, S. European Union legislation on sewage sludge management. Fresen. Environ. Bull. 2014, 23, 635–639. [Google Scholar]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Shafiq, S.; Zeb, Q.; Ali, A.; Sajjad, Y.; Nazir, R.; Widemann, E.; Liu, L. Lead, cadmium and zinc phytotoxicity alter DNA methylation levels to confer heavy metal tolerance in wheat. Int. J. Mol. Sci. 2019, 20, 4676. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Saleem, M.H.; Fahad, S.; Rehman, M.; Saud, S.; Jamal, Y.; Khan, S.; Liu, L. Morpho-physiological traits, biochemical response and phytoextraction potential of short-term copper stress on kenaf (Hibiscus cannabinus L.) seedlings. Peer J. 2020, 8, e8321. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Malik, K.M.; Khan, K.S.; Rukh, S.; Khan, A.; Akbar, S.; Billah, M.; Bashir, S.; Danish, S.; Alwahibi, M.S.; Elshikh, M.S.; et al. Immobilization of Cd, Pb and Zn through organic amendments in wastewater irrigated soils. Sustainability 2021, 13, 2392. [Google Scholar] [CrossRef]
- Zhou, W.; Ren, L.; Zhu, L. Reducement of cadmium adsorption on clay minerals by the presence of dissolved organic matter from animal manure. Environ. Pollut. 2017, 223, 247–254. [Google Scholar] [CrossRef]
- Marković, J.; Jović, M.; Smičiklas, I.; Šljivić-Ivanović, M.; Onjia, A.; Trivunac, K.; Popović, A. Cadmium retention and distribution in contaminated soil: Effects and interactions of soil properties, contamination level, aging time and in situ immobilization agents. Ecotoxicol. Environ. Saf. 2019, 174, 305–314. [Google Scholar] [CrossRef]
- Marques, J.P.; Rodrigues, V.G.S.; Raimondi, I.M.; Lima, J.Z. Increase in Pb and Cd adsorption by the application of peat in a tropical soil. Water Air Soil Pollut. 2020, 231, 136. [Google Scholar] [CrossRef]
- Smolinska, B. Green waste compost as an amendment during induced phytoextraction of mercury-contaminated soil. Environ. Sci. Pollut. Res. 2015, 22, 3528–3537. [Google Scholar] [CrossRef]
- Walker, D.J.; Clemente, R.; Roig, A.; Bernal, M.P. The effects of soil amendments on heavy metal bioavailability in two contaminated Mediterranean soils. Environ. Pollut. 2003, 122, 303–312. [Google Scholar] [CrossRef]
- Kim, R.; Yoon, J.; Kim, T.; Yang, J.; Owens, G.; Kim, K. Bioavailability of heavy metals in soils: Definitions and practical implementation—A critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef] [PubMed]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Huybrechts, M.; Cuypers, A.; Deckers, J.; Iven, V.; Vandionant, S.; Jozefczak, M.; Hendrix, S. Cadmium and plant development: An agony from seed to seed. Int. J. Mol. Sci. 2019, 20, 3971. [Google Scholar] [CrossRef] [PubMed]
- Eissa, M.A.; Abeed, A.H. Growth and biochemical changes in quail bush (Atriplex lentiformis (Torr.) S. Wats) under Cd stress. Environ. Sci. Pollut. Res. 2019, 26, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Eissa, M.A. Impact of compost on metals phytostabilization potential of two halophytes species. Int. J. Phytoremediat. 2015, 17, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernández, D.; Walker, D. The effects of soil amendments on the growth of Atriplex halimus and Bituminaria bituminosa in heavy metal-contaminated soils. Water Air Soil Pollut. 2012, 223, 63–72. [Google Scholar] [CrossRef]
- AL-Huqail, A.A.; El-Bondkly, A.M.A. Improvement of Zea mays L. growth parameters under chromium and arsenic stress by the heavy metal-resistant Streptomyces sp. NRC21696. Int. J. Environ. Sci. Technol. 2021, 18, 1–22. [Google Scholar] [CrossRef]
- AL-Huqail, A.A.; Ali, H.M.; Kushwaha, B.K.; Al-Huqail, A.A.; Singh, V.P.; Siddiqui, M.H. Ascorbic acid is essential for inducing chromium (VI) toxicity tolerance in tomato roots. J. Biotechnol. 2020, 322, 66–73. [Google Scholar] [CrossRef]
- AL-Huqail, A.A.; AL-Rashed, S.A.; Ibrahim, M.M.; El-Gaaly, G.A.; Qureshi, M.I. Arsenic induced eco-physiological changes in chickpea (Cicer arietinum) and protection by gypsum, a source of sulphur and calcium. Sci. Hortic. 2017, 217, 226–233. [Google Scholar] [CrossRef]
- Abou-Zaid, E.A.; Eissa, M.A. Thompson seedless grapevines growth and quality as affected by glutamic acid, vitamin b, and algae. J. Soil Sci. Plant Nutr. 2019, 19, 725–733. [Google Scholar] [CrossRef]
- Ali, A.M.; Awad, M.Y.; Hegab, S.A.; Gawad, A.M.A.E.; Eissa, M.A. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant Nutr. 2021, 44, 411–420. [Google Scholar] [CrossRef]
- Al-Sayed, H.; Hegab, S.A.; Youssef, M.; Khalafalla, M.; Almaroai, Y.A.; Ding, Z.; Eissa, M.A. Evaluation of quality and growth of roselle (Hibiscus sabdariffa L.) as affected by bio-fertilizers. J. Plant Nutr. 2021, 43, 1025–1035. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, Z.; Lin, X.; Zhao, F.; Wang, B.; Lin, F.; Ge, Y.; Eissa, M.A. Biochar impacts on NH3-volatilization kinetics and growth of sweet basil (Ocimum basilicum L.) under saline conditions. Ind. Crop. Prod. 2020, 157, 11290. [Google Scholar] [CrossRef]
- Liu, D.; Al-Fahd, M.H.; Ali, E.F.; Majrashi, A.; Ghoneim, A.M.; Ding, Z.; Eissa, M.A. Soil microbial biomass, CO2 and NH3 emission and nitrogen use efficiency in a sandy soil amended with recycled dairy products. Environ. Technol. Innov. 2021, 23, 101546. [Google Scholar] [CrossRef]
- Ding, Z.; Alharbi, S.; Ali, E.F.; Ghoneim, A.M.; Al-Fahd, M.; Wang, G.; Eissa, M.A. Effect of phosphorus-loaded biochar and nitrogen-fertilization on release kinetic of toxic heavy metals and tomato growth. Int. J. Phytorem. 2021, 1–10. [Google Scholar] [CrossRef]
- Alharbi, S.; Majrashi, A.; Ghoneim, A.M.; Ali, E.F.; Modahish, A.S.; Hassan, F.A.; Eissa, M.A. A new method to recycle dairy waste for the nutrition of wheat plants. Agronomy 2021, 11, 840. [Google Scholar] [CrossRef]
| Soil Properties | Value |
|---|---|
| Texture | Sandy loam |
| CaCO3 (g kg−1) | 60 |
| CEC (cmol kg−1) | 15 |
| pH (1:2) | 8.09 |
| EC (1:2) (dS m−1) | 2.11 |
| Organic carbon (g kg−1) | 3.50 |
| Total nitrogen (mg kg−1) | 190 |
| Total Zn (mg kg−1) | 800 |
| Available Zn (mg kg−1) | 8.2 |
| Total Cu (mg kg−1) | 300 |
| Available Cu (mg kg−1) | 2.5 |
| Total Pb (mg kg−1) | 850 |
| Available Pb (mg kg−1) | 7.0 |
| Total Cu (mg kg−1) | 300 |
| Available Cu (mg kg−1) | 2.5 |
| Organic Carbon (g kg−1) | Total Nitrogen (g kg−1) | pH 1:10 | EC (dS m−1) | Zn (mg kg−1) | Cu (mg kg−1) | Pb (mg kg−1) | Cd (mg kg−1) | |
|---|---|---|---|---|---|---|---|---|
| Compost | 400 | 22 | 8.02 | 4.5 | 160 | 40 | - | 0.33 |
| Manure | 320 | 18 | 7.82 | 6.5 | 155 | 35 | - | 0.42 |
| Soil Treatment | Amendments | EC (dS m−1) | pH (1:2) | SOC (g kg−1) | CEC (cmol kg−1) | Zn (mg kg−1) | Cu (mg kg−1) | Cd (mg kg−1) | Pb (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| Non-cultivatedSoil | Control | 2.20 ± 0.11 b | 7.90 ± 0.26 a | 3.5 ± 0.28 b | 16 ± 1.5 b | 8.00 ± 0.32 b | 2.48 ± 0.18 b | 3.40 ± 0.15 b | 7.00 ± 0.28 b |
| Compost | 3.00 ± 0.16 a | 7.78 ± 0.25 ab | 5.5 ± 0.26 a | 17 ± 1.8 ab | 6.52 ± 0.18 d | 2.28 ± 0.14 c | 3.00 ± 0.13 c | 6.10 ± 0.24 c | |
| Manure | 3.20 ± 0.15 a | 7.58 ± 0.26 b | 5.7 ± 0.25 a | 17 ± 1.7 ab | 8.63 ± 0.33 a | 2.86 ± 0.16 a | 4.00 ± 0.12 a | 8.00 ± 0.25 a | |
| Cultivated Soil | Control | 2.30 ± 0.14 b | 7.88 ± 0.27 a | 3.8 ± 0.24 b | 16 ± 1.3 b | 6.54 ± 0.25 d | 2.00 ± 0.14 d | 2.55 ± 0.15 e | 4.70 ± 0.29 e |
| Compost | 3.10 ± 0.18 a | 7.75 ± 0.24 ab | 5.8 ± 0.33 a | 18 ± 1.5 a | 5.86 ± 0.32 e | 1.76 ± 0.15 e | 1.96 ± 0.18 f | 4.00 ± 0.27 f | |
| Manure | 3.00 ± 0.11 a | 7.52 ± 0.22 b | 5.6 ± 0.42 a | 18 ± 1.4 a | 7.04 ± 0.19 c | 1.80 ± 0.11 e | 2.75 ± 0.17 d | 5.00 ± 0.26 d | |
| p | 0.0001 | 0.008 | 0.005 | 0.002 | 0.009 | 0.005 | 0.001 | 0.004 | |
| Treatments | Plant Length (cm) | Roots Dry Weight (g pot−1) | Shoots Dry Weight (g pot−1) | Leaves Number/Plant | Leaf Area/Plant (cm2) |
|---|---|---|---|---|---|
| Control | 130 ± 5.50 c | 30 ± 1.55 c | 100 ± 5.40 c | 50 ± 2.22 c | 100 ± 5.28 c |
| Compost | 180 ± 5.10 a | 45 ± 1.33 a | 138 ± 6.44 a | 80 ± 3.33 a | 150 ± 5.11 a |
| Manure | 165 ± 6.60 b | 40 ± 1.66 b | 125 ± 6.12 b | 75 ± 3.44 b | 138 ± 5.55 b |
| p | 0.01 | 0.001 | 0.003 | 0.01 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chang, Y.; AL-Huqail, A.A.; Ding, Z.; Al-Harbi, M.S.; Ali, E.F.; Abeed, A.H.A.; Rekaby, S.A.; Eissa, M.A.; Ghoneim, A.M.; et al. Effect of Manure and Compost on the Phytostabilization Potential of Heavy Metals by the Halophytic Plant Wavy-Leaved Saltbush. Plants 2021, 10, 2176. https://doi.org/10.3390/plants10102176
Li J, Chang Y, AL-Huqail AA, Ding Z, Al-Harbi MS, Ali EF, Abeed AHA, Rekaby SA, Eissa MA, Ghoneim AM, et al. Effect of Manure and Compost on the Phytostabilization Potential of Heavy Metals by the Halophytic Plant Wavy-Leaved Saltbush. Plants. 2021; 10(10):2176. https://doi.org/10.3390/plants10102176
Chicago/Turabian StyleLi, Jianjian, Yajun Chang, Arwa Abdulkreem AL-Huqail, Zheli Ding, Mohammad S. Al-Harbi, Esmat F. Ali, Amany H. A. Abeed, Saudi A. Rekaby, Mamdouh A. Eissa, Adel M. Ghoneim, and et al. 2021. "Effect of Manure and Compost on the Phytostabilization Potential of Heavy Metals by the Halophytic Plant Wavy-Leaved Saltbush" Plants 10, no. 10: 2176. https://doi.org/10.3390/plants10102176
APA StyleLi, J., Chang, Y., AL-Huqail, A. A., Ding, Z., Al-Harbi, M. S., Ali, E. F., Abeed, A. H. A., Rekaby, S. A., Eissa, M. A., Ghoneim, A. M., & Tammam, S. A. (2021). Effect of Manure and Compost on the Phytostabilization Potential of Heavy Metals by the Halophytic Plant Wavy-Leaved Saltbush. Plants, 10(10), 2176. https://doi.org/10.3390/plants10102176












