The Physiological and Biochemical Responses of European Chestnut (Castanea sativa L.) to Blight Fungus (Cryphonectria parasitica (Murill) Barr)
Abstract
:1. Introduction
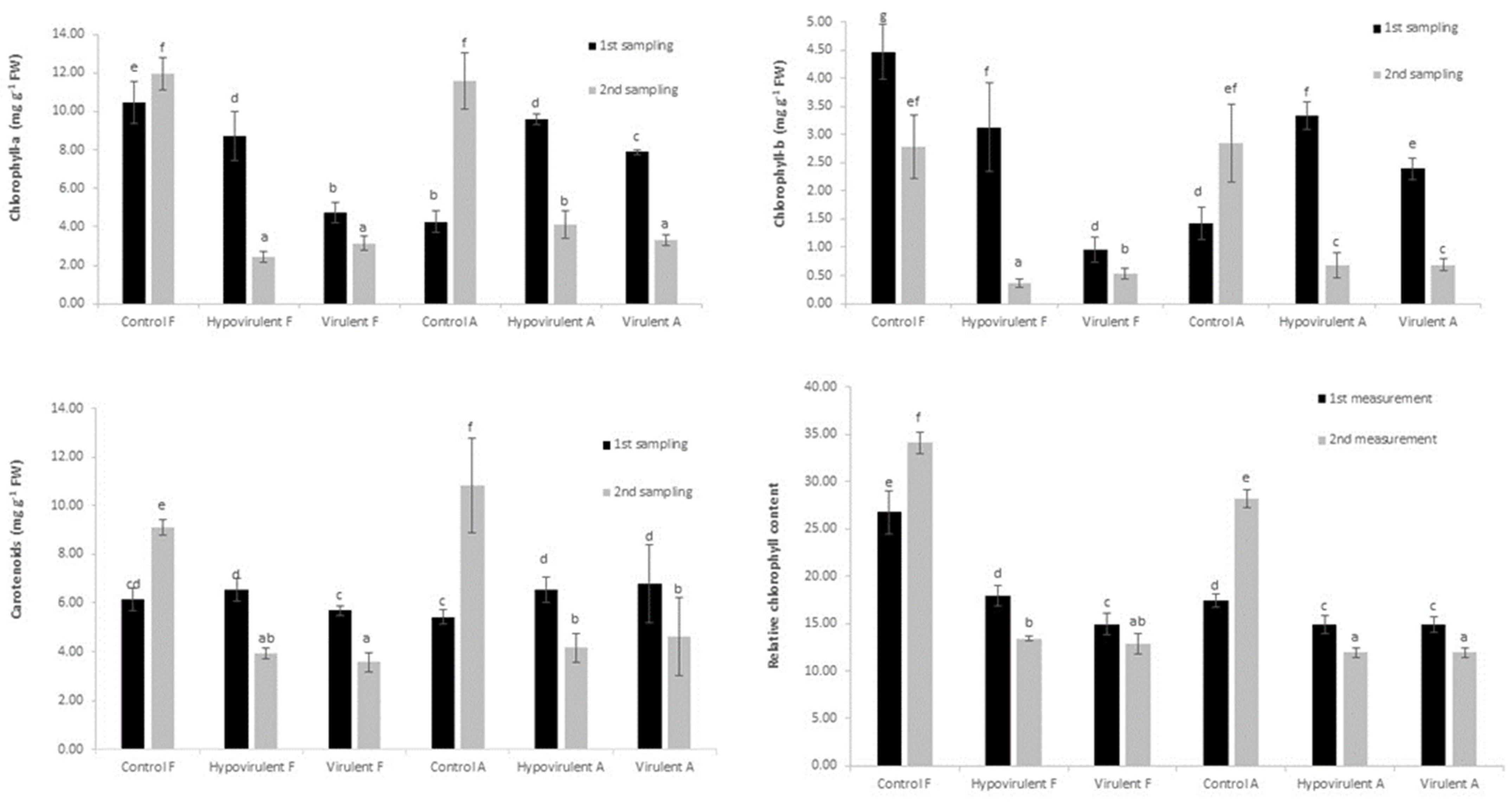
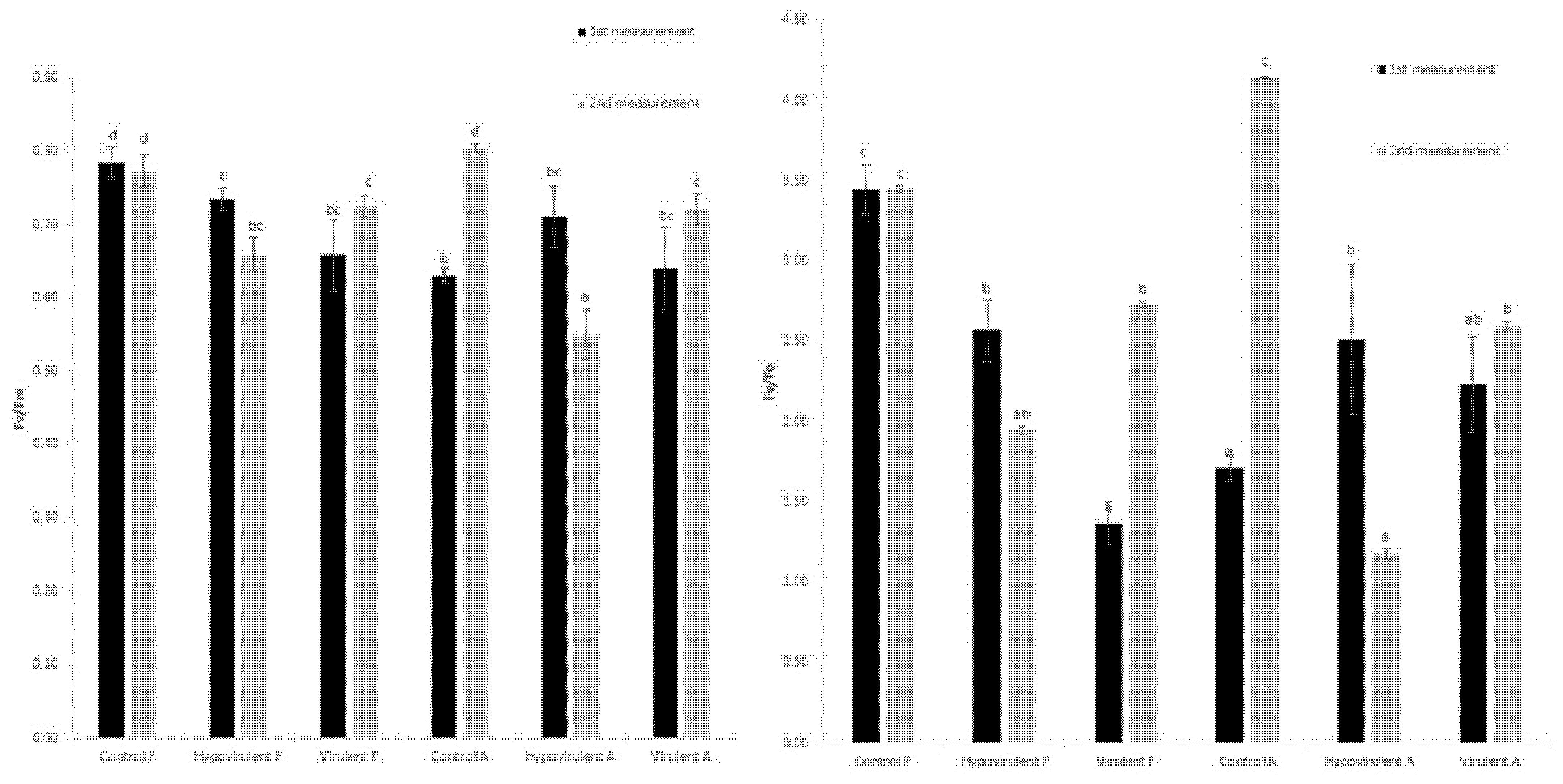
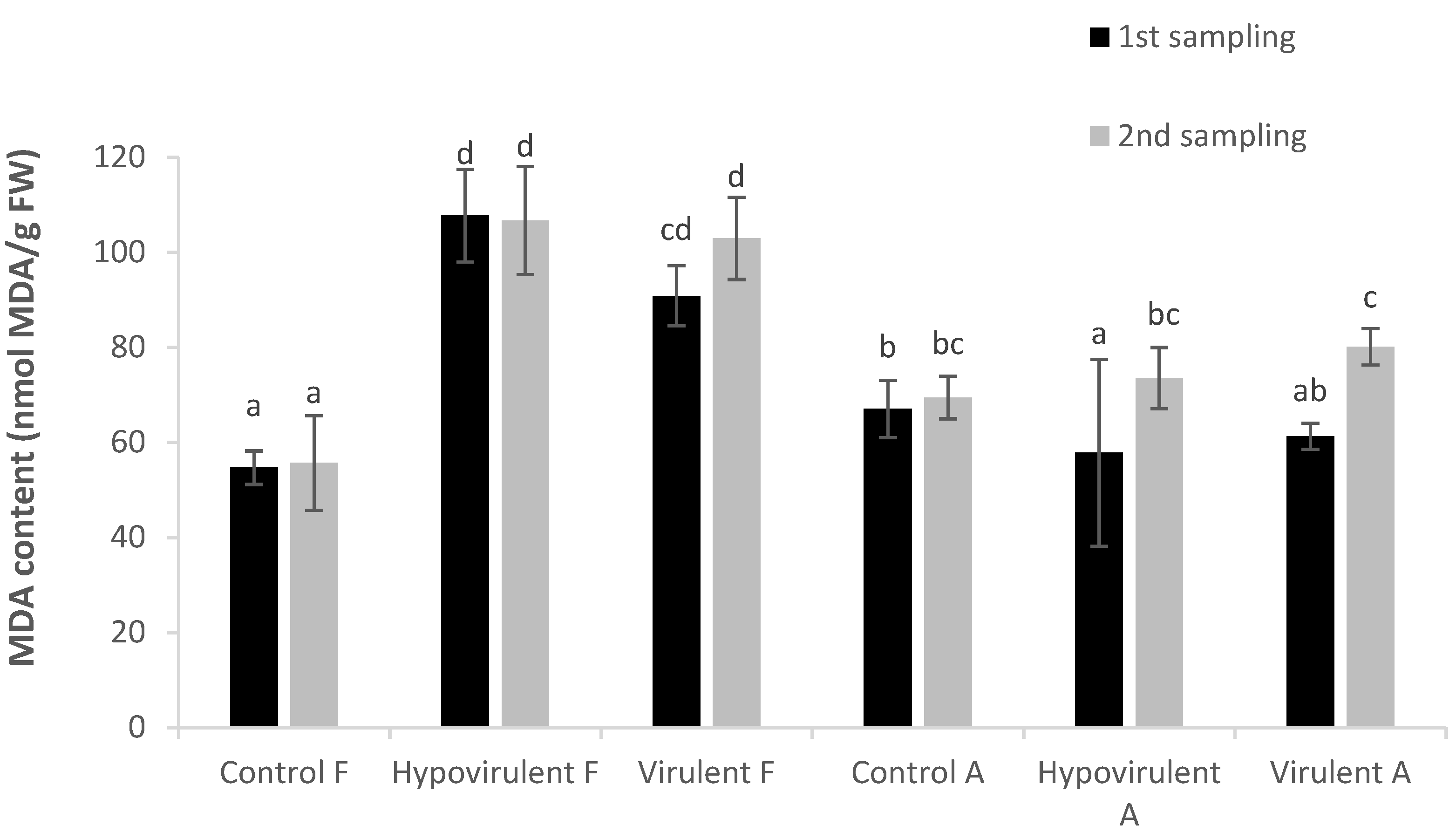
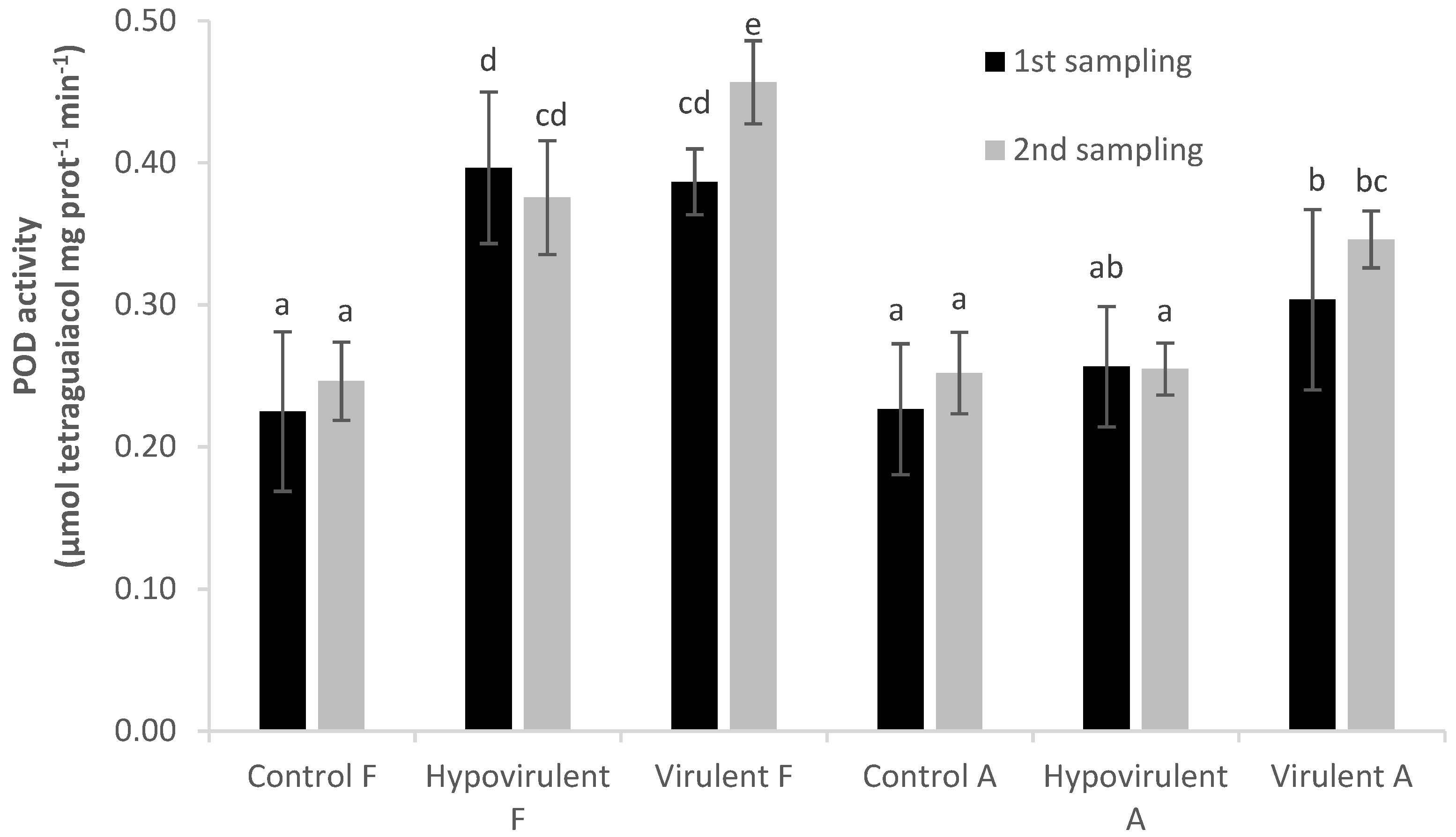
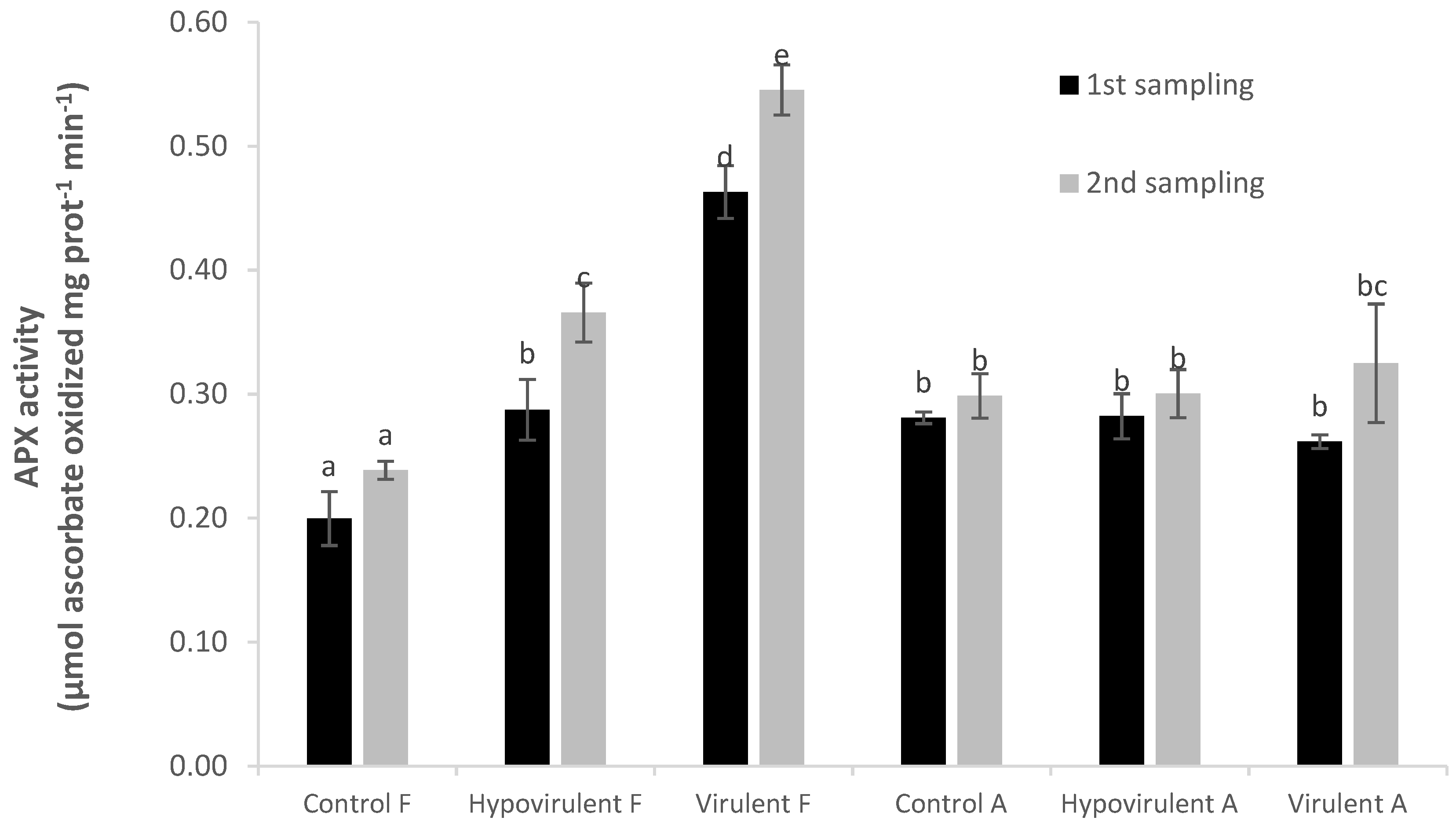
2. Results
3. Discussion
4. Materials and Methods
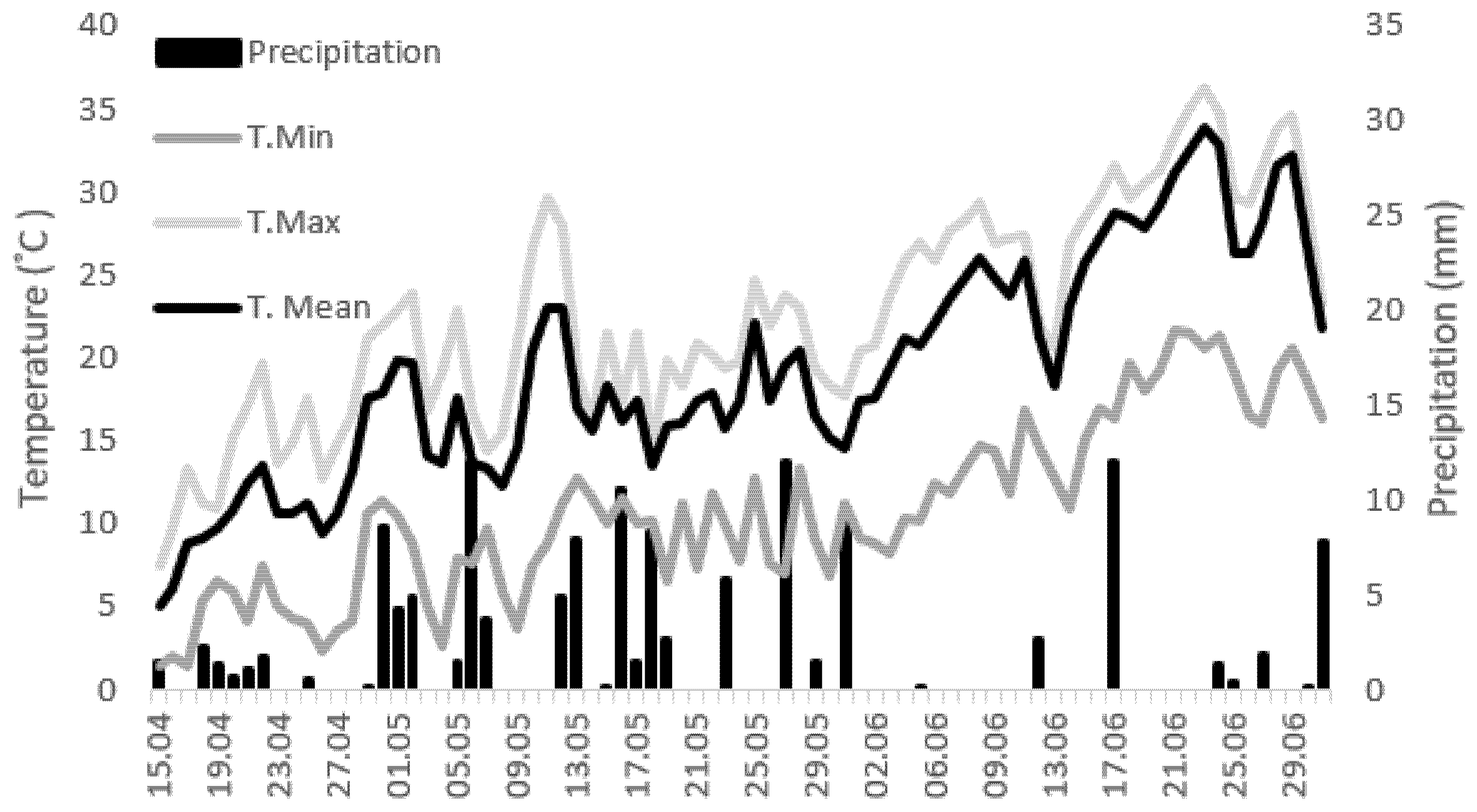
4.1. Experimental Conditions and Treatments
4.2. Quantity of the Photosynthetic Pigments
4.3. Measurement of Photochemical Efficiency
4.4. Enzyme Assays
4.5. Rate of Lipid Peroxidation
4.6. Proline Determination
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valipour, M.; Bateni, S.M.; Jun, C. Global surface temperature: A new insight. Climate 2021, 9, 81. [Google Scholar] [CrossRef]
- Hughes, L. Biological consequences of global warming: Is the signal already apparent? Trends Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Lenoir, J.; Svenning, J.-C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant and pathogen warfare under changing climate conditions. Curr. Biol. 2019, 28, R619–R634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Országos Meteorológiai Szolgálat (National Meteorogical Service Hungary). Available online: https://www.met.hu/eghajlat/eghajlatvaltozas/hatasok-alkalmazkodas/ (accessed on 20 July 2021).
- Skendži’c, S.; Zovko, M.; Živkovi’c, I.P.; Leši, V.; Lešić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Larson, E.L.; Tinghitella, R.M.; Taylor, S.A. Insect hybridization and climate change. Front. Ecol. Evol. 2019, 7, 348. [Google Scholar] [CrossRef] [Green Version]
- Murolo, S.; Angelini, R.M.D.M.; Faretra, F.; Romanazzi, G. Phenotypic and molecular investigations on hypovirulent Cryphonectria parasitica in Italy. Plant Dis. 2018, 103, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, T.R.; Santos, J.A.; Silva, A.P.; Fraga, H. Influence of climate change on chestnut trees: A review. Plants 2021, 10, 1463. [Google Scholar] [CrossRef]
- Országos Meteorológiai Szolgálat (National Meteorogical Service Hungary). Available online: https://www.met.hu/eghajlat/magyarorszag_eghajlata/eghajlati_visszatekinto/elmult_evek_idojarasa/ (accessed on 20 July 2021).
- Available online: https://dailynewshungary.com/2020-was-one-of-the-warmest-years-in-hungary-in-the-past-120-years/#:~:text=2020%20began%20with%20an%20average,breaking%20the%20daily%20heat%20record (accessed on 24 August 2021).
- Suzuki, N.; Cornejo, C.; Aulia, A.; Shahi, S.; Hillman, B.I.; Rigling, D. In-tree behavior of diverse viruses harbored in the chestnut blight fungus. Cryphonetria parasitica. J. Virol. 2021, 95, e01962-20. [Google Scholar] [CrossRef]
- Kolp, M.; Fulbright, D.W.; Jarosz, A.M. Inhibition of virulent and hypovirulent Cryphonectria parasitica growth in dual culture by fungi commonly isolated from chestnut blight cankers. Fungal Biol. 2018, 122, 935–942. [Google Scholar] [CrossRef]
- Eke, I.; Gál, T. Az Endothinia parasitica (Murr) Anderson elterjedése Magyarországon és a védekezés lehetőségei. Növényvédelem 1975, 11, 405–407. [Google Scholar]
- Havir, E.A.; Anagnostakis, S.L. Oxalate production by virulent but not by hypovirulent strains of Endothinia parasitica. Physiol. Plant Pathol. 1983, 23, 369–376. [Google Scholar] [CrossRef]
- Hebard, F.V.; Griffin, G.; Elkins, J.R. Developmental histopathology of cankers incited by hypovirulent and virulent isolates of Endothia parasitica on susceptible and resistant chestnut trees. Phytopathology 1984, 74, 140–149. [Google Scholar] [CrossRef]
- Roane, M.K.; Griffin, G.J.; Elkins, J.R. Chestnut Blight, Other Endothia Diseases, and the Genus Endothia; Monograph series; The American Phytopathologycal Society: St. Paul, MN, USA, 1986; p. 53. [Google Scholar]
- Radócz, L. Cryphonectria Parasitica in a Nagymarosi Szelídgesztenyések Története, Ápolása, Védelme, 1st ed.; DE ATC: Debrecen, Hungary, 2006; p. 34. ISBN 9789633180396. [Google Scholar]
- Heald, F.D.; Studhalter, R.A. Seasonal duration of ascospore explusion of Endothinia parasitica. Am. J. Bot. 1915, 2, 429–448. [Google Scholar] [CrossRef]
- Fulton, H.R. Recent Notes on the Chestnut Bark Disease. In Pennsylvania Chestnut Blight Conference Report; Penn State University Libaries: Harrisburg, PA, USA, 1912; pp. 48–56. Available online: https://digital.libraries.psu.edu/digital/collection/digitalbks2/id/13444 (accessed on 25 July 2021).
- Guérin, L.; Robin, C. Seasonal effect on infection and development of lesions caused by Cryphonectria parasitica in Castanea sativa. Forest Pathol. 2003, 33, 223–235. [Google Scholar] [CrossRef]
- Peters, F.S.; Holweg, C.L.; Rigling, D.; Meztler, B. Chestnut blight in south-western Germany: Multiple introductions of Cryphonectria parasitica and slow hypovirus spread. Forest Pathol. 2012, 42, 397–404. [Google Scholar] [CrossRef]
- Kovács, G.; Radócz, L. Effect of the media on morphology of Cryphonectria parasitica (Murr.) Barr isolates and their Vegetative Compatibility Groups. Acta Agrar. Debr. 2015, 66, 38–42. [Google Scholar] [CrossRef]
- Robin, C.; Heiniger, U. Chestnut blight in Europe: Diversity of Cryphonectria parasitica, hypovirulence and biocontrol. For. Snow Landsc. Res. 2001, 76, 361–367. [Google Scholar]
- Gimenez, E.; Salinas, M.; Manzano-Agugliaro, F. Worldwide research on plant defense against biotic stresses as improvement for sustainable agriculture. Sustainability 2018, 10, 391. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defence mechanisms in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Moloi, J.M.; van der Merwe, R. Drought tolerance responses in vegetable-type soybean involve a network of biochemical mechanisms at flowering and pod-filling stages. Plants 2021, 10, 1502. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. ARS 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qamar, A.; Mysore, K.S.; Senthil-Kumar, M. Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens. Front. Plant Sci. 2015, 6, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szőke, L.; Kovács, G.E.; Radócz, L.; Takácsné Hájos, M.; Kovács, B.; Tóth, B. The physiological changes of sweet corn infected by corn smut (Ustilago maydis (DC.) Corda) in greenhouse experiment, NRI Book of Abstracts. Proccedings of the Plant Protection Conference 61: Scientific Session of the Institute of Plant Protection, Poznan, Poland, 10–12 February 2021; p. 117. [Google Scholar]
- Lawson, S.S.; Ebrahimi, A.; McKenna, J.R. Differing responses to Cryphonectria parasitica at two Indiana locations. Forests 2021, 12, 794. [Google Scholar] [CrossRef]
- Biraghi, A. Caratteri di resistenza in “Castanea sativa” nei confronti di “Endothia parasitica”. Boll. Stn. Patol. Veg. 1951, 7, 161–171. [Google Scholar]
- Grente, J. Les formes hypovirulentes d’Endothia parasitica et les espoir de lutte contre le chancre du châtaignier. Acad. Agric. Fr. 1965, 51, 1033–1037. [Google Scholar]
- Krstin, L.; Katani, Z.; Ježi, M.; Poljak, I.; Nuskern, L.; Idžojti, M. Biological control of chestnut blight in Croatia: An interaction between host sweet chestnut, its pathogen Cryphonectria parasitica and the biocontrol agent Cryphonectria hypovirus 1. Pest. Manag. Sci. 2016, 73, 582–589. [Google Scholar] [CrossRef]
- Morris, T.J.; Dodds, J.A. Isolation and analyses of double-stranded RNA from virus-infected plant and fungal tissue. Phytopathology 1979, 87, 1026–1033. [Google Scholar]
- Zamora, B.P.; Martín, A.B.; Rigling, D.; Diez, J.J. Diversity of Cryphonectria parasitica in western Spain and identification of hypovirus-infected isolates. Forest Pathol. 2012, 42, 412–419. [Google Scholar] [CrossRef]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Kovács, G.E.; Radócz, L.A. Cryphonectria parasitica (Murr.) Barr kórokozó gomba fejlődési hőoptimumának meghatározása burgonya-dextróz agar táptalajon a hipovirulens törzsekkel végzendő biológiai védekezés optimalizálására. J. Agric. Sci. 2016, 71, 29–32. [Google Scholar]
- Tóth, B.; Juhász, C.; Labuschagne, M.; Moloi, M.J. The influence of soil acidity on the physiological responses of two bread wheat cultivars. Plants 2020, 9, 1472. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of salinity stress on growth and metabolomic prolfiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef]
- Hassan, M.J.; Raza, M.A.; Rehman, S.U.; Ansar, M.; Gitari, H.; Khan, I.; Wajid, M.; Ahmed, M.; Shah, G.A.; Peng, Y.; et al. Effect of cadmium toxicity on growth, oxidative damage, antioxidant defense system and cadmium accumulation in two sorghum cultivars. Plants 2020, 9, 1575. [Google Scholar] [CrossRef]
- Szőke, L.; Makoena, J.M.; Kovács, G.; Biro, G.; Radócz, L.; Takácsné, H.M.; Kovács, B.; Rácz, D.; Danter, M.; Tóth, B. The application of phytohormones as biostimulants in corn smut infected Hungarian sweet and fodder corn hybrids. Plants 2021, 10, 1822. [Google Scholar] [CrossRef]
- Sobiczewski, P.; Iakimova, E.; Mikiciński, A.; Wêgrzynowicz-Lesiak, E.; Dyki, B. Necrotrophic behaviour of Erwinia amylovora in apple and tobacco leaf tissue. Plant Pathol. 2017, 66, 842–855. [Google Scholar] [CrossRef]
- Pathi, K.M.; Rink, P.; Budhagatapalli, N.; Betz, R.; Saado, I.; Hiekel, S.; Becker, M.; Djamei, A.; Kumlehn, J. Engineering smut resistance in maize by site-directed mutagenesis of LIPOXYGENASE 3. Front. Plant Sci. 2020, 11, 543895. [Google Scholar] [CrossRef] [PubMed]
- Kumudini, B.S.; Patil, S.V. Antioxidant-mediated defense in triggering resistance against biotic stress in plants. Biocontrol Agents and Secondary Metabolites. Appl. Plant Growth Protec. 2021, 12, 383–399. [Google Scholar] [CrossRef]
- Nuskern, L.; Tkalec, M.; Jeric, M.; Katanic, Z.; Krstin, L.; Curkovic-Perica, M. Cryphonetria hypoviurs 1-induced changes of stress enzyme activity in transfected phytopathogenic fungus Cryphonetria parasitica. Microb. Ecol. 2017, 74, 302–3011. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Kaur, A.; Chopra, C.S. Gluten-free products for celiac susceptible people. Front. Nutr. 2018, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Szőke, L.; Kovács, G.E.; Radócz, L.; Hájos, M.T.; Kovács, B.; Tóth, B. Investigation of Ustilago maydis infection on some physiological parameters and phenotypic traits of maize. IJJIAAR 2020, 4, 396–406. [Google Scholar] [CrossRef]
- Frommer, D.; Radócz, L.; Veres, S. Relatív klorofill tartalom változása golyvás üszöggel fertőzött kukorica hibrideknél. Acta Agrar. Debr. 2017, 72, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Horst, R.J.; Engelsdorf, T.; Sonnewald, U.; Voll, L.M. Infection of maize leaves with Ustilago maydis prevents establishment of C4 photosynthesis. J. Plant Physiol. 2008, 165, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Lobato, A.K.S.; Goncalves-Vidigal, M.C.; Filho, P.S.V.; Andrade, C.A.B.; Kvitschal, M.V.; Bonato, C.M. Relationship between leaf pigments and photosynthesis in common bean plants infected by anthracnose. N. Z. J. Crop. Hortic. Sci. 2010, 38, 29–37. [Google Scholar] [CrossRef]
- Scholes, J.D.; Rolfe, S.A. Photosynthesis in localised regions of oat leaves infected with crown rust (Puccinia coronata): Quantitative imaging of chlorophyll fluorescence. Planta 1996, 199, 573–582. [Google Scholar] [CrossRef]
- Repka, V. Chlorophyll-deficient mutant in oak (Quercus petraea L.) displays an accelerated hypersensitive-like cell death and an enhanced resistance to powdery mildew disease. Photosynthetica 2002, 40, 183–193. [Google Scholar] [CrossRef]
- Savaci, G.N. Variation in chemical compounds of chestnut leaves infected by blight disease. In Proceedings of the International Ecology 2018 Syposium, Kastamonu, Turkey, 19–23 June 2018; Canbulat, S., Gültepe, N., Türkyilmaz, A., Eds.; Kastamonu University: Kastamonu, Turkey, 2018; p. 681, ISBN 9786054697175. [Google Scholar]
- Zarattini, M.; Forlani, G. Toward unveiling the mechanisms for transcriptional regulation of proline biosynthesis in the plant cell response to biotic and abiotic stress conditions. Front. Plant Sci. 2017, 8, 927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radócz, L., Jr. Tölgy (Quercus) Fajok Fogékonyságának Összehasonlító Vizsgálata és „Immunizálása” a Cryphonectria parasitica (Murr. Barr) Gombával Szemben. Master’ s Thesis, Institute of Plant Protection, Debrecen University, Debrecen, Hungary, 2020. [Google Scholar]
- Országos Meteorológiai Szolgálat (National Meteorogical Service Hungary). Available online: https://www.met.hu/eghajlat/magyarorszag_eghajlata/altalanos_eghajlati_jellemzes/csapadek/ (accessed on 20 July 2021).
- Moran, R.; Porath, D. Chlorophyll determination in intact tissues using N, N-dimethylformamide. Plant Physiol. 1980, 65, 478–479. [Google Scholar] [CrossRef] [Green Version]
- Wellburn, R.A. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvent with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fracheboud, Y. Using Chlorophyll Fluorescence to Study Photosynthesis; Institute of Plant Sciences ETH: Zürich, Switzerland, 2006. [Google Scholar]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Zeislin, N.; Ben-Zaken, R. Peroxidases, phenylalanine ammonia-lyase and lignification in peduncles of rose flowers. Plant Physiol. Biochem. 1991, 29, 147–151. [Google Scholar]
- Mishra, N.P.; Mishra, R.K.; Singhal, G.S. Changes in the activities of anti-oxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol. 1993, 102, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Gibon, Y. Protocol: Extraction and Determination of Proline. 2011. Available online: https://www.researchgate.net/publication/211353600_PROTOCOL_Extraction_and_determination_of_proline (accessed on 4 July 2021).
- Hummel, I.; Pantin, F.; Sulpice, R.; Piques, M.; Rolland, G.; Dauzat, M.; Christophe, A.; Pervent, M.; Bouteillé, M.; Stitt, M.; et al. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: An integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol. 2010, 154, 357–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, G.E.; Szőke, L.; Tóth, B.; Kovács, B.; Bojtor, C.; Illés, Á.; Radócz, L., Jr.; Moloi, M.J.; Radócz, L. The Physiological and Biochemical Responses of European Chestnut (Castanea sativa L.) to Blight Fungus (Cryphonectria parasitica (Murill) Barr). Plants 2021, 10, 2136. https://doi.org/10.3390/plants10102136
Kovács GE, Szőke L, Tóth B, Kovács B, Bojtor C, Illés Á, Radócz L Jr., Moloi MJ, Radócz L. The Physiological and Biochemical Responses of European Chestnut (Castanea sativa L.) to Blight Fungus (Cryphonectria parasitica (Murill) Barr). Plants. 2021; 10(10):2136. https://doi.org/10.3390/plants10102136
Chicago/Turabian StyleKovács, Gabriella Enikő, Lóránt Szőke, Brigitta Tóth, Béla Kovács, Csaba Bojtor, Árpád Illés, László Radócz, Jr., Makoena Joyce Moloi, and László Radócz. 2021. "The Physiological and Biochemical Responses of European Chestnut (Castanea sativa L.) to Blight Fungus (Cryphonectria parasitica (Murill) Barr)" Plants 10, no. 10: 2136. https://doi.org/10.3390/plants10102136
APA StyleKovács, G. E., Szőke, L., Tóth, B., Kovács, B., Bojtor, C., Illés, Á., Radócz, L., Jr., Moloi, M. J., & Radócz, L. (2021). The Physiological and Biochemical Responses of European Chestnut (Castanea sativa L.) to Blight Fungus (Cryphonectria parasitica (Murill) Barr). Plants, 10(10), 2136. https://doi.org/10.3390/plants10102136











