Long-Term Giant Hogweed Invasion Contributes to the Structural Changes of Soil Nematofauna
Abstract
:1. Introduction
2. Results
2.1. Soil Properties
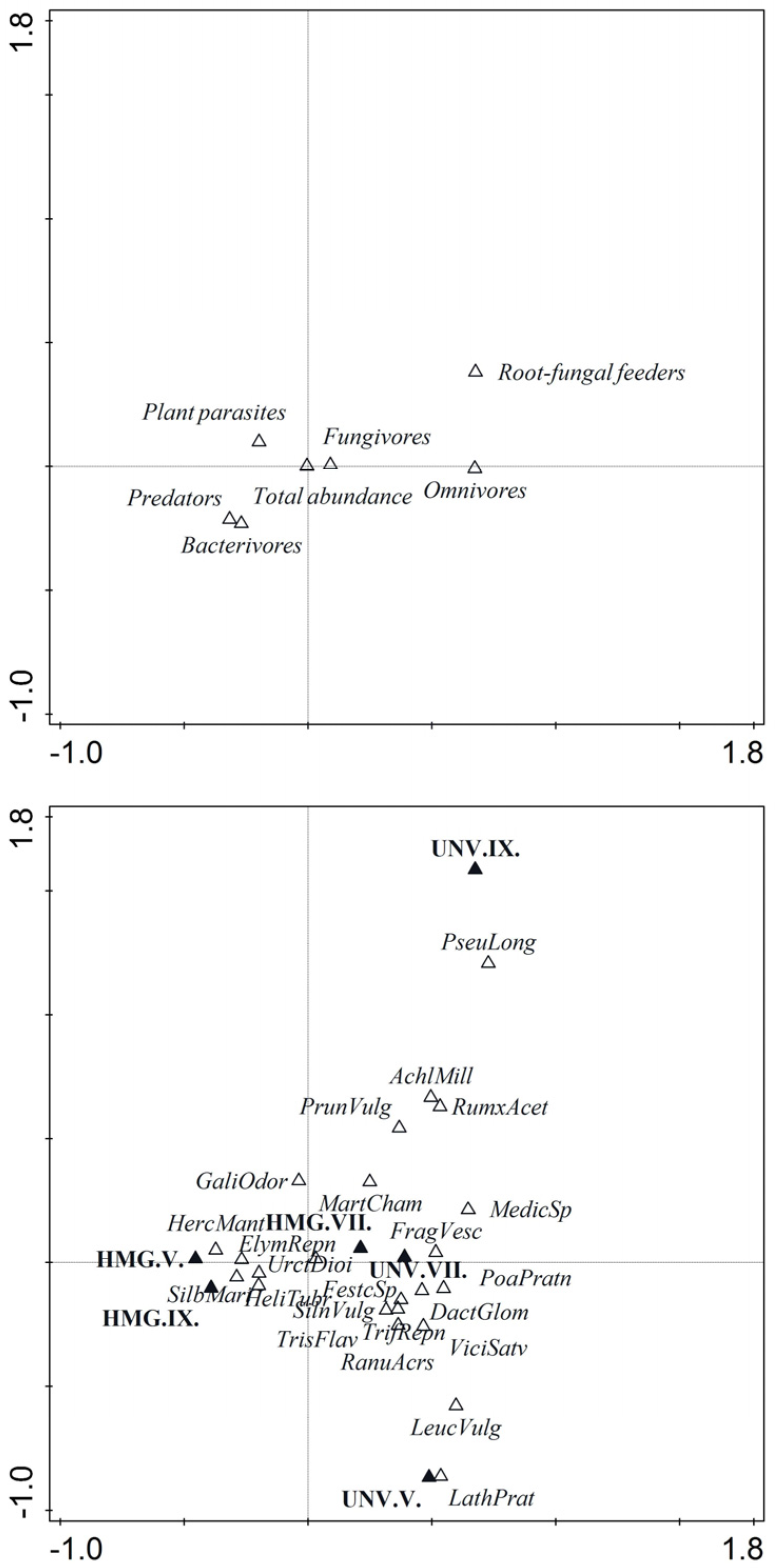
2.2. Plant–Community Analysis
2.3. Nematode Community Analysis
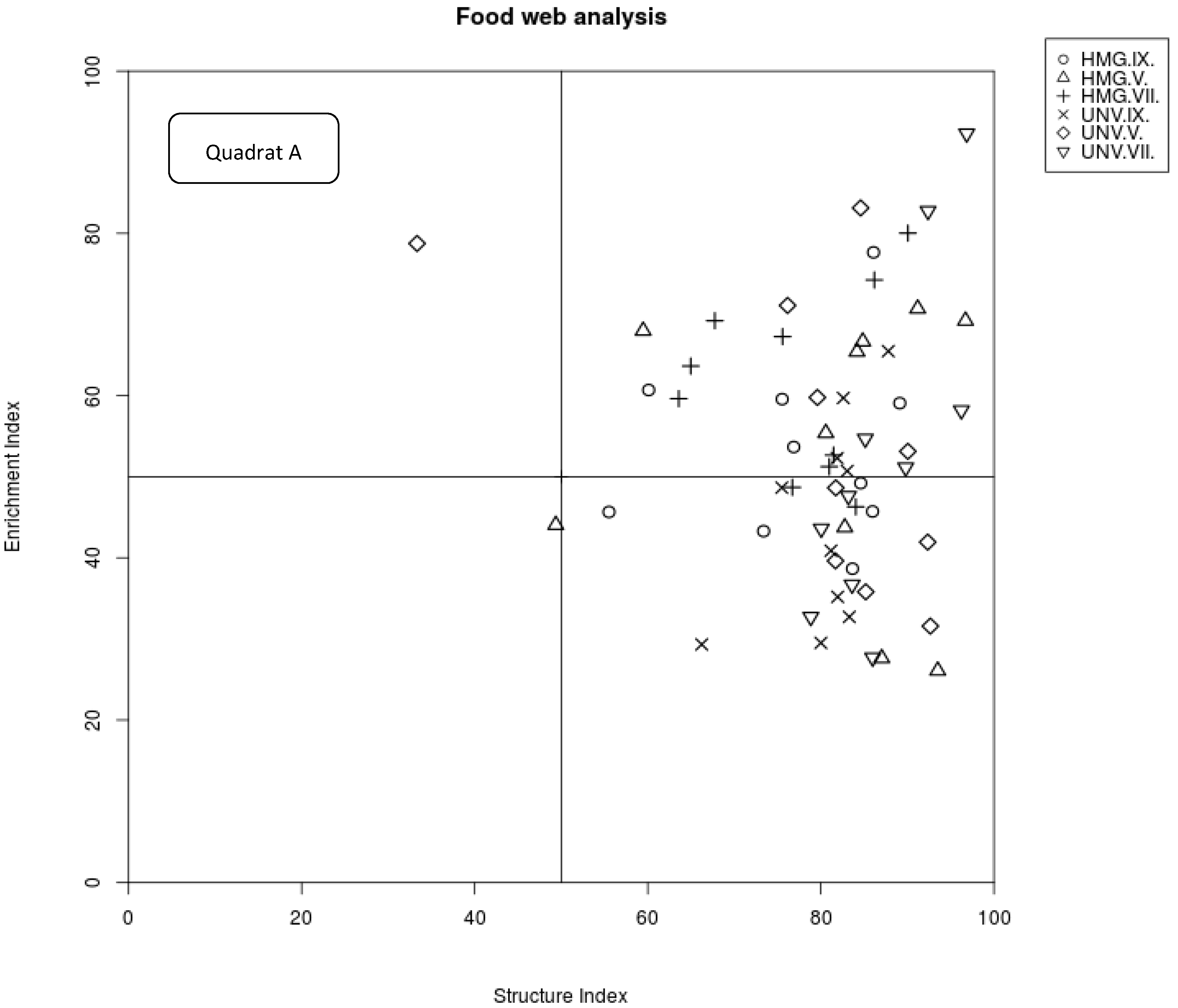
2.4. Analysis of Nematode Food Webs
3. Discussion
4. Materials and Methods
4.1. Study Area and Study Plots
4.2. Soil Sampling, Nematode Isolation, and Identification
4.3. Nematode Community Analysis
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shrestha, B.K.; Dangol, D.R. Impact of Mikania micrantha HBK invasion on diversity and abundance of plant species of Chitwan National Park, Nepal. J. Sci. Tech. 2014, 19, 30–36. [Google Scholar]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Essl, F. No saturation in the accumulation of alien species worldwide. Nat. Comm. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.G. The evolution of weeds. Annu. Rev. System. Evol. 1974, 5, 1–24. [Google Scholar] [CrossRef]
- Anderson, R.C.; Dhillion, S.S.; Kelley, T.M. Aspects of the ecology of an invasive plant, garlic mustard (Alliaria petiolata), in central Illinois. Restor. Ecol. 1996, 4, 181–191. [Google Scholar] [CrossRef]
- Pyšek, P. Clonality and plant invasions: Can a trait make a difference. In The Ecology and Evolution of Clonal Plants; de Kroon, H., Van Groenendael, J.M., Eds.; Backhuys Publisher: Leiden, The Netherlands, 1997; pp. 405–427. [Google Scholar]
- Droste, T.; Flory, S.; Clay, K. Variation for phenotypic plasticity among populations of an invasive exotic grass. Plant Ecol. 2010, 207, 297–306. [Google Scholar] [CrossRef]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.A.; Wrage, K.J.; Reich, P.B. Competition between tree seedlings and herbaceous vegetation: Support for a theory of resource supply and demand. J. Ecol. 1998, 86, 652–661. [Google Scholar] [CrossRef] [Green Version]
- Jahodová, Š.; Fröberg, L.; Pyšek, P.; Geltman, D.; Trybush, S.; Karp, A. Taxonomy, identification, genetic relationships and distribution of large Heracleum species in Europe. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); Pyšek, P., Cock, M.J.W., Nentwig, W., Rawn, H.P., Eds.; CABI: Wallingford UK, 2007; pp. 1–19. [Google Scholar]
- Otte, A.; Eckstein, R.L.; Thiele, J. Heracleum mantegazzianum in its primary distribution range of the Western Greater Caucasus. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); Pyšek, P., Cock, M.J.W., Nentwig, W., Rawn, H.P., Eds.; CABI: Wallingford, UK, 2007; pp. 20–41. [Google Scholar]
- Pyšek, P. Heracleum mantegazzianum in the Czech Republic: The dynamics of spreading from the historical perspective. Folia Geobot. Phytotaxon. 1991, 26, 439–454. [Google Scholar] [CrossRef]
- Pyšek, P. Ecological aspects of invasion by Heracleum mantegazzianum in the Czech Republic. In Ecology and Management of Invasive Riverside Plants; de Waal, L., Child, L.E., Wade, P.M., Brock, J.H., Eds.; Wiley: Hoboken, NJ, USA, 1994; pp. 439–454. [Google Scholar]
- Ochsmann, J. Heracleum mantegazzianum Sommier & Levier (Apiaceae) in Deutchland—Untersuchungen zur Biologie, Verbeitung, Morphologie, und Taxonomie. Feddes Repert. 1996, 107, 557–595. [Google Scholar]
- Darola, J. Heracleum speciosum Weinm., new important casual herb in Slovakia. Biológia 1965, 20, 555–556. [Google Scholar]
- Dobošová, A. Invasive Species, their Extension an Removal in the Competency Area of the Malá Fatra National Park Administration. Zivotn. Prostr. 2014, 48, 119–121. (In Slovak) [Google Scholar]
- Eliáš, P. Invasion and Invasive Plant Species; SEKOS: Trencin, Slovakia, 1997. [Google Scholar]
- Medvecká, J.; Kliment, J.; Majeková, J.; Halada, L.; Zaliberová, M.; Gojdičová, E.; Jarolimek, I. Inventory of the alien flora of Slovakia. Preslia 2012, 84, 257–309. [Google Scholar]
- Pauková, Ž.; Kapralová, R.; Hauptvogl, M. Mapping of occurrence and population dynamics of invasive plant species Heracleum mantegazzianum in the agricultural landscape. J. Cent. Eur. Agric. 2019, 20, 671–677. [Google Scholar] [CrossRef]
- Žlkovanová, K. The problems of alien plant Species in the competency area of Tatra National Park. Zivotn. Prostr. 2014, 48, 100–103. (In Slovak) [Google Scholar]
- Pyšek, P. Comparing the rate of invasion by Heracleum mantegazzianum at continental, regional and local scales. Divers. Distrib. 2008, 14, 355–363. [Google Scholar] [CrossRef]
- Lagey, K.; Duinslaeger, L.; Vanderkelen, A. Burns induced by plants. Burns 1995, 21, 542–543. [Google Scholar] [CrossRef]
- Jandová, K.; Klinerová, T.; Müllerová, J.; Pyšek, P.; Pergl, J.; Cajthaml, T.; Dostál, P. Long-term impact of Heracleum mantegazzianum invasion on soil chemical and biological characteristics. Soil Biol. Biochem. 2014, 68, 270–278. [Google Scholar] [CrossRef]
- Pyšek, P.; Pyšek, A. Invasion by Heracleum mantegazzianum in different habitats in the Czech Republic. J. Veg. Sci. 1995, 6, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Koutika, L.S.; Vanderhoeven, S.; Chapuis-Lardy, L.; Dassonville, N.; Meerts, P. Assessment of changes in soil organic matter following invasion by exotic plant species. Biol. Fertil. Soils 2007, 44, 331–341. [Google Scholar] [CrossRef]
- Zumkier, U. Impacts of the invasive alien Heracleum mantegazzianum on native plant-pollinator interactions. Ph.D. Thesis, der Fakultat fur Biologie, der Universitat Bielefeld, Bielefeld, Germany, December 2011; p. 116. [Google Scholar]
- Hansen, S.O.; Hattendorf, J.; Nentwig, W. Mutualistic relationship beneficial for aphids and ants on giant hogweed (Heracleum mantegazzianum). Community Ecol. 2006, 7, 43–52. [Google Scholar] [CrossRef]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Wasilewska, L. Soil invertebrates as bioindicators with special reference to soil-inhabiting nematodes. Russ. J. Nematol. 1997, 5, 113–126. [Google Scholar]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; De Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Wolfe, B.E.; Klironomos, J.N. Breaking new ground: Soil communities and exotic plant invasion. Bioscience 2004, 55, 477–487. [Google Scholar] [CrossRef]
- Chapin, F.S.; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Díaz, S. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- Renčo, M.; Baležentiené, L. An analysis of soil free-living and plant-parasitic nematode communities in three habitats invaded by Heracleum sosnowskyi in central Lithuania. Biol. Invasions 2015, 17, 1025–1039. [Google Scholar] [CrossRef]
- Renčo, M.; Kornobis, F.W.; Domaradzki, K.; Jakubska-Busse, A.; Jurová, J.; Homolová, Z. How does an invasive Heracleum sosnowskyi affect soil nematode communities in natural conditions? Nematology 2019, 21, 71–89. [Google Scholar] [CrossRef] [Green Version]
- Čerevková, A.; Ivashchenko, K.; Miklisová, D.; Ananyeva, N.; Renčo, M. Influence of invasion by Sosnowsky’s hogweed on nematode communities and microbial activity in forest and grassland ecosystems. Glob. Ecol. Conserv. 2020, 21, e00851. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Raaijmakers, C.E.; Van Ruijven, J.; Berendse, F.; Van Der Putten, W.H. Plant species identity and diversity effects on different trophic levels of nematodes in the soil food web. Oikos 2004, 106, 576–586. [Google Scholar] [CrossRef]
- Kostenko, O.; Duyts, H.; Grootemaat, S.; De Deyn, G.B.; Bezemer, T.M. Plant diversity and identity effects on predatory nematodes and their prey. Ecol. Evol. 2015, 5, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, N.; Reich, P.B. Above-and below-ground plant inputs both fuel soil food webs. Soil Biol. Biochem. 2012, 45, 156–160. [Google Scholar] [CrossRef]
- Cesarz, S.; Ciobanu, M.; Wright, A.J.; Ebeling, A.; Vogel, A.; Weisser, W.W.; Eisenhauer, N. Plant species richness sustains higher trophic levels of soil nematode communities after consecutive environmental perturbations. Oecologia 2017, 184, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Viketoft, M.; Bengtsson, J.; Sohlenius, B.; Berg, M.P.; Petchey, O.; Palmborg, C.; Huss-Danell, K. Long-term effects of plant diversity and composition on soil nematode communities in model grasslands. Ecology 2009, 90, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Cesarz, S.; Liu, T.; Roscher, C.; Eisenhauer, N. Effects of plant species diversity on nematode community composition and diversity in a long-term biodiversity experiment. Oecologia 2021, 1–15. [Google Scholar] [CrossRef]
- Seier, M.K.; Wittenberg, R.; Ellison, C.A.; Djeddour, D.H.; Evans, H.C. Surveys for natural enemies of giant hogweed (Heracleum mantegazzianum) in the Caucasus Region and assessment for their classical biological control potential in Europe. In XI International Symposium on Biological Control of Weeds; SCIRO Entomology: Canberra, Australia, 2004; p. 149. [Google Scholar]
- Reznik, S.Y.; Dolgovskaya, M.Y.; Zaitzev, V.F.; Davidian, G.E.; Nentwig, W. On the possibility of the use of the weevil Nastus fausti Reitter (Coleoptera, Curculionidae, Entiminae, Nastini) for biological control of invasive species of giant hogweeds (Heracleum spp.). Ėntomologicheskoe Obozr. 2008, 87, 489–502. [Google Scholar]
- Renčo, M.; Čerevková, A.; Homolová, Z. Nematode communities indicate the negative impact of Reynoutria japonica invasion on soil fauna in ruderal habitats of Tatra National park in Slovakia. Glob. Ecol. Conserv. 2021, 26, e01470. [Google Scholar] [CrossRef]
- Thiele, J.; Otte, A. Analysis of habitats and communities invaded by Heracleum mantegazzianum Somm. et Lev.(Giant Hogweed) in Germany. Phytocoenologia 2006, 36, 281. [Google Scholar] [CrossRef] [Green Version]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy is pervasive in invasive plants. Biol. Inv. 2021, 23, 367–371. [Google Scholar] [CrossRef]
- Grime, J.P.; Hodgson, J.G.; Hunt, R. Comparative Plant Ecology: A Functional Approach to Common British Species; Springer: Zug, Switzerland, 2014; p. 720. [Google Scholar]
- Lavoie, C. The impact of invasive knotweed species (Reynoutria spp.) on the environment: Review and research perspectives. Biol. Invasions 2017, 19, 2319–2337. [Google Scholar] [CrossRef]
- Dassonville, N.; Vanderhoeven, S.; Vanparys, V.; Hayez, M.; Gruber, W.; Meerts, P. Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 2008, 157, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz, A.M.; Banaś, A.; Stanek, M.; Woch, M.W.; Zubek, S. Large differences in biomass quantity and quality between invasive Reynoutria japonica and resident vegetation are not reflected in topsoil physicochemical properties. Geoderma 2020, 368, 114307. [Google Scholar] [CrossRef]
- Dostál, P.; Müllerová, J.; Pyšek, P.; Pergl, J.; Klinerová, T. The impact of an invasive plant changes over time. Ecol. Lett. 2013, 16, 1277–1284. [Google Scholar] [CrossRef]
- Belnap, J.; Phillips, S.L.; Sherrod, S.K.; Moldenke, A. Soil biota can change after exotic plant invasion: Does this affect ecosystem processes? Ecology 2005, 86, 3007–3017. [Google Scholar] [CrossRef]
- Zhang, P.; Neher, D.A.; Li, B.; Wu, J. The impacts of above-and belowground plant input on soil microbiota: Invasive Spartina alterniflora versus native Phragmites australis. Ecosystems 2018, 21, 469–481. [Google Scholar] [CrossRef]
- Čerevková, A.; Miklisová, D.; Bobuľská, L.; Renčo, M. Impact of the invasive plant Solidago gigantea on soil nematodes in a semi-natural grassland and a temperate broadleaved mixed forest. J. Helminth. 2019, 94, e51. [Google Scholar] [CrossRef]
- Jurová, J.; Renčo, M.; Gömöryová, E.; Čerevková, A. Effects of the invasive common milkweed (Asclepias syriaca) on nematode communities in natural grasslands. Nematology 2020, 22, 423–438. [Google Scholar] [CrossRef]
- Liang, W.; Li, F.; Li, Q.; Zhang, W. Temporal dynamics of soil nematode community structure under invasive Ambrosia trifida and native Chenopodium serotinum. Helminthologia 2007, 44, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere fauna: The functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Rønn, R.; Vestergard, M.; Ekelund, F. Interactions between bacteria, protozoa and nematodes in soil. Acta Protozool. 2012, 51, 223–235. [Google Scholar]
- Mincheva, T.; Barni, E.; Varese, G.C.; Brusa, G.; Cerabolini, B.; Siniscalco, C. Litter quality, decomposition rates and saprotrophic mycoflora in Fallopia japonica (Houtt.) Ronse Decraene and in adjacent native grassland vegetation. Acta Oecologica 2014, 54, 29–35. [Google Scholar] [CrossRef]
- Hattendorf, J.; Hansen, S.O.; Nentwig, W. Defense system of Heracleum mantegazzianum. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); Pyšek, P., Cock, M.J.W., Nentwig, W., Rawn, H.P., Eds.; CABI: Wallingford, UK, 2007; pp. 209–225. [Google Scholar]
- Abadollahi, A. Plant essential oils from Apiaceae family as alternatives to conventional insecticides. Ekol. Balk. 2013, 5, 149–172. [Google Scholar]
- Čerevková, A.; Bobuľská, L.; Miklisová, D.; Renčo, M. A case study of soil food web components affected by Fallopia japonica (Polygonaceae) in three natural habitats in Central Europe. J. Nematol. 2019, 51, e2019–e2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkman, J.J.; Doing, H.; Segal, S. Kritische bemerkungen und vorschläge zur quantitativen vegetationsanalyse. Acta Bot. Neerl. 1964, 13, 394–419. [Google Scholar] [CrossRef]
- Pen-Mouratov, S.; He, X.; Steinberger, Y. Spatial distribution and trophic diversity of nematode populations under Acacia raddiana along a temperature gradient in the Negev Desert ecosystem. J. Arid Environ. 2004, 56, 339–355. [Google Scholar] [CrossRef]
- Čerevková, A.; Renčo, M.; Cagáň, L. Short-term effects of forest disturbances on soil nematode communities in European mountain spruce forests. J. Helminth. 2013, 87, 376–385. [Google Scholar] [CrossRef]
- Renčo, M.; Čerevková, A.; Gömöryová, E. Soil nematode fauna and microbial characteristics in an early-successional forest ecosystem. Forests 2019, 10, 888. [Google Scholar] [CrossRef] [Green Version]
- Neher, D.A.; Peck, S.L.; Rawlings, J.O.; Campbell, C.L. Measures of nematode community structure and sources of variability among and within agricultural fields. Plant Soil 1995, 170, 167–181. [Google Scholar] [CrossRef]
- Neher, D.A.; Wu, J.; Barbercheck, M.E.; Anas, O. Ecosystem type affects interpretation of soil nematode community measures. Appl. Soil Ecol. 2005, 30, 47–64. [Google Scholar] [CrossRef]
- Cobb, N.A. Estimating the Nematode Population of the Soil. Agricultural Technology Circular 1; Bureau of Plant Industry: Washington DC, USA, 1918. [Google Scholar]
- Van Bezooijen, J. Methods and Techniques for Nematology; Wageningen University: Wageningen, The Netherlands, 2006. [Google Scholar]
- Renčo, M.; Čermák, V.; Čerevková, A. Composition of soil nematode communities in native birch forests in Central Europe. Nematology 2012, 14, 15–25. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Yeates, G.W.; Bongers, T.D.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—outline for soil ecologists. J. Nematol. 1993, 25, 315–335. [Google Scholar] [PubMed]
- Yeates, G.W. Modification and qualification of the nematode maturity index. Pedobiologia 1994, 38, 97–101. [Google Scholar]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Ter Braak, C.J.; Schaffers, A.P. Co-correspondence analysis: A new ordination method to relate two community compositions. Ecology 2004, 85, 834–846. [Google Scholar] [CrossRef] [Green Version]
- Neher, D.A.; Williams, K.M.; Lovell, S.T. Environmental indicators reflective of road design in a forested landscape. Ecosphere 2017, 8, e01734. [Google Scholar] [CrossRef]
- Ter Braak, C.J.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G. NINJA: An automated calculation system for nematode-based biological monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
| Spring (May) | Summer (July) | Autumn (September) | ||||
|---|---|---|---|---|---|---|
| HMG | UNV | HMG | UNV | HMG | UNV | |
| SM | 16.15 ± 2.88 | 12.82 ± 1.58 | 25.45 ± 2.11 | 26.78 ± 2.61 | 19.69 ± 1.28 | 18.93 ± 2.04 |
| pH (KCl) | 7.20 ± 0.10 * | 6.23 ± 0.28 | 7.02 ± 0.12 * | 6.11 ± 0.12 | 6.75 ± 0.24 * | 6.00 ± 0.12 |
| Ntot | 0.22 ± 0.01 * | 0.31 ± 0.02 | 0.23 ± 0.01 | 0.22 ± 0.02 | 0.24 ± 0.03 | 0.25 ± 0.03 |
| Cox | 1.81 ± 0.10 * | 2.38 ± 0.11 | 2.00 ± 0.07 * | 2.45 ± 0.18 | 1.92 ± 0.01 | 1.78 ± 0.20 |
| C/N | 7.90 ± 0.25 | 7.89 ± 0.36 | 8.25 ± 0.30 | 9.76 ± 0.78 | 8.19 ± 0.53 | 7.49 ± 0.69 |
| Plants | Invaded | Uninvaded Control | ||||
|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Spring | Summer | Autumn | |
| Achillea milleofolium | - | - | - | 1.5 | 1 | 2.6 |
| Dactylis glomerata | - | - | - | 18.1 | 10.9 | 6.5 |
| Elymus repens | 1.7 | 1.1 | 5.4 | - | - | - |
| Festuca sp. | - | - | - | 21.5 | 26 | 17 |
| Fragaria vesca | - | - | - | 7.8 | 5.5 | 2.5 |
| Galium odoratum | 8.9 | 7.5 | 4.7 | - | 0.5 | 2.2 |
| Heliantus tuberosus | 3.8 | 16 | 22.3 | - | - | - |
| Heracleum mantegazzianum | 83 | 80.1 | 53.9 | - | - | - |
| Lathyrum pratensis | - | - | - | 1.7 | 0.5 | 0.3 |
| Leucanthemum vulgare | - | - | - | 0.9 | 0.1 | - |
| Marticaria chamomilla | - | - | - | 0.4 | 1.2 | 0.5 |
| Medicago sp. | - | - | - | 5.2 | 1.1 | 3.9 |
| Poa pratensis | - | - | - | 2.4 | 1.6 | 2.3 |
| Prunella vulgaris | - | - | - | 0.3 | 0 | 0.2 |
| Pseudolysimachion longifolium | - | - | - | - | 0.3 | 0.5 |
| Pulmonaria officinalis | 0.2 | - | - | 0.3 | - | - |
| Ranunculus acris | - | - | - | 1.4 | 1.7 | - |
| Rumex acetosella | 0.1 | - | 0.1 | 1.4 | 2.5 | 2.4 |
| Silene vulgaris | - | - | - | 4.6 | 4 | 0.5 |
| Silybum marianum | 5.5 | 6 | 12.4 | 1.9 | 0.4 | 0.9 |
| Trifolium repens | - | - | - | 7.6 | 8.2 | 5.1 |
| Trisetum flavescens | - | - | - | 10.5 | 14 | 8.2 |
| Urctica dioica | 13.1 | 20.8 | 18.7 | 0.3 | 0.9 | 0.4 |
| Vicia sativa | - | - | - | 2.2 | 1.2 | 0.6 |
| Spring | Summer | Autumn | |||||
|---|---|---|---|---|---|---|---|
| c-p | HMG | UNV | HMG | UNV | HMG | UNV | |
| Bacterivores | |||||||
| Acrobeles ciliatus | 2 | - | 0.8 | 0.6 | 0.5 | - | - |
| Acrobeloides nanus | 2 | 6.0 | 2.7 | 6.9 | 8.1 | 8.7 | 10.4 |
| Alaimus parvus | 4 | 2.3 | 0.2 | 6.7 | 1.7 | 4.5 | 0.4 |
| Alaimus primitivus | 4 | 33.8 | 4.0 | 30.1 | 7.7 | 24.4 | 6.7 |
| Alaimus robustus | 4 | 7.4 | 1.7 | 8.5 | - | 3.2 | 0.5 |
| Amphidelus coronatus | 4 | 2.1 | - | - | - | 0.3 | - |
| Aulolaimus oxycephalus | 3 | - | 0.6 | - | 0.6 | - | - |
| Cephalobus parvus | 2 | 0.7 | - | - | - | 2.0 | 1.5 |
| Cephalobus persegnis | 2 | 5.2 | 3.3 | 21.6 | 4.9 | 16.9 | 1.7 |
| Cervidellus vexilliger | 2 | - | 0.5 | 0.6 | 1.2 | 0.2 | 0.2 |
| Drilocephalobus coomansi | 2 | - | - | - | - | - | - |
| Eucephalobus mucronatus | 2 | 14.2 | 8.6 | 13.7 | 1.5 | 9.0 | 5.4 |
| Eucephalobus oxyuroides | 2 | 2.6 | 0.3 | 1.0 | 0.8 | 2.7 | 1.3 |
| Eucephalobus striatus | 2 | 18.6 | 7.1 | 31.7 | 7.3 | 13.1 | 7.5 |
| Heterocephalobus elongatus | 2 | - | - | - | 0.2 | - | 0.4 |
| Heterocephalobus eurystoma | 2 | 1.6 | - | 1.0 | 1.4 | 3.5 | 4.2 |
| Chiloplacus propinquus | 2 | 0.5 | - | 2.3 | 2.1 | 3.8 | 2.5 |
| Mesorhabditis labiata | 1 | 4.0 | - | 0.9 | 0.8 | 3.2 | 1.8 |
| Panagrolaimus ridigus | 1 | 2.8 | - | 11.6 | 0.8 | 1.5 | 0.3 |
| Paramphidelus dolichurus | 4 | 4.0 | - | 0.1 | - | 1.0 | - |
| Plectus longicaudatus | 2 | 0.6 | 1.3 | - | 1.3 | - | - |
| Plectus opisthocirculus | 2 | - | - | - | - | 0.3 | 0.4 |
| Plectus parietinus | 2 | 0.6 | 0.5 | - | - | 0.7 | 0.1 |
| Plectus parvus | 2 | - | - | 2.6 | 14.4 | - | - |
| Prismatolaimus intermedius | 3 | 1.4 | 0.2 | - | 1.6 | 0.7 | 0.4 |
| Protorhabditis filiformis | 1 | - | - | - | - | 0.6 | - |
| Rhabditis spp. juvs. | 1 | 16.2 | 8.6 | 27.6 | 7.9 | 18.1 | 3.6 |
| Fungivores | |||||||
| Aphelenchoides parietinus | 2 | 18.3 | 1.8 | 4.6 | 0.4 | 5.9 | 0.9 |
| Aphelenchus avenae | 2 | 8.0 | 3.8 | 11.7 | 1.9 | 12.0 | 2.5 |
| Diptherophora communis | 3 | 23.1 | 10.6 | 14.1 | 16.9 | 18.8 | 16.3 |
| Ditylenchus intermedius | 2 | 3.2 | 3.6 | 3.4 | 3.6 | 5.9 | 2.5 |
| Ditylenchus longimetricalis | 2 | - | - | 1.2 | - | - | - |
| Tylencholaimellus striatus | 4 | 0.6 | 1.7 | - | 0.6 | - | 1.5 |
| Tylencholaimus minimus | 4 | 1.4 | 2.6 | - | 8.2 | - | - |
| Tylencholaimus stecki | 4 | - | 1.7 | 0.8 | 11.8 | 3.4 | 4.3 |
| Plant parasites | |||||||
| Bitylenchus dubius | 3 | 2.1 | 0.6 | - | - | 2.8 | - |
| Criconemoides informis | 3 | 4.5 | 11.9 | 2.0 | 18.1 | - | - |
| Geocenamus brevidens | 3 | 16.0 | 5.8 | 13.6 | 1.6 | 6.5 | 8.2 |
| Geocenamus microdorus | 3 | 7.3 | 0.7 | 10.5 | 4.0 | 15.7 | 2.7 |
| Geocenamus nanus | 3 | 5.5 | - | 5.6 | 0.6 | 2.3 | - |
| Gracilacus straeleni | 2 | - | - | - | 0.4 | - | 0.5 |
| Helicotylenchus canadensis | 3 | 2.2 | 10.1 | 3.5 | 1.8 | 4.0 | 4.8 |
| Helicotylenchus digonicus | 3 | 11.5 | 34.8 | 14.0 | 10.4 | 18.5 | 30.8 |
| Longidorus elongatus | 5 | - | 0.4 | - | 1.3 | - | 5.5 |
| Meloidogyne sp. | 3 | 1.2 | 1.2 | 2.0 | 6.0 | 3.0 | 1.4 |
| Mesocriconema curvatum | 3 | - | 0.6 | - | - | - | - |
| Paratylenchus microdorus | 2 | 72.6 | 0.5 | 30.6 | 1.5 | 59.4 | 0.4 |
| Paratylenchus projectus | 2 | 10.3 | 5.3 | 8.8 | 2.8 | 18.8 | 11.5 |
| Pratylenchoides crenicauda | 3 | 3.3 | 0.5 | 1.5 | 1.1 | 1.9 | 2.5 |
| Pratylenchus neglectus | 3 | 2.1 | 2.9 | - | - | - | 3.1 |
| Pratylenchus pratensis | 3 | 1.8 | 4.4 | 8.1 | 4.0 | 3.3 | 1.8 |
| Pratylenchus thornei | 3 | - | 3.7 | 0.8 | 0.8 | 0.3 | 5.7 |
| Xiphinema simile | 5 | - | - | - | 2.5 | - | 0.5 |
| Root-fungal feeders | |||||||
| Boleodorus thylactus | 2 | 2.9 | 5.1 | 8.3 | 0.2 | 2.6 | 2.8 |
| Basiria gracilis | 2 | - | 0.1 | 2.1 | - | - | 0.6 |
| Coslenchus costatus | 2 | 4.4 | 3.6 | 11.2 | 4.4 | 10.5 | 16.5 |
| Filenchus discrepans | 2 | 0.4 | - | 0.5 | 0.8 | 1.7 | 1.4 |
| Filenchus thornei | 2 | 1.1 | 0.6 | - | - | 0.4 | 5.0 |
| Filenchus vulgaris | 2 | 4.3 | 15.3 | 7.4 | 24.6 | 14.6 | 20.3 |
| Malenchus exiguus | 2 | 3.8 | 2.6 | 1.3 | 1.8 | 0.8 | 2.0 |
| Psilenchus hilarulus | 2 | 2.3 | 0.9 | 0.3 | 8.1 | 0.9 | 0.8 |
| Tylenchus elegans | 2 | - | 1.7 | - | 2.0 | - | 3.0 |
| Omnivores | |||||||
| Aporcelaimellus obtusicaudatus | 4 | 13.2 | 10.2 | 9.7 | 19.4 | 3.4 | 6.1 |
| Campydora demonstrans | 4 | 1.1 | 4.1 | - | 0.2 | 2.8 | 1.2 |
| Dorylaimoides mickoletskyi | 4 | - | 1.8 | 0.6 | 1.8 | 0.3 | 1.5 |
| Dorylaimus bryophilus | 4 | 11.9 | 13.3 | 19.4 | 26.4 | 10.0 | 11.2 |
| Dorylaimus microdorus | 4 | 1.4 | - | 0.3 | 1.3 | 0.3 | 0.2 |
| Ecumenicus monohystera | 4 | 0.8 | 2.4 | 4.1 | 0.2 | 2.8 | 0.4 |
| Eudorylaimus acuticauda | 4 | 3.4 | 0.4 | 0.4 | 3.4 | - | 0.7 |
| Eudorylaimus similis | 4 | - | - | 1.2 | 7.2 | 0.3 | 1.2 |
| Mesodorylaimus bastiani | 5 | 0.4 | 2.8 | 3.0 | - | - | - |
| Microdorylaimus parvus | 4 | 7.2 | 2.6 | 6.0 | 3.7 | 4.2 | 2.0 |
| Paraxonchium laetificans | 5 | - | 1.9 | - | 0.2 | - | 0.2 |
| Prodorylaimus brigdamensis | 5 | 0.7 | - | 1.8 | 3.8 | 2.7 | 5.5 |
| Pungentus engadinensis | 4 | - | 1.4 | 0.7 | 2.4 | 0.9 | 1.7 |
| Pungentus silvestris | 4 | - | 10.7 | - | 3.4 | - | - |
| Thonus ettersbersgensis | 4 | 0.4 | 0.7 | 0.3 | 1.4 | 0.2 | 1.7 |
| Predators | |||||||
| Clarkus papillatus | 4 | 2.5 | 1.0 | 0.3 | 2.8 | 0.3 | 1.1 |
| Discolaimoides bulbiferus | 5 | 10.4 | 2.7 | 5.3 | 2.4 | 10.2 | 4.2 |
| Discolaimus major | 5 | 0.8 | 2.2 | 0.8 | 0.6 | - | 0.9 |
| Enchondelus macrodorus | 4 | 1.1 | - | - | - | 0.9 | - |
| Mylonchulus brachyuris | 4 | 7.4 | 2.0 | 5.4 | 2.0 | 2.9 | 1.3 |
| Oxydirus oxycephalus | 5 | 0.2 | 0.8 | - | 0.2 | 1.1 | 0.7 |
| Trypila filicaudata | 3 | - | - | - | 3.4 | - | - |
| Spring (May) | Summer (July) | Autumn (September) | ||||
|---|---|---|---|---|---|---|
| HMG | UNV | HMG | UNV | HMG | UNV | |
| Abundance (100 g/dry soil) | 401.5 ± 174.2 * | 245.5 ± 59.9 | 412 ± 200.2 | 288.9 ± 150.5 | 375.4 ± 100.6 | 250.3 ± 54.2 |
| Nematode species number | 42.2 ± 3.6 | 38.7 ± 4.1 | 36 ± 3.1 * | 45 ± 2.5 | 36 ± 2.4 * | 43 ± 0.8 |
| Bacterivores | 124.6 ± 53.2 * | 39.8 ± 12.8 | 170.6 ± 100.2 * | 61.8 ± 30.7 | 115.8 ± 30.6 * | 52.5 ± 13.2 |
| Fungivores | 55.6 ± 15.9 * | 26.5 ± 10.1 | 34.5 ± 19.2 | 43.6 ± 20.3 | 46.5 ± 20.8 | 33.9 ± 15.4 |
| Omnivores | 42.5 ± 30.6 | 54.1 ± 38.7 | 39.8 ± 40.6 | 80.1 ± 23.6 | 25.4 ± 20.4 | 35.8 ± 10.1 |
| Predators | 20.1 ± 10.9 ** | 2.8 ± 2.0 | 10.9 ± 11.5 | 10.3 ± 6.6 | 13.8 ± 8.4 | 7.9 ± 3.8 |
| Root-fungal feeders | 18.9 ± 16.5 | 29.5 ± 20.9 | 36.8 ± 21.8 | 40.1 ± 30.7 | 30.1 ± 15.6 | 49.7 ± 13.4 |
| Plant parasites | 139.7 ± 55.8 | 89.9 ± 48.7 | 97.7 ± 48.3 | 50.2 ± 35.7 | 135.6 ± 74.5 | 82.7 ± 27.6 |
| Species diversity index | 2.63 ± 0.23 | 2.86 ± 0.20 | 2.83 ± 0.10 | 2.79 ± 2.68 | 2.75 ± 0.32 | 3.03 ± 0.12 |
| Maturity index | 2.95 ± 0.48 | 2.91 ± 0.57 | 2.65 ± 0.17 * | 3.11 ± 0.26 | 2.76 ± 0.03 * | 2.91 ± 0.14 |
| Maturity index (2–5) | 3.15 ± 0.44 | 3.12 ± 0.40 | 2.95 ± 0.28 * | 3.39 ± 0.07 | 2.95 ± 0.25 * | 3.25 ± 0.08 |
| Sum Maturity index | 2.70 ± 0.35 | 2.79 ± 0.42 | 2.65 ± 0.11 * | 3.17 ± 0.21 | 2.62 ± 0.13 | 2.86 ± 0.15 |
| Plant parasitic index | 2.40 ± 0.24 * | 2.75 ± 0.10 | 2.66 ± 0.09 * | 3.04 ± 0.21 | 2.45 ± 0.18 * | 2.89 ± 0.12 |
| Channel index | 30.32 ± 15.18 * | 50.11 ± 11.28 | 18.18 ± 8.33 * | 42.81 ± 18.19 | 35.97 ± 15.45 * | 66.81 ± 17.2 |
| Enrichment index | 53.24 ± 17.26 | 54.35 ± 18.84 | 61.81 ± 11.15 | 52.69 ± 20.37 | 53.31 ± 10.8 | 44.44 ± 12.83 |
| Structure index | 80.96 ± 15.42 | 79.52 ± 17.13 | 77.13 ± 9.15 | 87.21 ± 6.36 | 77.75 ± 11.8 | 80.34 ± 5.82 |
| Total nematode biomass | 0.95 ± 0.84 | 0.85 ± 0.0.72 | 1.37 ± 0.85 | 1.72 ± 0.64 | 0.73 ± 0.34 | 0.75 ± 0.57 |
| Composite footprint | 175.1 ± 127.2 | 145.9 ± 66.8 | 228.3 ± 181.9 | 259.5 ± 261.7 | 140.8 ± 70.7 | 128.7 ± 78.3 |
| Enrichment footprint | 36.2 ± 13.2 * | 19.8 ± 11.6 | 60.2 ± 40.5 * | 19.5 ± 12.8 | 40.7 ± 25.7 * | 10.8 ± 7.8 |
| Structure footprint | 118.9 ± 116.4 | 108.8 ± 39.2 | 142.3 ± 100.5 | 222.1 ± 189.5 | 70.8 ± 64.7 | 94.5 ± 37.8 |
| Herbivore footprint | 13.6 ± 4.8 | 14.7 ± 10.8 | 15.8 ± 9.6 | 11.8 ± 6.5 | 20.7 ± 24.6 | 23.8 ± 15.1 |
| Fungivore footprint | 4.9 ± 2.7 | 5.1 ± 2.3 | 4.9 ± 3.6 | 8.7 ± 5.4 | 7.4 ± 1.7 | 6.7 ± 3.1 |
| Bacterivore footprint | 45.8 ± 39.7 | 18.6 ± 10.8 | 70.9 ± 30.5 ** | 22.5 ± 11.8 | 45.7 ± 21.1 * | 12.1 ± 6.6 |
| Predator footprint | 5.8 ± 3.6 | 3.7 ± 3.8 | 4.4 ± 5.1 | 5.1 ± 5.6 | 4.9 ± 2.1 | 3.6 ± 2.8 |
| Omnivore footprint | 100.3 ± 27.9 | 97.5 ± 50.1 | 130.3 ± 68.7 | 215.1 ± 257.8 | 62.7 ± 43.3 | 74.4 ± 62.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renčo, M.; Jurová, J.; Gömöryová, E.; Čerevková, A. Long-Term Giant Hogweed Invasion Contributes to the Structural Changes of Soil Nematofauna. Plants 2021, 10, 2103. https://doi.org/10.3390/plants10102103
Renčo M, Jurová J, Gömöryová E, Čerevková A. Long-Term Giant Hogweed Invasion Contributes to the Structural Changes of Soil Nematofauna. Plants. 2021; 10(10):2103. https://doi.org/10.3390/plants10102103
Chicago/Turabian StyleRenčo, Marek, Jana Jurová, Erika Gömöryová, and Andrea Čerevková. 2021. "Long-Term Giant Hogweed Invasion Contributes to the Structural Changes of Soil Nematofauna" Plants 10, no. 10: 2103. https://doi.org/10.3390/plants10102103
APA StyleRenčo, M., Jurová, J., Gömöryová, E., & Čerevková, A. (2021). Long-Term Giant Hogweed Invasion Contributes to the Structural Changes of Soil Nematofauna. Plants, 10(10), 2103. https://doi.org/10.3390/plants10102103






