Arsenic Toxicity-Induced Physiological and Metabolic Changes in the Shoots of Pteris cretica and Spinacia oleracea
Abstract
:1. Introduction
2. Results
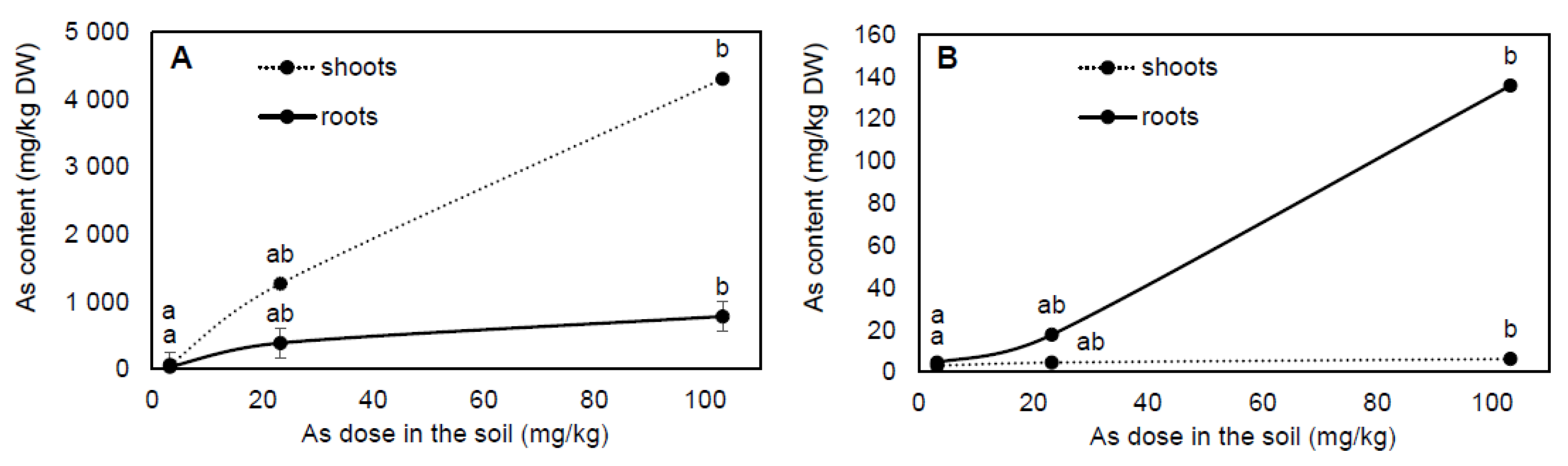
2.1. Arsenic Accumulation and Translocation
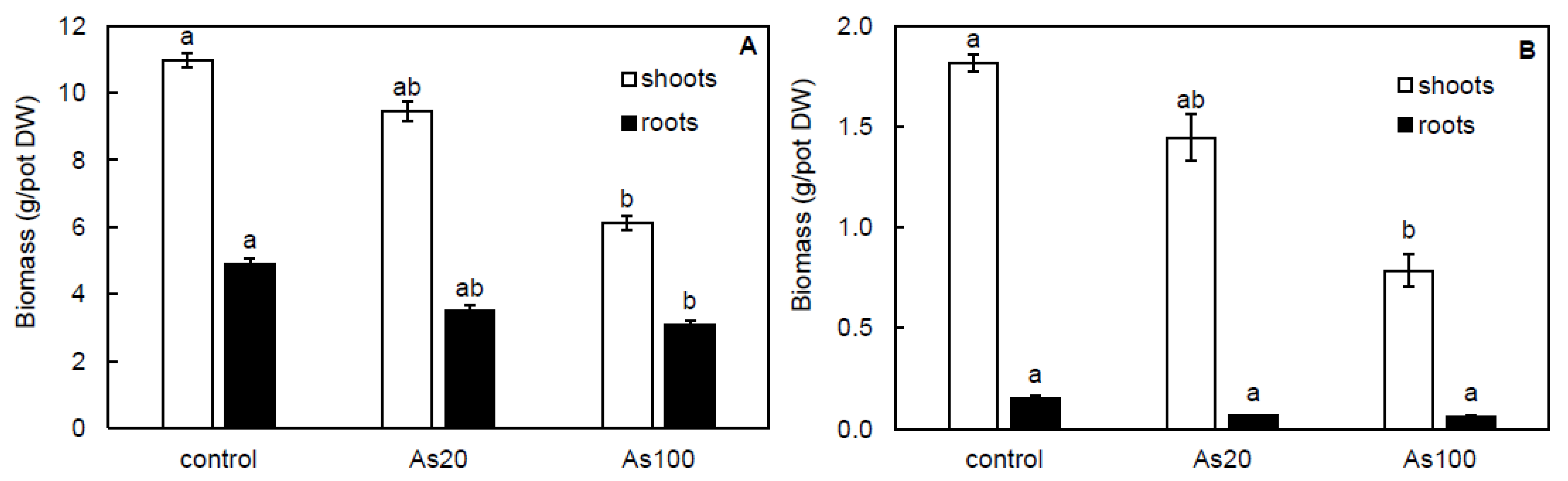
2.2. Effect of Arsenic on Plant Growth
2.3. Effect of Arsenic on Nutrient Content
2.4. Effect of Arsenic on Photosynthetic Pigments
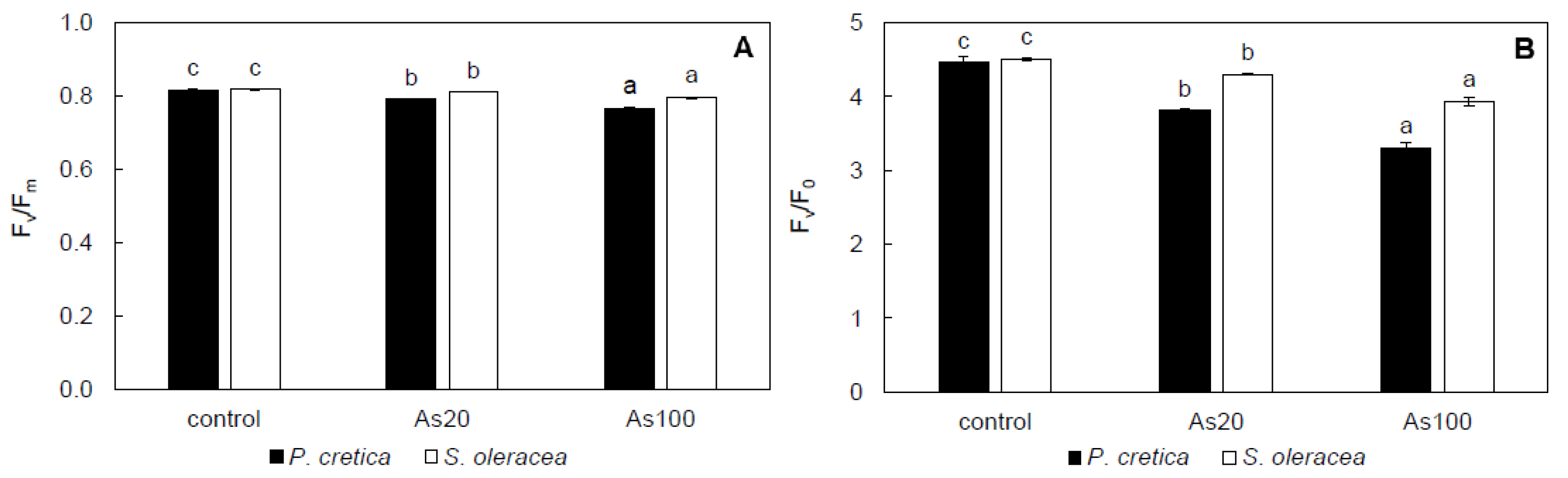
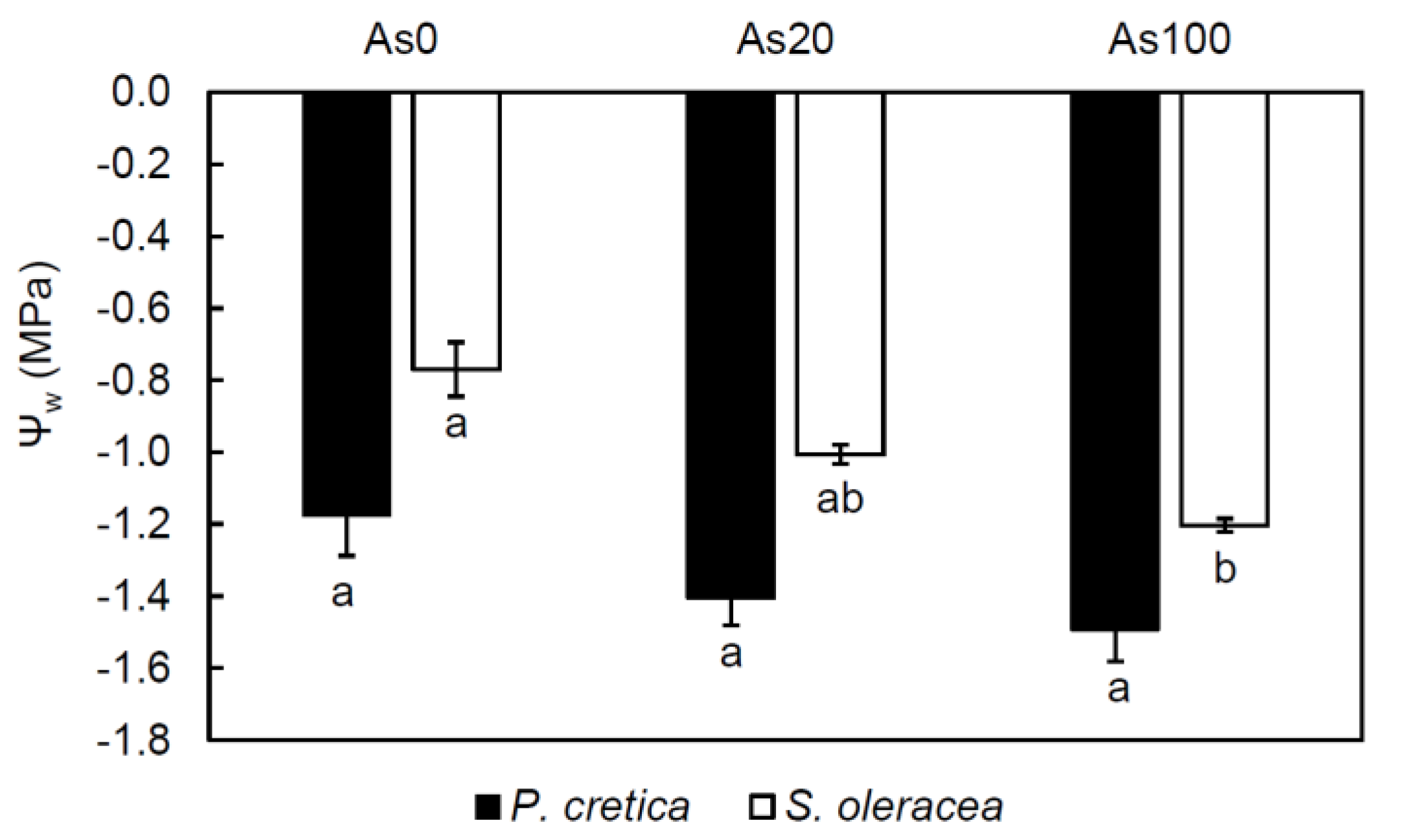
2.5. Effect of Arsenic on Leaf Gas-Exchange Parameters, Chlorophyll Fluorescence, and Water Potential
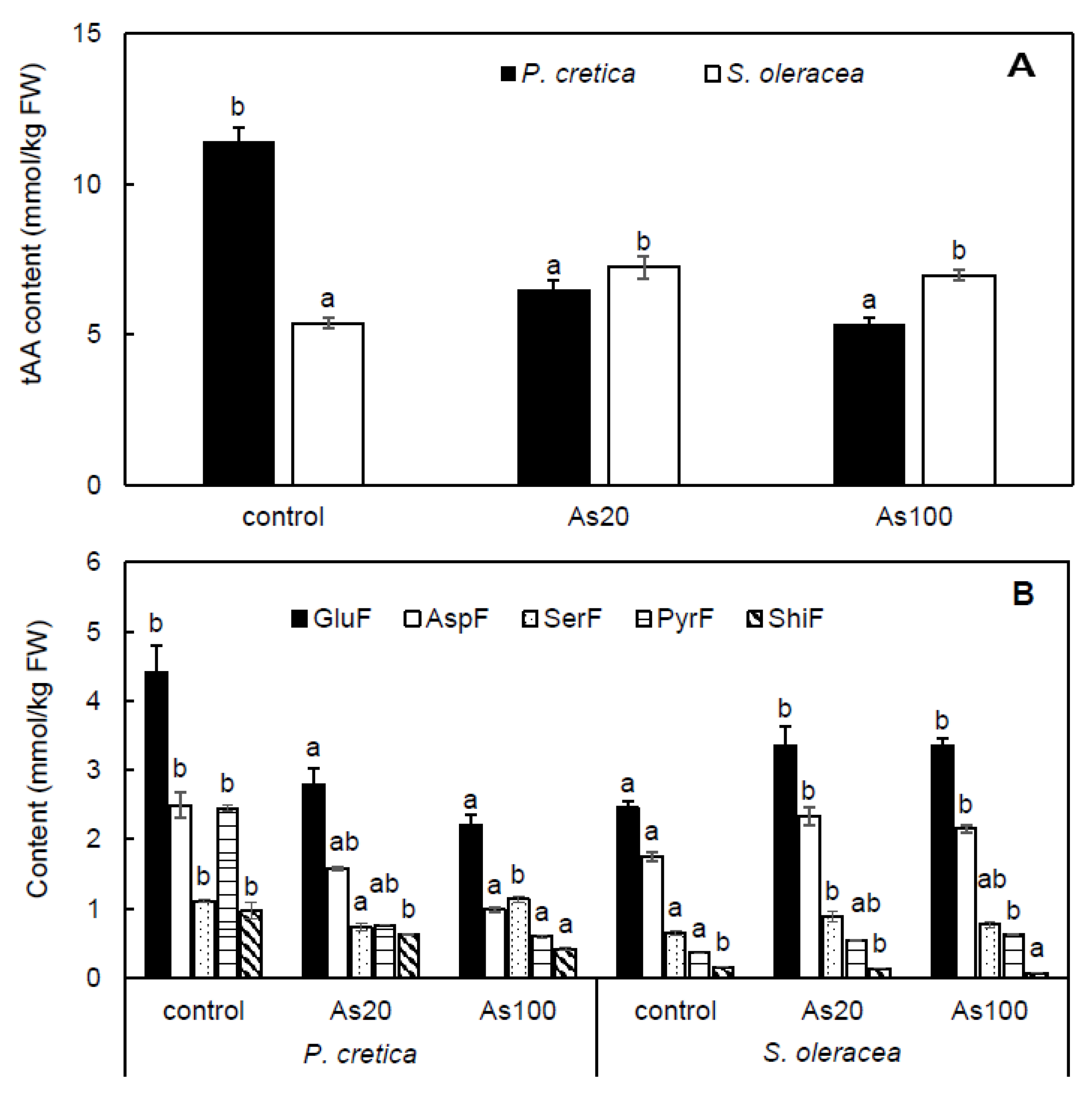
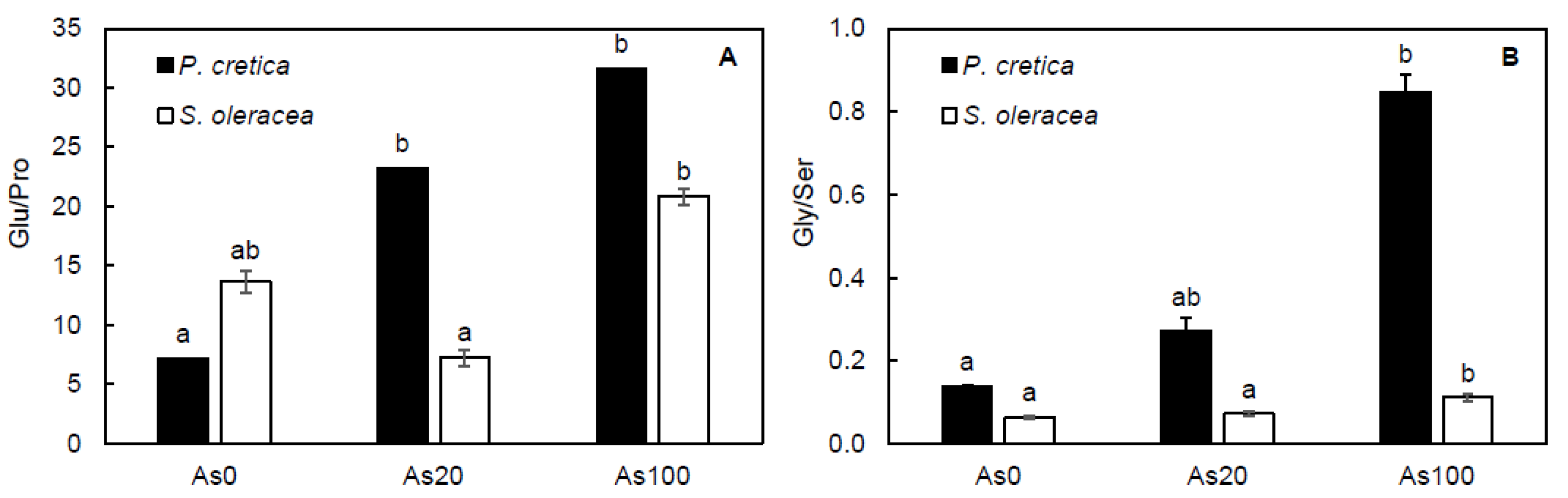
2.6. Effect of Arsenic on Free Amino Acid Metabolism
3. Discussion
4. Materials and Methods
4.1. Plant Material and Pot Experiment
4.2. Analysis of Arsenic and Elements
4.3. Analysis of Total Nitrogen
4.4. Gas-Exchange Parameter Measurements
4.5. Determination of Total Chlorophyll and Carotenoids
4.6. Chlorophyll Fluorescence Measurements
4.7. Water Potential
4.8. Analysis of Free Amino Acids
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Ahammed, G.J.; Zhang, X.N.; Zhang, L.; Yan, P.; Zhang, L.P.; Fu, J.Y.; Han, W.Y. Melatonin-mediated regulation of anthocyanin biosynthesis and antioxidant defense confer tolerance to arsenic stress in Camellia sinensis L. J. Hazard. Mater. 2021, 403, 123922. [Google Scholar] [CrossRef]
- Praveen, A.; Pandey, A.; Gupta, M. Protective role of nitric oxide on nitrogen-thiol metabolism and amino acids profiling during arsenic exposure in Oryza sativa L. Ecotoxicology 2020, 29, 825–836. [Google Scholar] [CrossRef]
- Quaghebeur, M.; Rengel, Z. Arsenic speciation governs arsenic uptake and transport in terrestrial plants. Microchim. Acta 2005, 151, 141–152. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.; Choudhary, S.; Majeed, A.; Singh, A.; Bhardwaj, P. Insights into the molecular mechanism of arsenic phytoremediation. J. Plant Growth Regul. 2020, 39, 532–543. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Kumar, M.; Agrawal, P.K.; Singh, D.K. Perspectives on arsenic toxicity, carcinogenicity and its systemic remediation strategies. Environ. Technol. Innov. 2019, 16, 100462. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, F.J. The roles of membrane transporters in arsenic uptake, translocation and detoxification in plants. Crit. Rev. Environ. Sci. Technol. 2020, in press. [Google Scholar] [CrossRef]
- Kofroňová, M.; Hrdinová, A.; Mašková, P.; Tremlová, J.; Soudek, P.; Petrová, Š.; Pinkas, D.; Lipavská, H. Multi-component antioxidative system and robust carbohydrate status, the essence of plant arsenic tolerance. Antioxidants 2020, 9, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, A.; Singh, A.P.; Kasote, D.; Sen, I.; Regina, A. Effect of phosphorus application on arsenic species accumulation and co-deposition of polyphenols in rice grain: Phyto and food safety evaluation. Plants 2021, 10, 281. [Google Scholar] [CrossRef]
- Fayiga, A.O.; Saha, U.K. Arsenic hyperaccumulating fern: Implications for remediation of arsenic contaminated soils. Geoderma 2016, 284, 132–143. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.; Aparicio-Chacón, M.V.; Palma, J.M.; Corpas, F.J. Arsenate disrupts ion balance, sulfur and nitric oxide metabolisms in roots and leaves of pea (Pisum sativum L.) plants. Environ. Exp. Bot. 2019, 161, 143–156. [Google Scholar] [CrossRef]
- Solórzano, E.; Corpas, F.J.; González-Gordo, S.; Palma, J.M. Reactive oxygen species (ROS) metabolism and nitric oxide (NO) content in roots and shoots of rice (Oryza sativa L.) plants under arsenic-induced stress. Agronomy 2020, 10, 1014. [Google Scholar] [CrossRef]
- Raab, A.; Feldmann, J.; Meharg, A.A. The nature of arsenic-phytochelatin complexes in Holcus lanatus and Pteris cretica. Plant. Physiol. 2004, 134, 1113–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Hu, S.; Zhao, X.; Kumar, S.; Li, Y.; Yang, J.; Hou, H. Mechanisms of the morphological plasticity induced by phytohormones and the environment in plants. Int. J. Mol. Sci. 2021, 22, 765. [Google Scholar] [CrossRef]
- Bandaru, V.; Hansen, D.J.; Codling, E.E.; Daughtry, C.S.; White-Hansen, S.; Green, C.E. Quantifying arsenic-induced morphological changes in spinach leaves: Implications for remote sensing. Int. J. Remote Sens. 2010, 31, 4163–4177. [Google Scholar] [CrossRef]
- Finnegan, P.M.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef] [Green Version]
- Pathare, V.; Srivastava, S.; Suprasanna, P. Evaluation of effects of arsenic on carbon, nitrogen, and sulfur metabolism in two contrasting varieties of Brassica juncea. Acta Physiol. Plant. 2013, 35, 3377–3389. [Google Scholar] [CrossRef]
- Pavlík, M.; Pavlíková, D.; Staszková, L.; Neuberg, M.; Kaliszová, R.; Száková, J.; Tlustoš, P. The effect of arsenic contamination on amino acids metabolism in Spinacia oleracea L. Ecotox. Environ. Saf. 2010, 73, 1309–1313. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, R.D.; Singh, R.P.; Dwivedi, S.; Chakrabarty, D.; Trivedi, P.K.; Adhikari, B. Arsenite tolerance in rice (Oryza sativa L.) involves coordinated role of metabolic pathways of thiols and amino acids. Environ. Sci. Pollut. Res. 2013, 20, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Campos, N.V.; Araújo, T.O.; Arcanjo-Silva, S.; Freitas-Silva, L.; Azevedo, A.A.; Nunes-Nesi, A. Arsenic hyperaccumulation induces metabolic reprogramming in Pityrogramma calomelanos to reduce oxidative stress. Physiol. Plant. 2016, 157, 135–146. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlíková, D.; Pavlík, M. Free amino acid regulation in fronds and roots of two Pteris cretica L. ferns under arsenic stress. Plant Soil Environ. 2020, 66, 483–492. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Ahmad, P. Differential distribution of amino acids in plants. Amino Acids 2017, 49, 821–869. [Google Scholar] [CrossRef]
- Zhao, F.J.; Dunham, S.J.; McGrath, S.P. Arsenic hyperaccumulation by different fern species. New Phytol. 2002, 156, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.B.; Xie, F.; Yao, Y.Z.; Zhao, B.; Xiao, Q.Q.; Pan, Y.H.; Wang, H.J. The effects of arsenic and induced-phytoextraction methods on photosynthesis in Pteris species with different arsenic-accumulating abilities. Environ. Exp. Bot. 2012, 75, 298–306. [Google Scholar] [CrossRef]
- Srivastava, M.; Ma, L.Q.; Santos, J.A.G. Three new arsenic hyperaccumulating ferns. Sci. Total Environ. 2006, 364, 24–31. [Google Scholar] [CrossRef]
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef] [Green Version]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: An overview on molecular aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Popov, M.; Zemanová, V.; Sácký, J.; Pavlík, M.; Leonhardt, T.; Matoušek, T.; Kaňa, A.; Pavlíková, D.; Kotrba, P. Arsenic accumulation and speciation in two cultivars of Pteris cretica L. and characterization of arsenate reductase PcACR2 and arsenite transporter PcACR3 genes in the hyperaccumulating cv. Albo-lineata. Ecotox. Ecotox. Environ. Saf. 2021, 216, 112196. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Pinelli, E.; Pourrut, B.; Silvestre, J.; Dumat, C. Lead-induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotox. Environ. Saf. 2011, 74, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, R.; Favas, P.J.C.; Pratas, J.; Varun, M.; Paul, M.S. Metal(loid) induced toxicity and defense mechanisms in Spinacia oleracea L. Ecological hazard and prospects for phytoremediation Ecotox. Environ. Saf. 2019, 183, 109570. [Google Scholar] [CrossRef]
- Alia, N.; Sardar, K.; Said, M.; Salma, K.; Sadia, A.; Sadaf, S.; Toqeer, A.; Miklas, S. Toxicity and bioaccumulation of heavy metals in spinach (Spinacia oleracea) grown in a controlled environment . Int. J. Environ. Res. Public Health 2015, 12, 7400–7416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubair, M.; Khan, Q.U.; Mirza, N.; Sarwar, R.; Khan, A.A.; Baloch, M.S.; Fahad, S.; Shah, A.N. Physiological response of spinach to toxic heavy metal stress. Environ. Sci. Pollut. Res. 2019, 26, 31667–31674. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and excluders—Strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Baker, A.J.M.; McGrath, S.P.; Reeves, R.D.; Smith, J.A.C. Metal hyperaccumulator plants: A review of the ecology and physiology of a biochemical resource for phytoremediation of metal-polluted soils. In Phytoremediation of Contaminated Soil and Water, 1st ed.; Terry, N., Bañuelos, G., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2000; pp. 85–107. [Google Scholar]
- Zhao, F.J.; Wang, J.R.; Barker, J.H.A.; Schat, H.; Bleeker, P.M.; McGrath, S.P. The role of phytochelatins in arsenic tolerance in the hyperaccumulator Pteris vittata. New Phytol. 2003, 159, 403–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.H.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic—A hardy, versatile, fast-growing plant helps to remove arsenic from contaminated soils. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Zemanová, V.; Popov, M.; Pavlíková, D.; Kotrba, P.; Hnilička, F.; Česká, J.; Pavlík, M. Effect of arsenic stress on 5-methylcytosine, photosynthetic parameters and nutrient content in arsenic hyperaccumulator Pteris cretica (L.) var. Albo-lineata. BMC Plant Biol. 2020, 20, 130. [Google Scholar] [CrossRef] [PubMed]
- Pavlíková, D.; Zemanová, V.; Pavlík, M.; Dobrev, P.I.; Hnilička, F.; Motyka, V. Response of cytokinins and nitrogen metabolism in the fronds of Pteris sp. under arsenic stress. PLoS ONE 2020, 15, e0233055. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Rafiq, M.; Niazi, N.K.; Dumat, C.; Shamshad, S.; Khalid, S.; Bibi, I. Arsenic accumulation and physiological attributes of spinach in the presence of amendments: An implication to reduce health risk. Environ. Sci. Pollut. Res. 2017, 24, 16097–16106. [Google Scholar] [CrossRef] [PubMed]
- Natasha; Shahid, M.; Khalid, S.; Saleem, M. Unrevealing arsenic and lead toxicity and antioxidant response in spinach: A human health perspective. Environ. Geochem. Health 2021, in press. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, J.; Zheng, J.; Zhao, J.; Qiu, C.; Xiao, D.; Mu, L.; Liu, X. Arsenate phytotoxicity regulation by humic acid and related metabolic mechanisms Ecotox. Environ. Saf. 2021, 207, 111379. [Google Scholar] [CrossRef]
- Amna, S.; Qamar, S.; Naqvi, A.A.T.; Al-Huqail, A.A.; Qureshi, M.I. Role of sulfur in combating arsenic stress through upregulation of important proteins, and in-silico analysis to study the interaction between phosphate transporter (PHO1), arsenic and phosphate in spinach. Plant Physiol. Biochem. 2020, 157, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction between macro- and micro-nutrients in plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.; Raza, H.; Murtaza, B.; Abbas, G.; Imran, M.; Shahid, M.; Naeem, M.A.; Zakir, A.; Iqbal, M.M. Nickel toxicity induced changes in nutrient dynamics and antioxidant profiling in two maize (Zea mays L.) hybrids. Plants 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascher, R.; Lippmann, B.; Holzinger, S.; Bergmann, H. Arsenate toxicity: Effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci. 2002, 163, 961–969. [Google Scholar] [CrossRef]
- Khan, S.; Khan, A.; Khan, M.A.; Aamir, M.; Li, G. Arsenic interaction and bioaccumulation in food crops grown on degraded soil: Effect on plant nutritional components and other dietary qualities. Land Degrad. Dev. 2019, 30, 1954–1967. [Google Scholar] [CrossRef]
- Rofkar, J.R.; Dwyer, D.F. Irrigation of three wetland species and a hyperaccumulating fern with arsenic-laden solutions: Observations of growth, arsenic uptake, nutrient status, and chlorophyll content. Int. J. Phytoremediat. 2013, 15, 561–572. [Google Scholar] [CrossRef]
- Tu, C.; Ma, L.Q. Effects of arsenic on concentration and distribution of nutrients in the fronds of the arsenic hyperaccumulator Pteris vittata L. Environ. Pollut. 2005, 135, 333–340. [Google Scholar] [CrossRef]
- Bashir, H.; Ahmad, J.; Bagheri, R.; Nauman, M.; Qureshi, M.I. Limited sulfur resource forces Arabidopsis thaliana to shift towards non-sulfur tolerance under cadmium stress. Environ. Exp. Bot. 2013, 94, 19–32. [Google Scholar] [CrossRef]
- Ribera, A.; Bai, Y.; Wolters, A.M.A.; van Treuren, R.; Kik, C. A review on the genetic resources, domestication and breeding history of spinach (Spinacia oleracea L.). Euphytica 2020, 216, 48. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Castor, J.M.; Guzmán-Mar, J.L.; Hernández-Ramírez, A.; Garza-González, M.T.; Hinojosa-Reyes, L. Arsenic accumulation in maize crop (Zea mays): A review. Sci. Total Environ. 2014, 488, 176–187. [Google Scholar] [CrossRef]
- Singh, R.; Jha, A.B.; Misra, A.N.; Sharma, P. Differential responses of growth, photosynthesis, oxidative stress, metals accumulation and NRAMP genes in contrasting Ricinus communis genotypes under arsenic stress. Environ. Sci. Pollut. Res. 2019, 26, 31166–31177. [Google Scholar] [CrossRef]
- Whittaker, J.W. Molecular relaxation and metalloenzyme active site Modeling. Int. J. Quantum Chem. 2002, 90, 1529–1535. [Google Scholar] [CrossRef]
- Ye, X.; Chen, X.F.; Deng, C.L.; Yang, L.T.; Lai, N.W.; Guo, J.X.; Chen, L.S. Magnesium-deficiency effects on pigments, photosynthesis and photosynthetic electron transport of leaves, and nutrients of leaf blades and veins in Citrus sinensis seedlings. Plants 2019, 8, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemanová, V.; Pavlíková, D.; Hnilička, F.; Pavlík, M.; Zámečníková, H.; Hlavsa, T. A comparison of the photosynthesis response to arsenic stress in two Pteris cretica ferns. Photosynthetica 2021, 59, 228–236. [Google Scholar] [CrossRef]
- Karimi, N.; Shayesteh, L.S.; Ghasmpour, H.; Alavi, M. Effects of arsenic on growth, photosynthetic activity, and accumulation in two new hyperaccumulating populations of Isatis cappadocica Desv. J. Plant Growth Regul. 2013, 32, 823–830. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, P.; Sharma, Y.K. Status of photosynthetic pigments, lipid peroxidation and anti-oxidative enzymes in Vigna mungo in presence of arsenic. J. Plant Nutr. 2017, 40, 298–306. [Google Scholar] [CrossRef]
- Singh, V.P.; Srivastava, P.K.; Prasad, S.M. Nitric oxide alleviates arsenic-induced toxic effects in ridged Luffa seedlings. Plant Physiol. Biochem. 2013, 71, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srivastava, A.K.; Singh, B.; Suprasanna, P.; D’Souza, S.F. The effect of arsenic on pigment composition and photosynthesis in Hydrilla verticillata. Biol. Plant. 2013, 57, 385–389. [Google Scholar] [CrossRef]
- Joardar, J.C.; Afrin, N.; Halder, M. Arsenic stress on photosynthesis and growth in Ipomoea aquatica. Plant Sci. Today 2019, 6, 420–426. [Google Scholar] [CrossRef]
- Gago, J.; Coopman, R.E.; Cabrera, H.M.; Hermida, C.; Molins, A.; Conesa, M.A.; Galmes, J.; Ribas-Carbo, M.; Flexas, J. Photosynthesis limitations in three fern species. Physiol. Plant. 2013, 149, 599–611. [Google Scholar] [CrossRef]
- Kofroňová, M.; Mašková, P.; Lipavská, H. Two facets of world arsenic problem solution: Crop poisoning restriction and enforcement of phytoremediation. Planta 2018, 248, 19–35. [Google Scholar] [CrossRef]
- Sicher, R.C.; Bunce, J.A. Adjustments of net photosynthesis in Solanum tuberosum in response to reciprocal changes in ambient and elevated growth CO2 partial pressures. Physiol. Plant. 2001, 112, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; Qi, Y.; Song, W.; Xu, H. Effects of di-n-butyl phthalate and di (2-ethylhexyl) phthalate on the growth, photosynthesis, and chlorophyll fluorescence of wheat seedlings. Chemosphere 2016, 151, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, N.; Berova, M.; Zlatev, Z. Effect of arsenic on some physiological parameters in bean plants. Biol. Plant. 2005, 49, 293–296. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ashraf, U.; Khan, I.; Wang, L. Alteration in growth, leaf gas exchange, and photosynthetic pigments of maize plants under combined cadmium and arsenic stress. Water Air Soil Pollut. 2017, 228, 13. [Google Scholar] [CrossRef]
- Vezza, M.E.; Llanes, A.; Travaglia, C.; Agostini, E.; Talano, M.A. Arsenic stress effects on root water absorption in soybean plants: Physiological and morphological aspects. Plant Physiol. Biochem. 2018, 123, 8–17. [Google Scholar] [CrossRef]
- Yang, N.; Wang, X.; Cotrozzi, L.; Chen, Y.; Zheng, F. Ozone effects on photosynthesis of ornamental species suitable for urban green spaces of China. Urban For. Urban Green. 2016, 20, 437–447. [Google Scholar] [CrossRef]
- Ros, R.; Muñoz-Bertomeu, J.; Krueger, S. Serine in plants: Biosynthesis, metabolism, and functions. Trends Plant Sci. 2014, 19, 564–569. [Google Scholar] [CrossRef]
- González-Orenga, S.; Ferrer-Gallego, P.P.; Laguna, E.; López-Gresa, M.P.; Donat-Torres, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Insights on salt tolerance of two endemic Limonium species from Spain. Metabolites 2019, 9, 294. [Google Scholar] [CrossRef] [Green Version]
- Okumoto, S.; Funck, D.; Trovato, M.; Forlani, G. Editorial: Amino acids of the glutamate family: Functions beyond primary metabolism. Front. Plant Sci. 2016, 7, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Showalter, A.M. Structure and function of plant cell wall proteins. Plant Cell 1993, 5, 9–23. [Google Scholar] [CrossRef]
- Cassab, G.I. Plant cell wall proteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 281–309. [Google Scholar] [CrossRef] [PubMed]
- Zemanová, V.; Pavlík, M.; Pavlíková, D.; Hnilička, F.; Vondráčková, S. Responses to Cd stress in two Noccaea species (Noccaea praecox and Noccaea caerulescens) originating from two contaminated sites in Mežica, Slovenia and Redlschlag, Austria. Arch. Environ. Contam. Toxicol. 2016, 70, 464–474. [Google Scholar] [CrossRef]
- Pavlíková, D.; Pavlík, M.; Staszková, L.; Motyka, V.; Száková, J.; Tlustoš, P.; Balík, J. Glutamate kinase as a potential biomarker of heavy metal stress in plants. Ecotox. Environ. Saf. 2008, 70, 223–230. [Google Scholar] [CrossRef]
- Campos, N.V.; Arcanjo-Silva, S.; Viana, I.B.; Batista, B.L.; Barbosa, F.; Loureiro, M.E.; Ribeiro, C.; Azevedo, A.A. Arsenic-induced responses in Pityrogramma calomelanos (L.) Link: Arsenic speciation, mineral nutrition and antioxidant defenses. Plant Physiol. Biochem. 2015, 97, 28–35. [Google Scholar] [CrossRef]
- Novitskaya, L.; Trevanion, S.J.; Driscoll, S.; Foyer, C.H.; Noctor, G. How does photorespiration modulate leaf amino acid contents? A dual approach through modelling and metabolite analysis. Plant Cell Environ. 2002, 25, 821–835. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yang, C.; Zhang, L.; Li, L.; Liu, S.; Yu, J.; You, L.; Zhou, D.; Xia, C.; Zhao, J.; et al. Metabolic profiling of cadmium-induced effects in one pioneer intertidal halophyte Suaeda salsa by NMR-based metabolomics. Ecotoxicology 2011, 20, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Azevedo Neto, A.D.; Prisco, J.T.; Gomes-Filho, E. Changes in soluble amino-N, soluble proteins and free amino acids in leaves and roots of salt-stressed maize genotypes. J. Plant Interact. 2009, 4, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Rocha, M.; Licausi, F.; Araújo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; van Dongen, J.T. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef] [Green Version]
- Hjorth, M.; Mathiassen, S.K.; Kudsk, P.; Ravn, H.W. Amino acids in loose silky-bent (Apera spica-venti (L.) Beauv.) responding to prosulfocarb exposure and the correlation with physiological effects. Pestic. Biochem. Physiol. 2006, 86, 138–145. [Google Scholar] [CrossRef]
- Tripathi, R.D.; Singh, R.; Tripathi, P.; Dwivedi, S.; Chauhan, R.; Adhikari, B.; Trivedi, P.K. Arsenic accumulation and tolerance in rootless macrophyte Najas indica are mediated through antioxidants, amino acids and phytochelatins. Aquat. Toxicol. 2014, 157, 70–80. [Google Scholar] [CrossRef]
- Kumar, A.; Dwivedi, S.; Singh, R.P.; Chakrabarty, D.; Mallick, S.; Trivedi, P.K.; Adhikari, B.; Tripathi, R.D. Evaluation of amino acid profile in contrasting arsenic accumulating rice genotypes under arsenic stress. Biol. Plant. 2014, 58, 733–742. [Google Scholar] [CrossRef]
- Pavlíková, D.; Pavlík, M.; Procházková, D.; Zemanová, V.; Hnilička, F.; Wilhelmová, N. Nitrogen metabolism and gas exchange parameters associated with zinc stress in tobacco expressing an ipt gene for cytokinin synthesis. J. Plant Physiol. 2014, 171, 559–564. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kudrna, J.; Hnilička, F.; Kubeš, J.; Váchová, P.; Hniličková, H.; Kuklová, M. Effect of acetaminophen (APAP) on physiological indicators in Lactuca sativa. Life 2020, 10, 303. [Google Scholar] [CrossRef] [PubMed]
| P. cretica | S. oleracea | |||||
|---|---|---|---|---|---|---|
| TF | BF (Shoots) | BF (Roots) | TF | BF (Shoots) | BF (Roots) | |
| control | 1.82 ± 0.06 a | 2.65 ± 0.02 a | 1.46 ± 0.06 a | 0.67 ± 0.02 b | 0.13 ± 0.01 b | 0.20 ± 0.01 a |
| As20 | 3.28 ± 0.06 ab | 29.34 ± 0.13 ab | 8.96 ± 0.11 b | 0.26 ± 0.01 ab | 0.10 ± 0.01 ab | 0.41 ± 0.01 ab |
| As100 | 5.53 ± 0.25 b | 34.96 ± 0.12 b | 6.35 ± 0.32 ab | 0.05 ± 0.01 a | 0.05 ± 0.01 a | 1.10 ± 0.01 b |
| P. cretica | S. oleracea | |||||
|---|---|---|---|---|---|---|
| Control | As20 | As100 | Control | As20 | As100 | |
| N (g/kg DW) | 26.22 ± 0.14 a | 28.54 ± 0.10 ab | 30.43 ± 0.02 b | 42.42 ± 0.29 a | 44.12 ± 0.22 ab | 47.19 ± 0.25 b |
| Fe (g/kg DW) | 0.12 ± 0.01 a | 0.22 ± 0.01 b | 0.19 ± 0.01 ab | 0.25 ± 0.01 b | 0.16 ± 0.01 a | 0.21 ± 0.01 ab |
| Mg (g/kg DW) | 3.44 ± 0.02 a | 3.87 ± 0.02 ab | 4.25 ± 0.03 b | 12.01 ± 0.03 b | 11.56 ± 0.01 ab | 9.06 ± 0.11 a |
| S (g/kg DW) | 1.61 ± 0.01 a | 2.19 ± 0.02 ab | 2.95 ± 0.01 b | 2.28 ± 0.01 a | 2.88 ± 0.01 ab | 3.46 ± 0.01 b |
| P (g/kg DW) | 2.96 ± 0.01 a | 4.15 ± 0.02 b | 3.86 ± 0.02 ab | 5.43 ± 0.02 b | 5.15 ± 0.08 ab | 3.6 ± 0.05 a |
| Mn (mg/kg DW) | 56.40 ± 0.40 a | 63.68 ± 0.69 ab | 65.39 ± 0.29 b | 145.02 ± 0.54 b | 108.68 ± 0.57 ab | 106.48 ± 0.90 a |
| Zn (mg/kg DW) | 24.38 ± 0.19 a | 29.00 ± 0.12 ab | 33.68 ± 0.14 b | 88.00 ± 0.55 b | 72.70 ± 0.05 ab | 48.32 ± 0.15 a |
| Cu (mg/kg DW) | 8.10 ± 0.05 a | 11.04 ± 0.01 b | 10.64 ± 0.04 ab | 8.91 ± 0.04 b | 7.99 ± 0.06 ab | 7.71 ± 0.09 a |
| Ni (mg/kg DW) | 0.88 ± 0.08 a | 0.88 ± 0.04 a | 1.90 ± 0.02 a | 0.94 ± 0.08 a | 0.84 ± 0.02 a | 0.60 ± 0.04 a |
| P. cretica | S. oleracea | |||
|---|---|---|---|---|
| Chltot (g/m2) | Car (g/m2) | Chltot (g/m2) | Car (g/m2) | |
| control | 0.35 ± 0.08 a | 0.05 ± 0.01 a | 0.30 ± 0.02 b | 0.05 ± 0.01 a |
| As20 | 0.23 ± 0.06 a | 0.03 ± 0.01 a | 0.25 ± 0.01 ab | 0.05 ± 0.01 a |
| As100 | 0.22 ± 0.04 a | 0.03 ± 0.01 a | 0.21 ± 0.03 a | 0.04 ± 0.01 a |
| P. cretica | PN (μmol/m2 s) | Ci [μmol(CO2)/mol] | E [mmol(H2O)/m2s] | gs [mol(H2O)/m2s] | WUE [mol(CO2)/mol(H2O)] |
|---|---|---|---|---|---|
| control | 8.08 ± 0.03 c | 298.60 ± 12.15 a | 0.78 ± 0.02 b | 0.03 ± 0.001 b | 10.39 ± 0.21 ab |
| As20 | 7.72 ± 0.01 b | 319.60 ± 16.59 a | 0.60 ± 0.03 a | 0.02 ± 0.002 a | 13.45 ± 0.76 b |
| As100 | 7.31 ± 0.01 a | 302.13 ± 9.51 a | 0.77 ± 0.01 b | 0.03 ± 0.001 b | 9.48 ± 0.17 a |
| S. oleracea | |||||
| control | 11.13 ± 0.26 b | 297.25 ± 4.53 b | 2.65 ± 0.03 ab | 0.20 ± 0.004 b | 4.20 ± 0.09 b |
| As20 | 10.73 ± 0.14 b | 273.39 ± 3.63 a | 2.78 ± 0.03 b | 0.18 ± 0.004 a | 3.87 ± 0.07 a |
| As100 | 10.14 ± 0.18 a | 279.32 ± 1.07 ab | 2.54 ± 0.04 a | 0.18 ± 0.005 a | 4.01 ± 0.06 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemanová, V.; Pavlíková, D.; Hnilička, F.; Pavlík, M. Arsenic Toxicity-Induced Physiological and Metabolic Changes in the Shoots of Pteris cretica and Spinacia oleracea. Plants 2021, 10, 2009. https://doi.org/10.3390/plants10102009
Zemanová V, Pavlíková D, Hnilička F, Pavlík M. Arsenic Toxicity-Induced Physiological and Metabolic Changes in the Shoots of Pteris cretica and Spinacia oleracea. Plants. 2021; 10(10):2009. https://doi.org/10.3390/plants10102009
Chicago/Turabian StyleZemanová, Veronika, Daniela Pavlíková, František Hnilička, and Milan Pavlík. 2021. "Arsenic Toxicity-Induced Physiological and Metabolic Changes in the Shoots of Pteris cretica and Spinacia oleracea" Plants 10, no. 10: 2009. https://doi.org/10.3390/plants10102009






