Chemical Profile, Antioxidant, Anti-Proliferative, Anticoagulant and Mutagenic Effects of a Hydroalcoholic Extract of Tuscan Rosmarinus officinalis
Abstract
1. Introduction
2. Results
2.1. Chemical Analysis of Rosmarinus Officinalis Extract (Roex)
Extraction Yield and Chemical Characterization of Tuscan R. officinalis Extract
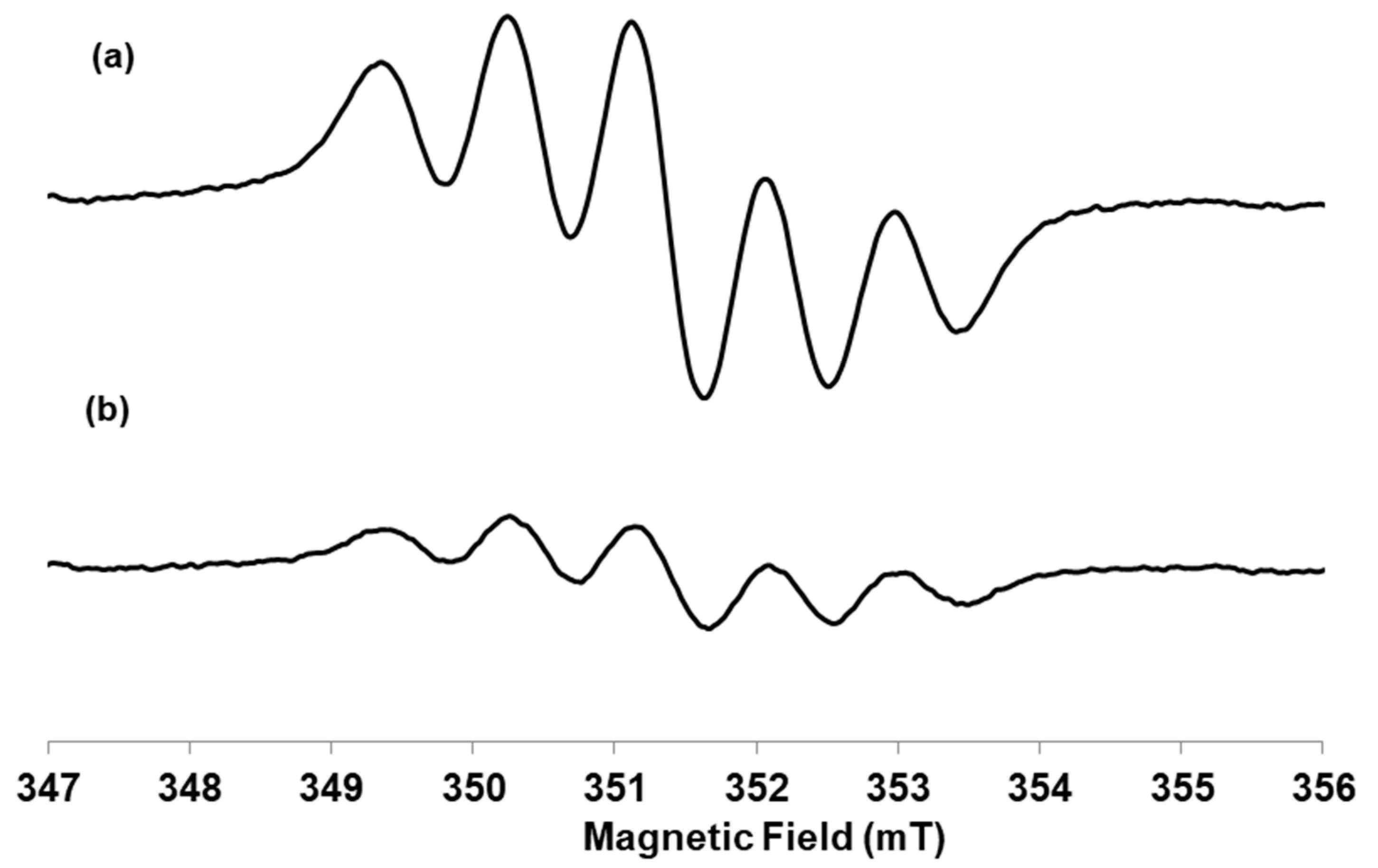
2.2. Antiradical Activity: DPPH Assay and EPR Analysis
2.3. Hydrogen Peroxide Scavenging Activity
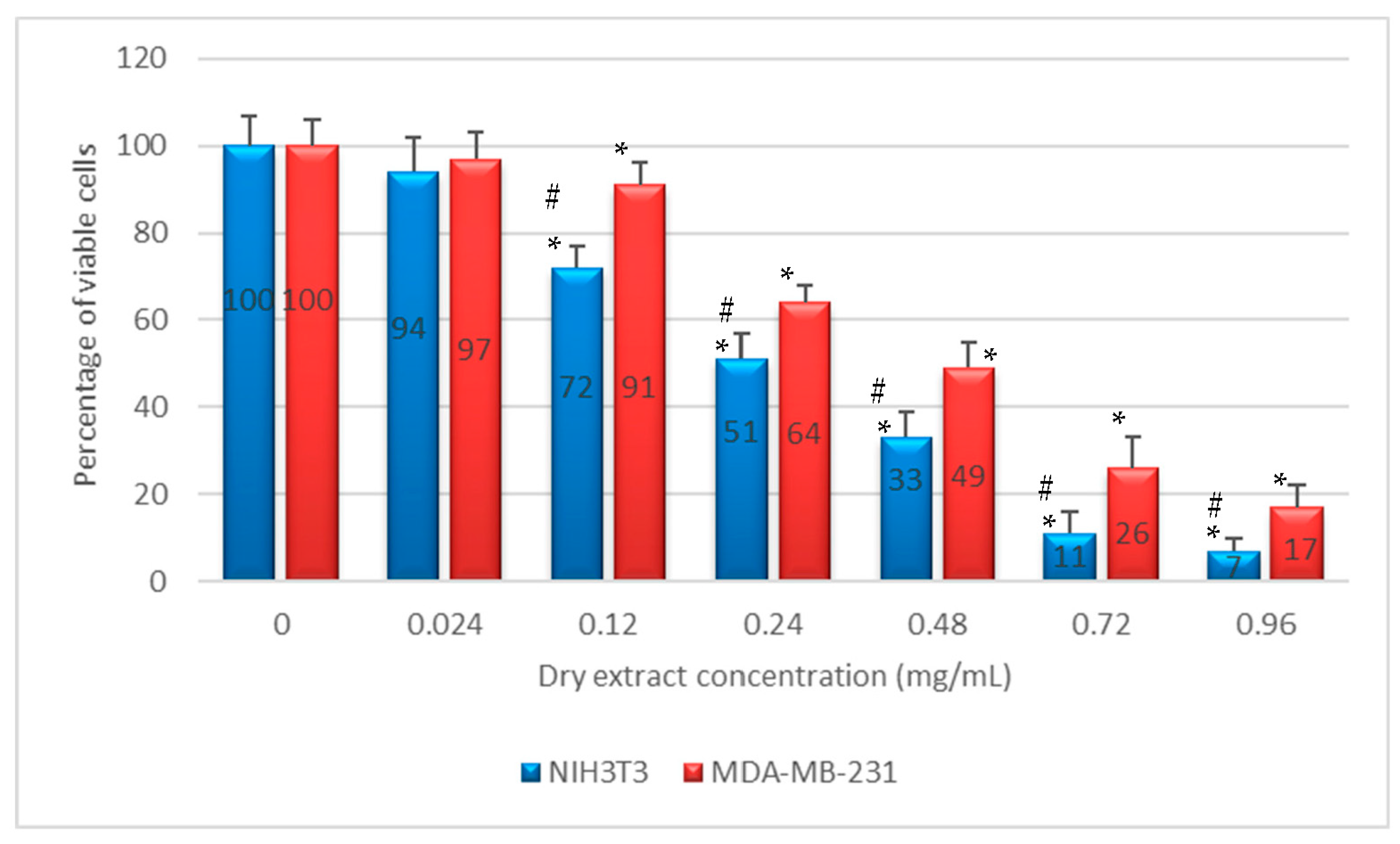
2.4. In Vitro Anti-Proliferative Activity
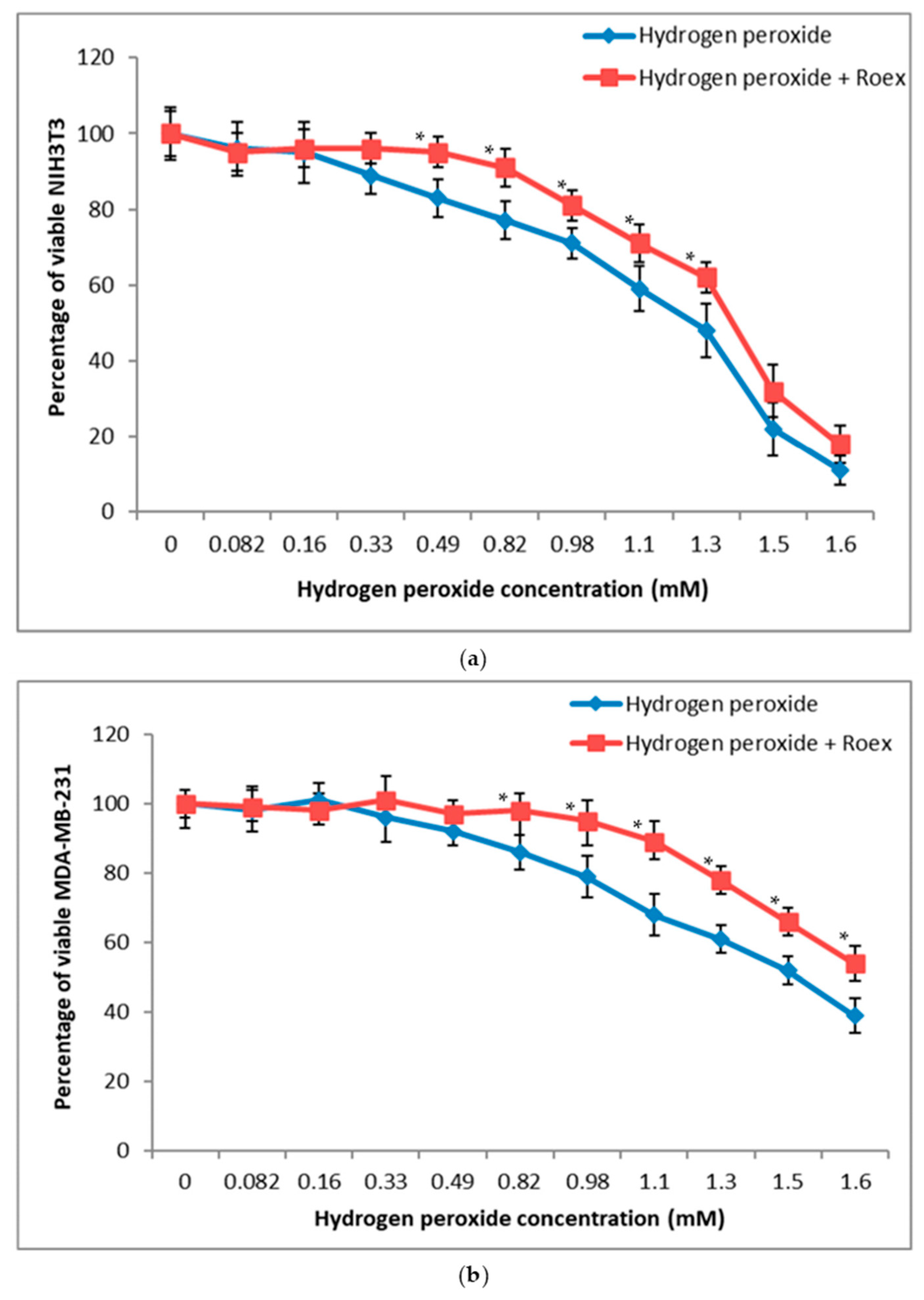
2.5. Protective Effect against Hydrogen Peroxide Induced Oxidative Stress
2.6. Mutagenicity Assay: Ames Test
2.7. Antithrombotic Activity: Thrombin Time (TT)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Rosmarinus officinalis Extract (Roex) Preparation
4.3. Chemical Analysis
4.3.1. Determination of Total Phenolic and Flavonoid Content
4.3.2. Determination of Total Triterpenes
4.3.3. High-Performance Liquid Chromatography Analysis of Phenolics Compounds
4.4. Antiradical Capacity: DPPH Assay
4.4.1. Spectrophotometric Assay
4.4.2. Scavenger Activity by Electron Paramagnetic Resonance (EPR) Analysis
4.4.3. Gallic Acid-Equivalent Antioxidant Activity through DPPH Assay
4.5. Hydrogen Peroxide Scavenging Assay
4.6. Anti-Proliferative Assay and Protective Effect against Hydrogen Peroxide Induced Oxidative Stress
4.6.1. Cell Cultures and Anti-Proliferative Test
4.6.2. Protective Effect against Hydrogen Peroxide Induced Oxidative Stress
4.6.3. Evaluation of Cell Viability: NRU Assay
- Neutral Red (NR) stock solution: 0.33 g NR dye powder in 100 mL sterile H2O
- NR medium: 1.0 mL NR stock solution + 99.0 routine culture medium pre-warmed to 37 °C
- NR desorb solution: 1% glacial acetic acid solution + 50% ethanol + 49% H2O
4.7. Mutagenicity Assay: Ames Test
4.8. Antithrombotic Activity: Thrombin Time (TT)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavalcanti, R.N.; Forster-Carneiro, T.; Gomes, M.T.M.S.; Rostagno, M.A.; Prado, J.M.; Meireles, M.A.A. Uses and Applications of Extracts from Natural Sources. In Natural Product Extraction: Principles and Applications, Green Chemistry Series; Rostagno, M.A., Prado, J.M., Eds.; The Royal Society of Chemistry: London, UK, 2013; pp. 1–57. [Google Scholar]
- Segneanu, A.E.; Velciov, S.M.; Olariu, S.; Cziple, F.; Damian, D.; Grozescu, I. Bioactive Molecules Profile from Natural Compounds. In Amino Acid—New Insights and Roles in Plant and Animal; Asao, T., Ed.; IntechOpen: London, UK, 2017; pp. 209–228. [Google Scholar] [CrossRef]
- Gonzalez-Trujano, M.E.; Peña, E.I.; Martınez, A.L.; Moreno, J.; Guevara-Feferc, P.; Déciga-Camposd, M.; López-Muñoz, F.J. Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents. J. Ethnopharmacol. 2007, 111, 476–482. [Google Scholar] [CrossRef]
- Nogueira de Melo, G.A.; Grespan, J.; Fonseca, R.P.; Farinha, T.O.; Silva, E.L.; Romero, A.L.; Bersani-Amado, C.A.; Cuman, R.K.N. Rosmarinus officinalis L. essential oil inhibits in vivo and in vitro leukocyte migration. J. Med. Food 2011, 14, 944–949. [Google Scholar] [CrossRef]
- Estévez, M.; Ramırez, R.; Ventanas, S.; Cava, R. Sage and rosemary essential oils versus BHT for the inhibition of lipid oxidative reactions in liver pâté. LWT Food Sci. Technol. 2007, 40, 58–65. [Google Scholar] [CrossRef]
- Pérez-Fons, L.; Garzón, M.T.; Micol, V. Relationship between the antioxidant capacity and effect of rosemary (Rosmarinus officinalis L.) polyphenols on membrane phospholipid order. J. Agric. Food Chem. 2010, 58, 161–171. [Google Scholar] [CrossRef]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Deguin, B.; Cappello, A.R.; Cappello, M.S. Chemical Profile, Antioxidant, Anti-Inflammatory, and Anti-Cancer Effects of Italian Salvia rosmarinus Spenn. Methanol Leaves Extracts. Antioxidants 2020, 9, 826. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Huang, L.; Ding, B.; Zhang, H.; Kong, B.; Xiong, Y.L. Textural and sensorial quality protection in frozen dumplings through the inhibition of lipid and protein oxidation with clove and rosemary extracts. J. Sci. Food Agric. 2019, 10, 4739–4747. [Google Scholar] [CrossRef]
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Pérez-Sánchez, A.; Micol, V. Skin photoprotective and antiageing effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr. Res. 2016, 60, 31871. [Google Scholar] [CrossRef]
- Williams, G.M.; Iatropoulos, M.J.; Whysner, J. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. J. Food Chem. Toxicol. 1999, 37, 1027–1038. [Google Scholar] [CrossRef]
- Tai, J.; Cheung, S.; Wu, M.; Hasman, D. Antiproliferation effect of rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine 2012, 19, 436–443. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamada, K.; Naemura, A.; Yamashita, T.; Arai, R. Testing various herbs for antithrombotic effect. Nutrition 2005, 21, 580–587. [Google Scholar] [CrossRef]
- Cheung, S.; Tai, J. Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol. Rep. 2007, 17, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef]
- Harach, T.; Aprikian, O.; Monnard, I.; Moulin, J.; Membrez, M.; Béolor, J.C.; Raab, T.; Macè, K.; Darimont, C. Rosemary (Rosmarinus officinalis L.) Leaf extract limits weight gain and liver steatosis in mice fed a high-fat diet. Planta Med. 2010, 76, 566–571. [Google Scholar] [CrossRef]
- Bakirel, T.; Bakirel, U.; Keles, O.U.; Ulgen, S.-G.; Yardibi, H. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J. Ethnopharmacol. 2008, 116, 64–73. [Google Scholar] [CrossRef]
- Afonso, M.S.; De, O.; Silva, A.M.; Carvalho, E.B.; Rivelli, D.P.; Barros, S.B.; Rogero, M.M.; Lottenberg, A.M.; Torres, R.P.; Mancini-Filho, J. Phenolic compounds from Rosemary (Rosmarinus officinalis L.) attenuate oxidative stress and reduce blood cholesterol concentrations in diet-induced hypercholesterolemic rats. Nutr. Metab. 2013, 10, 19. [Google Scholar] [CrossRef]
- Sasaki, K.; El Omri, A.; Kondo, S.; Han, J.; Isoda, H. Rosmarinus officinalis polyphenols produce anti-depressant like effect through monoaminergic and cholinergic functions modulation. Behav. Brain. Res. 2013, 238, 86–94. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 2014, 15, 585. [Google Scholar] [CrossRef]
- Okamura, N.; Haraguchi, H.; Hashimoto, K.; Yagi, A. Flavonoids in Rosmarinus officinalis leaves. Phytochemistry 1994, 37, 1463–1466. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Al-Shihry, S.S.; Son, B.W. Diterpenoid quinones from rosemary (Rosmarinus officinalis L.). Phytochemistry 2005, 66, 1685–1690. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; García-Villalba, R.; Larrosa, M.; Yáñez-Gascón, M.J.; Fromentin, E.; Flanagan, J.; Roller, M.; Tomás-Barberán, F.A.; Espín, J.C.; García-Conesa, M.T. Bioavailability of the major bioactive diterpenoids in a rosemary extract: Metabolic profile in the intestine, liver, plasma, and brain of Zucker rats. Mol. Nutr. Food. Res. 2013, 57, 1834–1846. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodríguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Rodríguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Fernández Ochoa, Á.; Borrás, I.; Pérez-Sánchez, A.; Barrajón-Catalán, E.; Gonzalez-Alvarez, I.; Arráez-Román, D.; Micol, V.; Segura, C. Phenolic compounds in rosemary as potential source of bioactive compounds against colorectal cancer: In situ absorption and metabolism study. J. Funct. Food 2017, 33, 202–210. [Google Scholar] [CrossRef]
- Ribeiro, A.; Caleja, C.; Barros, L.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. Rosemary extracts in functional foods: Extraction, chemical characterization and incorporation of free and microencapsulated forms in cottage cheese. Food Funct. 2016, 7, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Innocenti, M.; Bellumori, M.; Giaccherini, C.; Martini, V.; Michelozzi, M. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta 2011, 85, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Bicchi, C.; Binello, A.; Rubiolo, P. Determination of phenolic diterpene antioxidants in rosemary (Rosmarinus officinalis L.) with different methods of extraction and analysis. Phytochem. Anal. 2000, 11, 236–242. [Google Scholar] [CrossRef]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J. Full-Spectrum Analysis of Bioactive Compounds in Rosemary (Rosmarinus officinalis L.) as Influenced by Different Extraction Methods. Molecules 2020, 25, 4599. [Google Scholar] [CrossRef] [PubMed]
- Grillo, A.; Chemi, G.; Brogi, S.; Brindisi, M.; Relitti, N.; Fezza, F.; Fazio, D.; Castelletti, L.; Perdona, E.; Wong, A.; et al. Development of novel multipotent compounds modulating endocannabinoid and dopaminergic systems. Eur. J. Med. Chem. 2019, 1, 111674. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Danguien, M.; Bily, A.; Chemat, F. Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary. Ultrason. Sonochem. 2015, 27, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Ancillotti, C.; Ciofi, L.; Pucci, D.; Sagona, E.; Giordani, E.; Biricolti, S.; Gori, M.; Petrucci, W.A.; Giardi, F.; Bartoletti, R.; et al. Polyphenolic profiles and antioxidant and antiradical activity of Italian berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. subsp. gaultherioides (Bigelow) S.B. Young. Food Chem. 2016, 204, 176–184. [Google Scholar] [CrossRef]
- Liu, T.; Sui, X.; Zhang, R.; Yang, L.; Zu, Y.; Zhang, L.; Zhang, Y.; Zhang, Z. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from Rosmarinus officinalis. J. Chromatogr. A 2011, 1218, 8480–8489. [Google Scholar] [CrossRef] [PubMed]
- Biagi, M.; Noto, D.; Corsini, M.; Baini, G.; Cerretani, D.; Cappellucci, G.; Moretti, E. Antioxidant Effect of the Castanea sativa Mill. Leaf Extract on Oxidative Stress Induced upon Human Spermatozoa. Oxid. Med. Cell Longev. 2019, 2019, 8926075. [Google Scholar] [CrossRef] [PubMed]
- Jacotet-Navarro, M.; Laguerre, M.; Fabiano-Tixier, A.S.; Tenon, M.; Feuillère, N.; Bily, A.; Chemat, F. What is the best ethanol-water ratio for the extraction of antioxidants from rosemary? Impact of the solvent on yield, composition, and activity of the extracts. Electrophoresis 2018, 39, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Megateli, S.; Krea, M. Enhancement of total phenolic and flavonoids extraction from Rosmarinus officinalis L using electromagnetic induction heating (EMIH) process. Physiol. Mol. Biol. Plants 2018, 24, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ho, C.-T. Antioxidant Activities of Caffeic Acid and Its Related Hydroxycinnamic Acid Compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Dueñas, M.; Surco-Laos, F.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Antioxidant properties of major metabolites of quercetin. Eur. Food Res. Technol. 2011, 232, 103–111. [Google Scholar] [CrossRef]
- Deiana, M.; Corona, G.; Incani, A.; Loru, D.; Rosa, A.; Atzeri, A.; Paola Melis, M.; Assunta Dessì, M. Protective effect of simple phenols from extravirgin olive oil against lipid peroxidation in intestinal Caco-2 cells. Food Chem. Toxicol. 2010, 48, 3008–3016. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.J.; Kim, K.B.; An, I.S.; Ahn, K.J.; Han, H.J. Protective effects of rosmarinic acid against hydrogen peroxide-induced cellular senescence and the inflammatory response in normal human dermal fibroblasts. Mol. Med. Rep. 2017, 16, 9763–9769. [Google Scholar] [CrossRef]
- Song, J.; Guo, D.; Bi, H. Chlorogenic acid attenuates hydrogen peroxide-induced oxidative stress in lens epithelial cells. Int. J. Mol. Med. 2018, 41, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Kook, D.; Wolf, A.H.; Yu, A.L.; Neubauer, A.S.; Priglinger, S.G.; Kampik, A.; Welge-Lussen, U.C. The Protective Effect of Quercetin against Oxidative Stress in the Human RPE In Vitro. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1712–1720. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; Mânica da Cruz, I.B.; Pillat, M.M.; Mânica, A.; Stefanello, N.; CastagnaCezimbra Weis, G.; de Oliveira Alves, A.; Melazzo de Andrade, C.; et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, A.; Sakanashi, Y.; Matsui, H.; Oyama, T.; Nishimura, Y.; Masuda, T.; Oyama, Y. Cytometric Analysis of Cytotoxicity of Polyphenols and Related Phenolics to Rat Thymocytes: Potent Cytotoxicity of Resveratrol to Normal Cells. Basic Clin. Pharmacol. Toxicol. 2009, 104, 455–462. [Google Scholar] [CrossRef]
- Makhafola, T.J.; Elgorashi, E.E.; McGaw, L.J.; Verschaeve, L.; Eloff, N.J. The correlation between antimutagenic activity and total phenolic content of extracts of 31 plant species with high antioxidant activity. BMC Complementary Altern. Med. 2016, 16, 490. [Google Scholar] [CrossRef]
- TarikKhouya, T.; Ramchoun, M.; Hmidani, A.; Amrani, S.; Harnafi, H.; Benlyas, M.; Zegzouti, Y.F.; Alem, C. Anti-inflammatory, anticoagulant and antioxidant effects of aqueous extracts from Moroccan thyme varieties. Asian Pac. J. Trop. Biomed 2015, 5, 636–644. [Google Scholar] [CrossRef]
- Pawlaczyk, I.; Lewik-Tsirigotis, M.; Capek, P.; Matulová, M.; Sasinková, V.; Dąbrowski, P.; Witkiewicz, W.; Gancarz, R. Effects of extraction condition on structural features and anticoagulant activity of F. vesca L. conjugates. Carbohydr. Pol. 2013, 92, 741–750. [Google Scholar] [CrossRef]
- Biagi, M.; Manca, D.; Barlozzini, B.; Miraldi, E.; Giachetti, D. Optimization of extraction: Of drugs containing polyphenols using an innovative technique. Agro Food Ind. Hi Tech 2014, 25, 60–65. [Google Scholar]
- Sosa, S.; Bornancin, A.; Tubaro, A.; Loggia, R.D. Topical antiinflammatory activity of an innovative aqueous formulation of actichelated propolis vs two commercial propolis formulations. Phytother. Res. 2007, 21, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Marchi, M.; Cocetta, V.; De Leo, B.; Saunders, P.T.K.; Catanzaro, D.; Miraldi, E.; Montopoli, M.; Biagi, M. Effects of Boswellia Serrata Roxb. and Curcuma longa L. in an In Vitro Intestinal Inflammation Model Using Immune Cells and Caco-2. Pharmaceuticals 2018, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Biagi, M.; Collodel, G.; Corsini, M.; Pascarelli, N.A.; Moretti, E. Protective effect of Propolfenol® on induced oxidative stress in human spermatozoa. Andrologia 2018, 50. [Google Scholar] [CrossRef] [PubMed]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- ISO 10995-5:2009, Biological Evaluation of Medical Devices—Part 5: Tests for Cytotoxicity: In Vitro Methods. Available online: https://www.iso.org/standard/36406.html (accessed on 4 January 2021).
- Lamponi, S.; Leone, G.; Consumi, M.; Greco, G.; Magnani, A. In vitro biocompatibility of new PVA-based hydrogels as vitreous body substitutes. J. Biomater. Sci. Polym. Ed. 2012, 23, 555–575. [Google Scholar] [CrossRef] [PubMed]
- Lamponi, S.; Leone, G.; Consumi, M.; Greco, G.; Nelli, N.; Magnani, A. Porous multi-layered composite hydrogel as cell substrate for in vitro culture of chondrocytes. Int. J. Polym. Mater. 2020. [Google Scholar] [CrossRef]
- Purves, D.; Harvey, C.; Tweats, D.; Lumley, C.E. Genotoxicity testing: Current practices and strategies used by. the pharmaceutical industry. Mutagenesis 1995, 10, 297–312. [Google Scholar] [CrossRef]
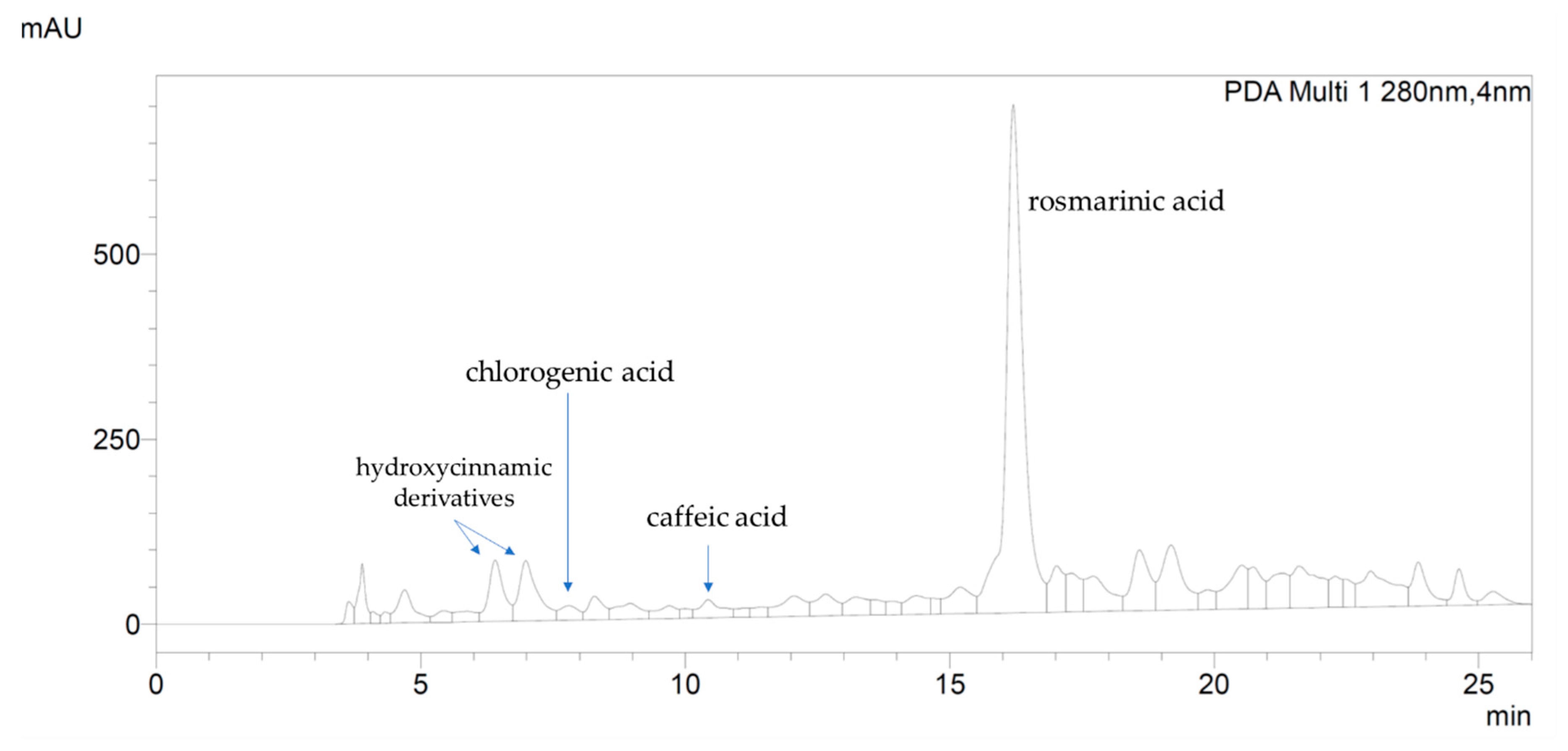
| Dry Extract for mL of Suspension (mg/mL ± SD) | Extraction Yield (% ± SD) | Total Phenolic Content (mg GAE/g d.e. ± SD) | Total Flavonoids Content (mg Hyperoside/g d.e. ± SD) | Total Triterpenoids Content (mg β-sitosterol/g d.e. ± SD) |
|---|---|---|---|---|
| 24.3 ± 1.2 | 11.2 ± 0.5 | 95.8 ± 1.1 | 6.5 ± 0.7 | 71.7 ± 7.2 |
| Compound | mg/g d.e. |
|---|---|
| Rosmarinic acid | 46.3 ± 5.0 |
| Chlorogenic acid | 2.0 ± 0.1 |
| Caffeic acid | 0.7 ± 0.1 |
| Total hydroxycinnamic derivatives (expressed as rosmarinic acid) | 69.4 ± 5.2 |
| Concentration of d.e. (mg/mL) | % Scavenged H2O2 ± SD | Total Phenolic Content (mg/mL) | Total Hydroxycinnamic Acids Content (mg/mL) | Total Flavonoid Content (mg/mL) | Total Triterpenoids (mg/mL) |
|---|---|---|---|---|---|
| 0.024 | 15 ± 3 | 2.3 × 10−3 | 1.1 × 10−3 | 1.6 × 10−4 | 1.7 × 10−3 |
| 0.12 | 28 ± 4 | 1.2 × 10−2 | 5.6 × 10−3 | 7.8 × 10−4 | 8.6 × 10−3 |
| 0.24 | 41 ± 4 | 2.3 × 10−2 | 1.1 × 10−2 | 1.6 × 10−3 | 1.7 × 10−2 |
| 0.48 | 53 ± 6 | 4.6 × 10−2 | 2.2 × 10−2 | 3.1 × 10−3 | 3.4 × 10−2 |
| 0.72 | 66 ± 3 | 6.9 × 10−2 | 3.3 × 10−2 | 4.7 × 10−3 | 5.2 × 10−2 |
| 0.96 | 73 ± 5 | 9.2 × 10−2 | 4.4 × 10−2 | 6.2 × 10−3 | 6.9 × 10−2 |
| Salmonella typhimurium TA 98 | Salmonella typhimurium TA 100 | |||
|---|---|---|---|---|
| Revertant (Mean ± SD) | Revertant (Mean ± SD) | |||
| Roex d.e. Concentration (mg/mL) | −S9 | +S9 | −S9 | +S9 |
| 0 | 1.33 ± 0.52 | 1.67 ± 1.21 | 1.33 ± 1.37 | 1.33 ± 1.21 |
| 2.4 × 10−2 | 1.67 ± 1.53 | 1.33 ± 0.58 | 1.33 ± 0.58 | 1.33 ± 1.53 |
| 1.2 × 10−1 | 1.67 ± 1.15 | 2.33 ± 0.58 | 1.00 ± 0.00 | 2.00 ± 0.00 |
| 2.4 × 10−1 | 2.00 ± 1.73 | 3.00 ± 1.00 | 1.33 ± 1.53 | 2.33 ± 2.08 |
| 4.8 × 10−1 | 2.33 ± 1.53 | 333 ± 1.15 | 1.67 ± 2.08 | 1.67 ± 0.58 |
| 7.2 × 10−1 | 2.67 ± 1.15 | 1.33 ± 1.15. | 2.67 ± 1.15 | 2.33 ± 1.53 |
| 24 | 1.33 ± 1.53 | 2.33 ± 0.58 | 2.33 ± 1.15 | 2.33 ± 0.58 |
| Positive Control | 31.33 ± 3.06 | 34.00 ± 2.00 | 47.33 ± 1.15 | 47.67 ± 0.58 |
| Baseline | 1.85 | 2.88 | 2.54 | 2.70 |
| Roex d.e. Concentration (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 2.4 × 10−2 | 1.2 × 10−1 | 2.4 × 10−1 | 4.8 × 10−1 | 7.2 × 10−1 | 9.6 × 10−1 | |
| Thrombin Time (second ± SD) | 18 ± 2 | >120″ (*) | >120″ (*) | >120″ (*) | >120″ (*) | >120″ (*) | >120″ (*) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamponi, S.; Baratto, M.C.; Miraldi, E.; Baini, G.; Biagi, M. Chemical Profile, Antioxidant, Anti-Proliferative, Anticoagulant and Mutagenic Effects of a Hydroalcoholic Extract of Tuscan Rosmarinus officinalis. Plants 2021, 10, 97. https://doi.org/10.3390/plants10010097
Lamponi S, Baratto MC, Miraldi E, Baini G, Biagi M. Chemical Profile, Antioxidant, Anti-Proliferative, Anticoagulant and Mutagenic Effects of a Hydroalcoholic Extract of Tuscan Rosmarinus officinalis. Plants. 2021; 10(1):97. https://doi.org/10.3390/plants10010097
Chicago/Turabian StyleLamponi, Stefania, Maria Camilla Baratto, Elisabetta Miraldi, Giulia Baini, and Marco Biagi. 2021. "Chemical Profile, Antioxidant, Anti-Proliferative, Anticoagulant and Mutagenic Effects of a Hydroalcoholic Extract of Tuscan Rosmarinus officinalis" Plants 10, no. 1: 97. https://doi.org/10.3390/plants10010097
APA StyleLamponi, S., Baratto, M. C., Miraldi, E., Baini, G., & Biagi, M. (2021). Chemical Profile, Antioxidant, Anti-Proliferative, Anticoagulant and Mutagenic Effects of a Hydroalcoholic Extract of Tuscan Rosmarinus officinalis. Plants, 10(1), 97. https://doi.org/10.3390/plants10010097







