Identification of QTLs Controlling Salt Tolerance in Cucumber (Cucumis sativus L.) Seedlings
Abstract
1. Introduction
2. Results
2.1. Phenotypic Variations and Correlation Analysis
2.2. Linkage Map Construction
2.3. QTL Mapping and Analysis
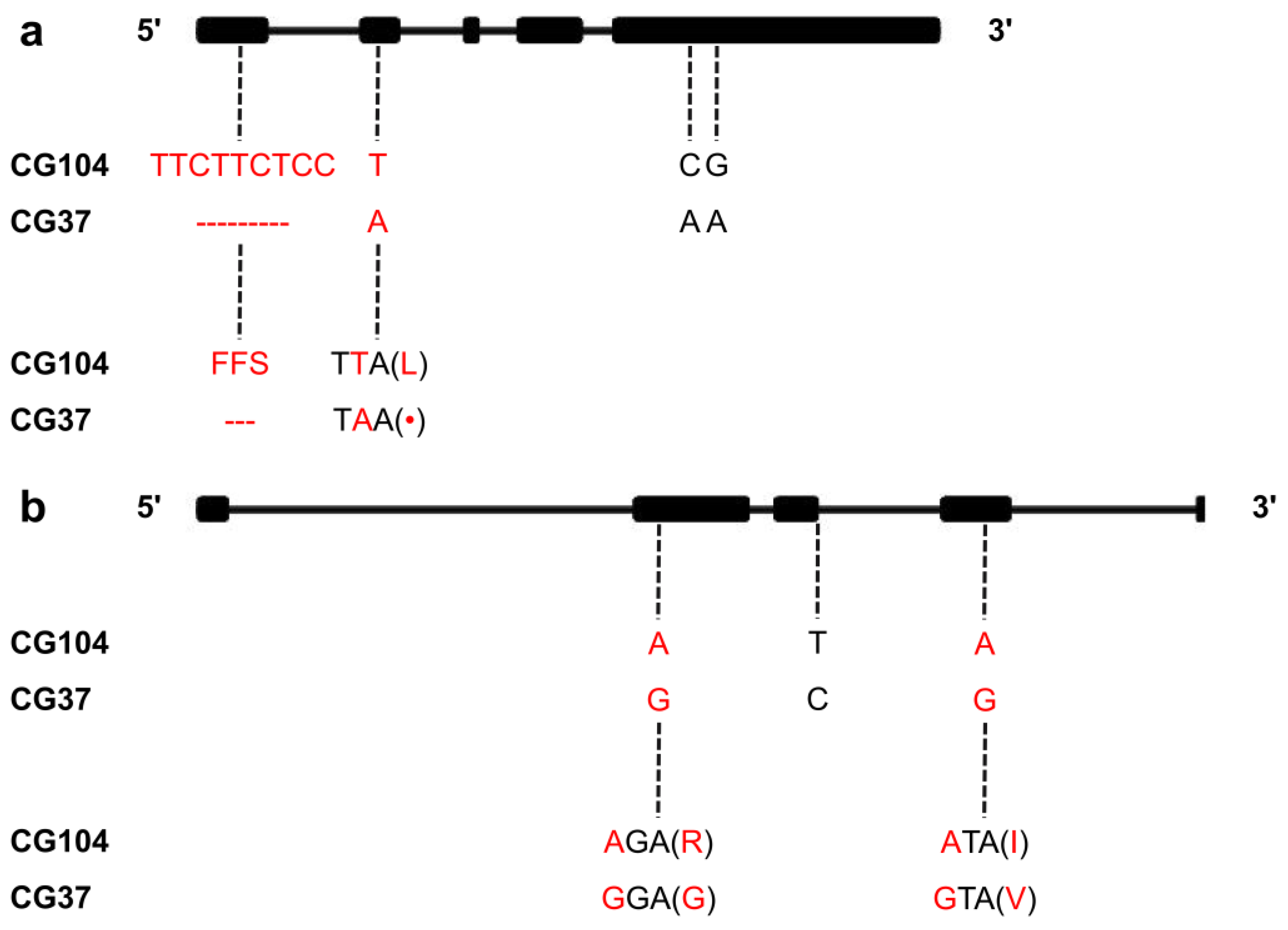
2.4. Prediction and Molecular Cloning of the Candidate Gene
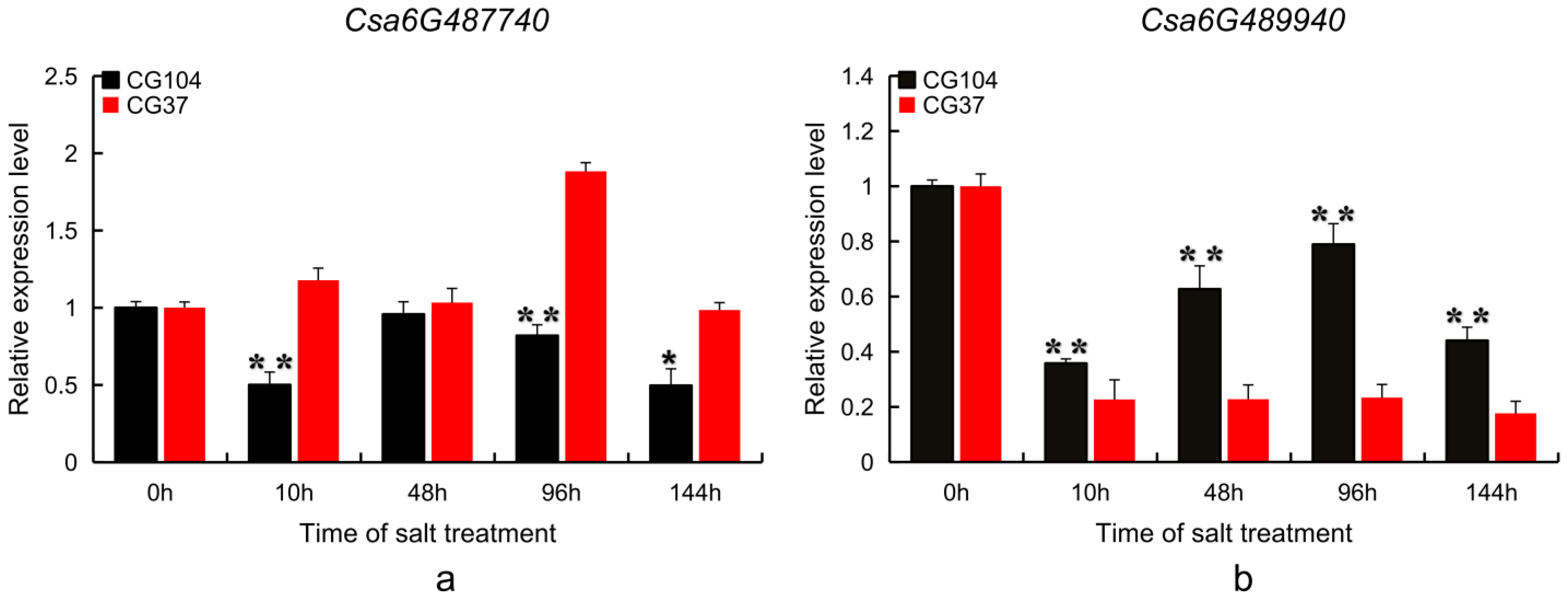
2.5. Candidate Gene Expression Pattern Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Population Development
4.2. Evaluation of Salt Tolerance Ability and Data Analysis
4.3. Molecular Marker Analysis
4.4. Linkage Map Construction and QTL Analysis
4.5. Prediction and Molecular Cloning of the Candidate Gene
4.6. Extraction of Nucleic Acids and qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Zhao, Q.Y.; Huang, H. Current states and challenges of salt-affected soil remediation by cyanobacteria. Sci. Total Environ. 2019, 669, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.C.; Wang, W.P.; Shi, L.X.; Zhang, S.; Xie, Q.; Wei, S.; Wang, Y.; Bo, K.L.; Miao, H.; Zhang, S.P.; et al. Identification of QTLs controlling low-temperature tolerance during the germination stage in cucumber (Cucumis sativus L.). Plant Breed. 2018, 137, 629–637. [Google Scholar] [CrossRef]
- Baysal, G.; Tipirdamaz, R. The effect of salinity on lipid peroxidation and some antioxidant enzyme activities in two cucumber cultivars. Acta Hortic. 2007, 729, 199–203. [Google Scholar] [CrossRef]
- Shen, J.X.; Jiang, J.L.; Tian, S.; Liu, X. Effects of NaCl stress on growth and cell membrane permeability of cucumber seedlings. Guizhou Agric. Sci. 2016, 8, 19–24. [Google Scholar]
- Chartzoulakis, K.S. Effects of NaCl salinity on germination, growth and yield of greenhouse cucumber. J. Hortic. Sci. 1992, 67, 115–119. [Google Scholar] [CrossRef]
- Li, Y.R.; Si, L.T. Genetic analysis of salt-tolerance of cucumber at seedling stage. J. Northwest A F Univ. 2011, 39, 127–131. [Google Scholar] [CrossRef]
- Wang, X.T. Evaluation and Genetic Analysis of Salt Tolerance of Cucumber Germplasm Resources. Ph.D. Thesis, Northeast Agricultural University, Harbin, China, 2014. [Google Scholar]
- Gong, J.M.; He, P.; Qian, Q.; Shen, L.S.; Zhu, L.H.; Chen, S.Y. Identification of salt-tolerance QTL in rice (Oryza sativa L.). Chin. Sci. Bull. 1999, 44, 68–71. [Google Scholar] [CrossRef]
- Quesada, V.C.; García-Martínez, S.; Piqueras, P.; Ponce, M.A.R.; Micol, J.L. Genetic architecture of NaCl tolerance in Arabidopsis. Plant Physiol. 2002, 130, 951–963. [Google Scholar] [CrossRef]
- DeRose-Wilson, L.; Gaut, B.S. Mapping salinity tolerance during Arabidopsis thaliana germination and seedling growth. PLoS ONE 2011, 6, e22832. [Google Scholar] [CrossRef]
- Quan, R.D.; Wang, J.; Hui, J.; Bai, H.; Lyu, X.; Zhu, Y.; Zhang, H.; Zhang, Z.; Li, S.; Huang, R. Improvement of salt tolerance using wild rice genes. Front. Plant Sci. 2018, 8, 2269. [Google Scholar] [CrossRef]
- Lei, L.; Zheng, H.; Bi, Y.; Yang, L.; Liu, H.; Wang, J.; Sun, J.; Zhao, H.; Li, X.; Li, J.; et al. Identification of a Major QTL and candidate gene analysis of salt tolerance at the bud burst stage in rice (Oryza sativa L.) using QTL-Seq and RNA-Seq. Rice (N. Y.) 2020, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.P.; Dai, X.G.; Chen, J.D.; Ji, Q.Q.; Zeng, Z.L. Research progress on salt stress effect on Brassica napus and QTL reaserch of salt tolerance related traits. Chin. J. Oil Crop Sci. 2020, 42, 536–544. [Google Scholar] [CrossRef]
- Li, J.M.; Liu, L.; Bai, Y.L.; Zhang, P.J.; Finkers, R.; Du, Y.C.; Visser, R.G.F.; Heusden, A.W.V. Seedling salt tolerance in tomato. Euphytica 2011, 178, 403–414. [Google Scholar] [CrossRef]
- Huang, S.; Li, R.; Zhang, Z.; Li, L.; Gu, X.; Fan, W.; Lucas, W.J.; Wang, X.; Xie, B.; Ni, P.; et al. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 2009, 41, 1275–1281. [Google Scholar] [CrossRef]
- Shi, L.X.; Yang, Y.; Xie, Q.; Miao, H.; Bo, K.; Song, Z.; Wang, Y.; Xie, B.; Zhang, S.; Gu, X. Inheritance and QTL mapping of cucumber mosaic virus resistance in cucumber (Cucumis Sativus L.). PLoS ONE 2018, 13, e0200571. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liu, S.Q.; Yang, F.J.; Li, D.F. Study on the screening of salt-tolerant watermelon stock and mechanism of salt-tolerance. Acta Agric. Boreali-Occident. Sin. 2003, 12, 105–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.Q.; Yan, X.D. Rules for Identifying Salt Tolerance of Cucumber Lines; Standard code DB13/T 1471-2011; Hebei Provincial Bureau of Quality and Technical Supervision: Cangzhou, China, 2011. [Google Scholar]
- Yang, Y.Q.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Jaiswal, S.; Gautam, R.K.; Singh, R.K.; Krishnamurthy, S.L.; Ali, S.; Sakthivel, K.; Iquebal, M.A.; Rai, A.; Kumar, D. Harmonizing technological advances in phenomics and genomics for enhanced salt tolerance in rice from a practical perspective. Rice (N. Y.) 2019, 12, 89. [Google Scholar] [CrossRef]
- Chen, H.T.; Liu, X.Q.; Zang, H.M.; Yuan, X.X.; Gu, H.P.; Cui, X.Y.; Chen, X. Advances in salinity tolerance of soybean: Genetic diversity, heredity, and gene identification contribute to improving salinity tolerance. J. Integr. Agric. 2018, 17, 2215–2221. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Genetics of yield, abiotic stress tolerance and biofortification in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2020, 133, 1569–1602. [Google Scholar] [CrossRef]
- Luo, M.J.; Zhang, Y.X.; Chen, K.; Kong, M.S.; Song, W.; Lu, B.S.; Shi, Y.X.; Zhao, Y.X.; Zhao, J.R. Mapping of quantitative trait loci for seedling salt tolerance in maize. Mol. Breed. 2019, 39, 64. [Google Scholar] [CrossRef]
- Ren, Z.H.; Gao, J.P.; Li, L.G.; Cai, X.L.; Huang, W.; Chao, D.Y.; Zhu, M.Z.; Wang, Z.Y.; Luan, S.; Lin, H.X. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005, 37, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liao, X.; Cui, Y.; Ma, W.; Zhang, X.; Du, H.; Ma, Y.; Ning, L.; Wang, H.; Huang, F.; et al. A cation diffusion facilitator, GmCDF1, negatively regulates salt tolerance in soybean. PLoS Genet. 2019, 15, e1007798. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.X.; Yue, Q.; Yong, G.; Lili, Y.; Ying, L.; Jinghan, J.; Jiangang, C.; Yulong, R.; Guangyu, L.; Lei, T.; et al. Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J. 2014, 80, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Robles, P.; Micol, J.L.; Quesada, V. Arabidopsis MDA1, a nuclear-encoded protein, functions in chloroplast development and abiotic stress responses. PLoS ONE 2012, 7, e42924. [Google Scholar] [CrossRef]
- Robles, P.; Micol, J.L.; Quesada, V. Mutations in the plant-conserved MTERF9 alter chloroplast gene expression, development and tolerance to abiotic stress in Arabidopsis thaliana. Physiol. Plant. 2015, 154, 297–313. [Google Scholar] [CrossRef]
- Nú Ez Delegido, E.; Robles, P.; Ferrández Ayela, A.; Quesada, V. Functional analysis of mTERF5 and mTERF9 contribution to salt tolerance, plastid gene expression and retrograde signalling in Arabidopsis thaliana. Plant Biol. 2020, 22, 459–471. [Google Scholar] [CrossRef]
- Ma, S.; Gong, Q.; Bohnert, H.J. Dissecting salt stress pathways. J. Exp. Bot. 2006, 57, 1097–1107. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Meng, L.; Li, H.H.; Zhang, L.Y.; Wang, J.K. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Wang, J.K. Inclusive composite interval mapping of quantitative trait genes. Acta Agron. Sin. 2009, 35, 239–245. [Google Scholar] [CrossRef]
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1943, 12, 172–175. [Google Scholar] [CrossRef]
- Miao, H.; Zhang, S.; Wang, X.; Zhang, Z.; Li, M.; Mu, S.; Cheng, Z.; Zhang, R.; Huang, S.; Xie, B.; et al. A linkage map of cultivated cucumber (Cucumis sativus L.) with 248 microsatellite marker loci and seven genes for horticulturally important traits. Euphytica 2011, 182, 167–176. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Z.; Liu, J.; Staub, J.E.; Han, Y.; Cheng, Z.; Li, X.; Lu, J.; Miao, H.; Kang, H.; et al. An integrated genetic and cytogenetic map of the cucumber genome. PLoS ONE 2009, 4, e5795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.P.; Liu, M.M.; Miao, H.; Zhang, S.Q.; Yang, Y.H.; Xie, B.Y.; Wehner, T.C.; Gu, X.F. Chromosomal mapping and QTL analysis of resistance to downy mildew in Cucumis sativus. Plant Dis. 2013, 97, 245–251. [Google Scholar] [CrossRef] [PubMed]

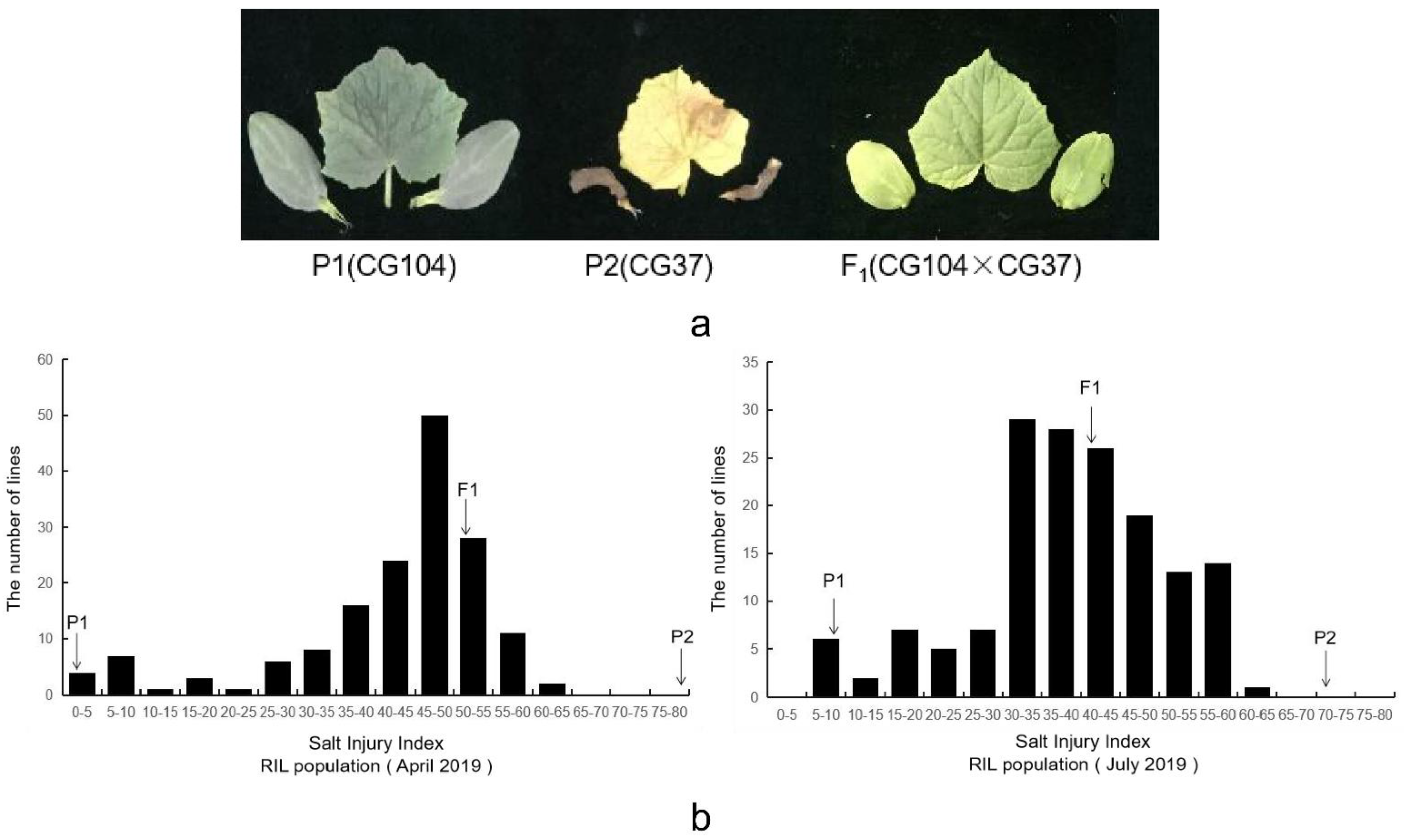


| Time | Parental Lines (Mean ± S.E) | F1 Plants | RIL Populations | ||||
|---|---|---|---|---|---|---|---|
| ‘CG104’ | ‘CG37’ | (Mean ± S.E) | Mean ± S.E | SD 1 | Skewness | Kurtosis | |
| April 2019 | 2.25 ± 0.12 | 79.50 ± 0.24 | 51.00 ± 0.31 | 42.03 ± 1.03 | 13.06 | −1.54 | 2.00 |
| July 2019 | 8.00 ± 0.16 | 72.00 ± 0.83 | 41.00 ± 0.56 | 38.48 ± 0.98 | 12.26 | −0.56 | 0.21 |
| Time | QTL | Chr. | Position (cM) | Marker Interval | LOD | R2 (%) |
|---|---|---|---|---|---|---|
| April 2019 | qST2.1 | 2 | 66.0 | 2SSR22203-2SSR16135 | 3.45 | 8.35 |
| qST6.1 | 6 | 15.0 | 6SSR10476-6SSR11343 | 4.24 | 7.18 | |
| qST6.2 | 6 | 107.0 | 6SSR13741-6SSR19970 | 11.97 | 21.32 | |
| July 2019 | qST3.1 | 3 | 65.0 | 3SSR11699-3SSR06235 | 3.21 | 7.20 |
| qST6.2 | 6 | 107.0 | 6SSR13741-6SSR19970 | 8.11 | 18.71 |
| Gene ID | Position on Chr.6 | Homologous Gene in Arabidopsis | Predicated Gene Function |
|---|---|---|---|
| Csa6G483260 | 22147783-22153709 | AT4G33470 | Histone deacetylase, putative |
| Csa6G483280 | 22159504-22162733 | AT5G63980 | 3’(2’),5’-bisphosphate nucleotidase like protein |
| Csa6G486760 | 22594296-22600877 | AT2G17520 | Serine threonine protein kinase, putative |
| Csa6G487000 | 22792092-22793544 | AT1G16850 | Transmembrane protein |
| Csa6G487690 | 22933332-22934682 | AT2G23780 | RING finger protein |
| Csa6G487740 | 22955329-22957793 | AT4G14605 | Mitochondrial transcription termination factor family protein |
| Csa6G488350 | 23038795-23040540 | AT3G50830 | Cold acclimation protein-like protein |
| Csa6G489940 | 23128924-23132268 | AT4G34400 | B3 domain-containing protein |
| Csa6G490230 | 23301401-23303177 | AT1G49570 | Peroxidase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Dong, S.; Bo, K.; Miao, H.; Li, C.; Zhang, Y.; Zhang, S.; Gu, X. Identification of QTLs Controlling Salt Tolerance in Cucumber (Cucumis sativus L.) Seedlings. Plants 2021, 10, 85. https://doi.org/10.3390/plants10010085
Liu D, Dong S, Bo K, Miao H, Li C, Zhang Y, Zhang S, Gu X. Identification of QTLs Controlling Salt Tolerance in Cucumber (Cucumis sativus L.) Seedlings. Plants. 2021; 10(1):85. https://doi.org/10.3390/plants10010085
Chicago/Turabian StyleLiu, Dongrang, Shaoyun Dong, Kailiang Bo, Han Miao, Caixia Li, Yanyan Zhang, Shengping Zhang, and Xingfang Gu. 2021. "Identification of QTLs Controlling Salt Tolerance in Cucumber (Cucumis sativus L.) Seedlings" Plants 10, no. 1: 85. https://doi.org/10.3390/plants10010085
APA StyleLiu, D., Dong, S., Bo, K., Miao, H., Li, C., Zhang, Y., Zhang, S., & Gu, X. (2021). Identification of QTLs Controlling Salt Tolerance in Cucumber (Cucumis sativus L.) Seedlings. Plants, 10(1), 85. https://doi.org/10.3390/plants10010085





