Evaluation of Different Bacterial Wilt Resistant Eggplant Rootstocks for Grafting Tomato
Abstract
1. Introduction
2. Results
2.1. Graft Compatibility
2.2. Wilt Percentage, Disease Index and Field Plant Survival
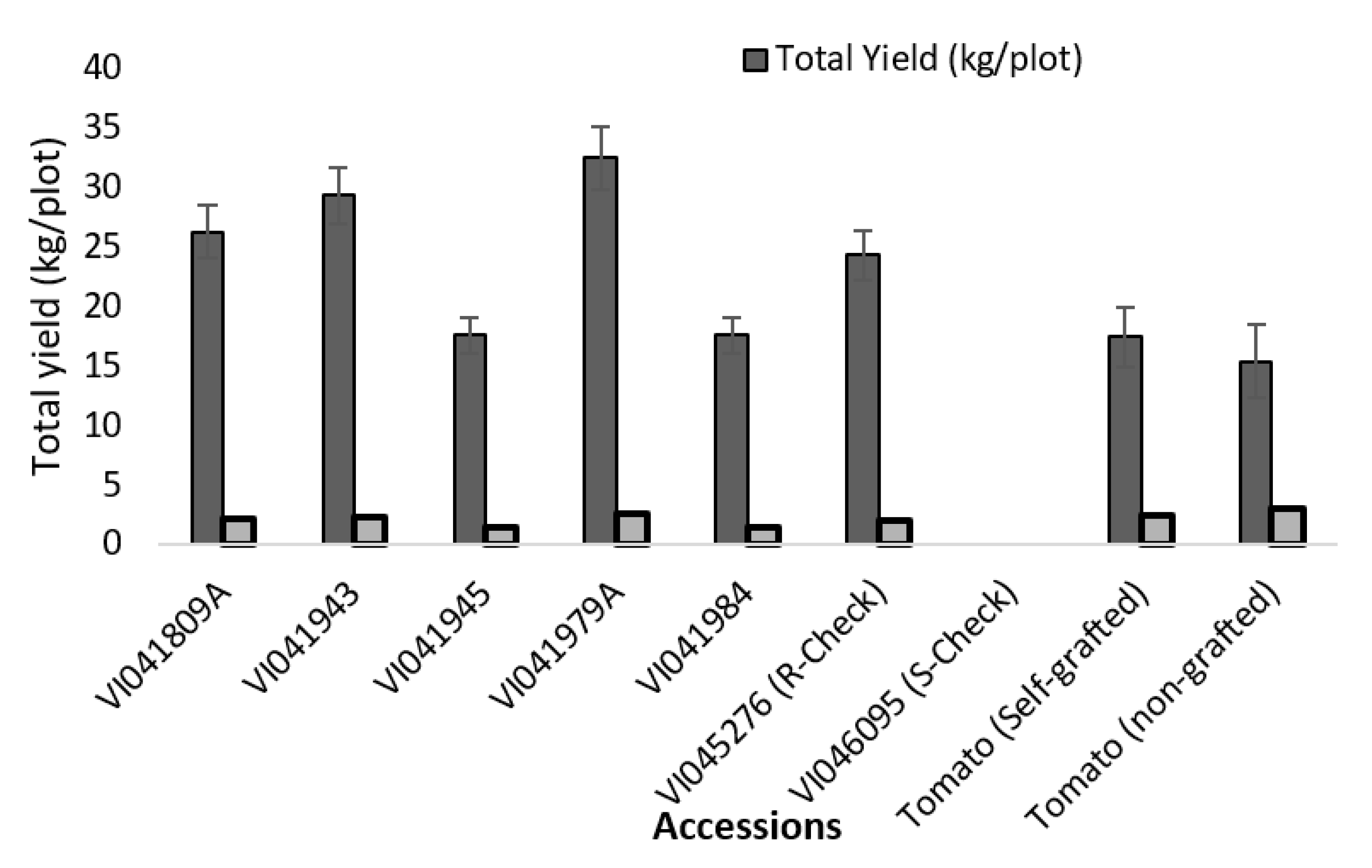
2.3. SPAD Value, Fruit Yield, Marketable Fruit Weight, Fruit Length, Fruit Width and Fruit Length to Width Ratio
2.4. Fruit Quality Parameters (pH, Soluble Solid, Acidity, Color, β-Carotene, Lycopene, Antioxidant Activity)
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Experimental Location
4.2. Seedling Production, Grafting and Pathogen Inoculation
4.3. Experimental Design
4.4. Graft Compatibility
4.5. Wilting Percentage (W%) and Disease Index (DI)
4.6. Chlorophyll Content
4.7. Fruit Yield
4.8. Fruit Quality
Sample Preparation
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- García-Alonso, F.J.; Bravo, S.; Casas, J.; Pérez-Conesa, D.; Jacob, K.; Periago, M.J. Changes in Antioxidant Compounds during the Shelf Life of Commercial Tomato Juices in Different Packaging Materials. J. Agric. Food Chem. 2009, 57, 6815–6822. [Google Scholar] [CrossRef]
- Perveen, R.; Suleria, H.A.R.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (Solanum lycopersicum) Carotenoids and Lycopenes Chemistry; Metabolism, Absorption, Nutrition, and Allied Health Claims—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef]
- Weinberger, K.M.; Lumpkin, T.A. Horticulture for Poverty Alleviation—The Unfunded Revolution. AVRDC Working Paper No. 15. SSRN J. 2005, 15, 28. [Google Scholar] [CrossRef]
- Fan, S.; Brzeska, J.; Keyzer, M.; Halsema, A. From Subsistence to Profit: Transforming Smallholder Farms; Food Policy Report; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2013; p. 30. [Google Scholar] [CrossRef]
- Schreinemachers, P.; Wu, M.; Uddin, M.N.; Ahmad, S.; Hanson, P. Farmer Training in Off-Season Vegetables: Effects on Income and Pesticide Use in Bangladesh. Food Policy 2016, 61, 132–140. [Google Scholar] [CrossRef]
- Hanssen, I.M.; Lapidot, M.; Thomma, B.P.H.J. Emerging Viral Diseases of Tomato Crops. MPMI 2010, 23, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging Virus Diseases Transmitted by Whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.C. Biology and Epidemiology of Bacterial Wilt Caused by Pseudomonas Solanacearum. Annu. Rev. Phytopathol. 1991, 29, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 Plant Pathogenic Bacteria in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Fry, W. Phytophthora Infestans: The Plant (AndRgene) Destroyer. Mol. Plant Pathol. 2008, 9, 385–402. [Google Scholar] [CrossRef]
- Nowicki, M.; Foolad, M.R.; Nowakowska, M.; Kozik, E.U. Potato and Tomato Late Blight Caused by Phytophthora Infestans: An Overview of Pathology and Resistance Breeding. Plant Dis. 2012, 96, 4–17. [Google Scholar] [CrossRef]
- Boncato, T.; Ellamar, J. Off-season tomato production: A new technology in Tarlac province of Philippines. Acta Hortic. 2015, 1086, 261–267. [Google Scholar] [CrossRef]
- Smith, E.F. A Bacterial Disease of the Tomato, Eggplant, and Irish Potato (Bacillus solanacearum nov. sp.); Bulletin (United States. Division of Vegetable Physiology and Pathology),G.P.O.: Washington, DC, USA, 1896; p. 26. [Google Scholar]
- Adebayo, O.S. Control of bacterial wilt disease of tomato: A review of research efforts in Nigeria. Acta Hortic. 2011, 914, 35–37. [Google Scholar] [CrossRef]
- Kumar, M.; Srinivasa, V.; Kumari, M. Screening of Tomato Line/Varieties for Bacterial Wilt (Ralstonia solanacearum) Resistance in Hill Zone of Karnataka, India. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 1451–1455. [Google Scholar]
- Elphinstone, J.G. The current bacterial wilt situation: A global overview. In Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex; Allen, C., Prior, P., Hayward, A.C., Eds.; APS Press: St. Paul, MN, USA, 2005; pp. 9–28. [Google Scholar]
- Peeters, N.; Guidot, A.; Vailleau, F.; Valls, M. Ralstonia Solanacearum, a Widespread Bacterial Plant Pathogen in the Post-Genomic Era. Mol. Plant Pathol. 2013, 14, 651–662. [Google Scholar] [CrossRef]
- Fegan, M.; Prior, P. How complex is the Ralstonia solanacearum species complex? In Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex; Allen, C., Prior, P., Hayward, A.C., Eds.; APS Press: St. Paul, MN, USA, 2005; pp. 449–461. [Google Scholar]
- Hayward, A.C.; Pegg, K.G. Bacterial Wilt of Ginger in Queensland: Reappraisal of a Disease Outbreak. Australas. Plant Pathol. 2013, 42, 235–239. [Google Scholar] [CrossRef]
- Prior, P.; Fegan, M. Recent developments in the phylogeny and classification of Ralstonia solanacearum. Acta Hortic. 2005, 695, 127–136. [Google Scholar] [CrossRef]
- Fegan, M.; Prior, P. Diverse members of the Ralstonia solanacearum species complex cause bacterial wilts of banana. Australas. Plant Pathol. 2006, 35, 93–101. [Google Scholar] [CrossRef]
- Safni, I.; Cleenwerck, I.; De Vos, P.; Fegan, M.; Sly, L.; Kappler, U. Polyphasic Taxonomic Revision of the Ralstonia Solanacearum Species Complex: Proposal to Emend the Descriptions of Ralstonia Solanacearum and Ralstonia Syzygii and Reclassify Current, R. Syzygii Strains as Ralstonia Syzygii Subsp. Syzygii Subsp. Nov., R. Solanacearum Phylotype IV Strains as Ralstonia Syzygii Subsp. Indonesiensis Subsp. Nov., Banana Blood Disease Bacterium Strains as Ralstonia Syzygii Subsp. Celebesensis Subsp. Nov. and R. Solanacearum Phylotype I and III Strains as Ralstonia Pseudosolanacearum Sp. Nov. Int. J. Sys. Evol. Microbiol. 2014, 64 Pt 9, 3087–3103. [Google Scholar] [CrossRef]
- Huet, G. Breeding for Resistances to Ralstonia Solanacearum. Front. Plant Sci. 2014, 5, 715. [Google Scholar] [CrossRef]
- Pradhanang, P.M.; Ji, P.; Momol, M.T.; Olson, S.M.; Mayfield, J.L.; Jones, J.B. Application of Acibenzolar-S-Methyl Enhances Host Resistance in Tomato Against Ralstonia Solanacearum. Plant Dis. 2005, 89, 989–993. [Google Scholar] [CrossRef]
- Fujiwara, A.; Fujisawa, M.; Hamasaki, R.; Kawasaki, T.; Fujie, M.; Yamada, T. Biocontrol of Ralstonia Solanacearum by Treatment with Lytic Bacteriophages. Appl. Environ. Microbiol. 2011, 77, 4155–4162. [Google Scholar] [CrossRef] [PubMed]
- Keatinge, J.D.H.; Lin, L.-J.; Ebert, A.W.; Chen, W.Y.; Hughes, J.A.; Luther, G.C.; Wang, J.-F.; Ravishankar, M. Overcoming Biotic and Abiotic Stresses in the Solanaceae through Grafting: Current Status and Future Perspectives. Biol. Agric. Hortic. 2014, 30, 272–287. [Google Scholar] [CrossRef]
- Wenneker, M.; Verdel, M.S.W.; Groeneveld, R.M.W.; Kempenaar, C.; van Beuningen, A.R.; Janse, J.D. Ralstonia (Pseudomonas) solanacearum race 3 (biovar 2) in surface water and natural weed hosts: First report on stinging nettle (Urtica dioica). Eur. J. Plant Pathol. 1999, 105, 307–315. [Google Scholar] [CrossRef]
- Murakoshi, S.; Takahashi, M. Trials of some control of tomato bacterial wilt caused by Pseudomonas solanacearum. Bull. Kanagawa Hortic. Exp. Stn. 1984, 31, 50–56. [Google Scholar]
- Cardoso, S.C.; Soares, A.C.F.; dos Santos Brito, A.; dos Santos, A.P.; Laranjeira, F.F.; de Carvalho, L.A. Evaluation of Tomato Rootstocks and Its Use to Control Bacterial Wilt Disease. Sem. Ci. Agr. 2012, 33, 595–604. [Google Scholar] [CrossRef][Green Version]
- Lee, M.H.; Kim, J.K.; Lee, H.K.; Kim, K.-J.; Yu, S.-H.; Kim, Y.S.; Lee, Y.S. Reduction of Bacterial Wilt Diseases with Eggplant Rootstock EG203-Grafted Tomatoes in the Field Trials. Res. Plant Dis. 2013, 19, 108–113. [Google Scholar] [CrossRef]
- Wu, M.T.; Lin, M.W. Studies on the grafting of Solanaceae fruit vegetables. Chinese Soc. Hortic. Sci. J. 1998, 44, 160–167. [Google Scholar]
- Leonardi, C.; Giuffrida, F. Variation of plant growth and macronutrient uptake in grafted tomatoes and eggplants on three different rootstocks. Eur. J. Hortic. Sci. 2006, 71, 97–101. [Google Scholar]
- Romano, D.; Paratore, A. Effects of grafting on tomato and eggplant. Acta Hortic. 2001, 559, 149–154. [Google Scholar] [CrossRef]
- Grieneisen, M.L.; Aegerter, B.J.; Scott Stoddard, C.; Zhang, M. Yield and Fruit Quality of Grafted Tomatoes, and Their Potential for Soil Fumigant Use Reduction. A Meta-Analysis. Agron. Sustain. Dev. 2018, 38. [Google Scholar] [CrossRef]
- Higashide, T.; Nakano, A.; Yasuba, K. Yield and Dry Matter Production of a Japanese Tomato ‘Momotaro York’ Are Improved by Grafting onto a Dutch Rootstock ‘Maxifort’. J. Jpn. Soc. Hort. Sci. 2014, 83, 235–243. [Google Scholar] [CrossRef]
- Djidonou, D.; Zhao, X.; Brecht, J.K.; Cordasco, K.M. Influence of Interspecific Hybrid Rootstocks on Tomato Growth, Nutrient Accumulation, Yield, and Fruit Composition under Greenhouse Conditions. HortTechnology 2017, 27, 868–877. [Google Scholar] [CrossRef]
- Mišković, A.; Ilin, Z.; Marković, V. Effect of different rootstock type on quality and yield of tomato fruits. Acta Hortic. 2009, 807, 619–624. [Google Scholar] [CrossRef]
- Qaryouti, M.M.; Qawasmi, W.; Hamdan, H.; Edwan, M. Tomato fruit yield and quality as affected by grafting and growing system. Acta Hortic. 2007, 741, 199–206. [Google Scholar] [CrossRef]
- Poudel, S.R.; Lee, W.S. Response of eggplant (Solanum melongena L.) as rootstock for tomato (Solanum lycopersicum L.). Hortic. NCHU 2009, 34, 39–52. [Google Scholar]
- Miguel, A.; Marsal, J.I.; Goto, R.; San Bautista, A.; López-Galarza, S.; Pascual, B.; Maroto, J.V. Improving the affinity of tomato grafted on Solanum torvum using an intermediate rootstock. Acta Hortic. 2011, 898, 291–295. [Google Scholar] [CrossRef]
- Flores, F.B.; Sanchez-Bel, P.; Estañ, M.T.; Martinez-Rodriguez, M.M.; Moyano, E.; Morales, B.; Campos, J.F.; Garcia-Abellán, J.O.; Egea, M.I.; Fernández-Garcia, N.; et al. The Effectiveness of Grafting to Improve Tomato Fruit Quality. Sci. Hortic. 2010, 125, 211–217. [Google Scholar] [CrossRef]
- Khah, E.M.; Kakava, E.; Mavromatis, A.; Chachalis, D.; Goulas, C. Effect of Grafting on Growth and Yield of Tomato (Lycopersicon Esculentum Mill.) in Greenhouse and Open-Field. J. Appl. Hortic. 2006, 8, 3–7. [Google Scholar] [CrossRef]
- Mohammed, S.M.T.; Humidan, M.; Boras, M.; Abdalla, O.A. Effect of Grafting Tomato on Different Rootstocks on Growth and Productivity under Glasshouse Conditions. Asian J. Agric. Res. 2009, 3, 47–54. [Google Scholar] [CrossRef]
- Turhan, A.; Ozmen, N.; Serbeci, M.S.; Seniz, V. Effects of Grafting on Different Rootstocks on Tomato Fruit Yield and Quality. Hort. Sci. 2011, 38, 142–149. [Google Scholar] [CrossRef]
- Neocleous, D. Yield, Nutrients, and Antioxidants of Tomato in Response to Grafting and Substrate. Int. J. Veg. Sci. 2010, 16, 212–221. [Google Scholar] [CrossRef]
- Vrcek, V.I.; Samobor, V.; Bojic, M.; Medic-Saric, M.; Vukobratovic, M.; Erhatic, R.; Horvat, D.; Matotan, Z. The Effect of Grafting on the Antioxidant Properties of Tomato (Solanum lycopersicum L.). Span. J. Agric. Res. 2011, 9, 844. [Google Scholar] [CrossRef]
- Arwiyanto, T.; Nurcahyanti, S.D.; Indradewa, D.; Widada, J. Grafting local commercial tomato cultivars with H-7996 and EG-203 to suppress bacterial wilt (Ralstonia solanacearum) in Indonesia. Acta Hortic. 2015, 1069, 173–178. [Google Scholar] [CrossRef]
- Namisy, A.; Chen, J.-R.; Prohens, J.; Metwally, E.; Elmahrouk, M.; Rakha, M. Screening Cultivated Eggplant and Wild Relatives for Resistance to Bacterial Wilt (Ralstonia solanacearum). Agriculture 2019, 9, 157. [Google Scholar] [CrossRef]
- Nordey, T.; Deletre, E.; Mlowe, N.; Martin, T. Small Mesh Nets Protect Tomato Plants from Insect Pests and Increase Yields in Eastern Africa. J. Hortic. Sci. Biotechnol. 2019, 95, 222–228. [Google Scholar] [CrossRef]
- Black, L.L.; Wu, D.L.; Wang, J.F.; Kalb, T.; Abbass, D.; Chen, J.H. Grafting Tomatoes for Production in the Hot-Wet Season; International Cooperators’ Guide; AVRDC Publication number 03-551; Asian Vegetable Research & Development Center AVRDC: Tainan, Taiwan, 2003; p. 6. Available online: https://worldveg.tind.io/record/39571 (accessed on 26 May 2020).
- Jaunet, T.X.; Wang, J.-F. Variation in Genotype and Aggressiveness of Ralstonia Solanacearum Race 1 Isolated from Tomato in Taiwan. Phytopathology 1999, 89, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.M.; Licardo, O.; Hanudin; Wang, J.-F.; Chen, J. Diallel Analysis of Bacterial Wilt Resistance in Tomato Derived from Different Sources. Plant Dis. 1998, 82, 74–78. [Google Scholar] [CrossRef]
- Kado, C.I. Selective Media for Isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas, and Xanthomonas. Phytopathology 1970, 60, 969. [Google Scholar] [CrossRef]
- Winstead, N.N.; Kelman, A. Inoculation techniques for evaluating resistance to Pseudomonas solanacearum. Phytopathology 1952, 42, 628–634. [Google Scholar]
- AVRDC. Preliminary Screening of Resistance to Bacterial Wilt in Eggplant; AVRDC 1993 Progress Report; AVRDC: Tainan, Taiwan, 1994; pp. 96–98. Available online: http://worldveg.tind.io/record/20396 (accessed on 26 May 2020).
- Hanson, P.M.; Yang, R.; Wu, J.; Chen, J.; Ledesma, D.; Tsou, S.C.S.; Lee, T.-C. Variation for Antioxidant Activity and Antioxidants in Tomato. J. Am. Soc. Hortic. Sci. 2004, 129, 704–711. [Google Scholar] [CrossRef]
- Ryan, J.J.; Dupont, J.A. Identification and Analysis of the Major Acids from Fruit Juices and Wines. J. Agric. Food Chem. 1973, 21, 45–49. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
| Accession | Graft Compatibility (%) * | Wilting (%) | Disease Index (%) | Field Survival (out of 12 Plants) | ||||
|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | |
| VI041809A | 100 a | 100 | 2.1 c | 10.4 c | 2.1 b | 10.4 b | 11.8 a | 9.5 a |
| VI041943 | 100 a | 100 | 0.0 c | 5.8 c | 0.0 b | 8.3 b | 12.0 a | 10.8 a |
| VI041945 | 93 b | 100 | 0.0 c | 14.6 c | 0.0 b | 16.7 b | 12.0 a | 5.5 abc |
| VI041979A | 96 ab | 100 | 2.1 c | 7.9 c | 0.8 b | 10.4 b | 12.0 a | 9.0 ab |
| VI041984 | 99 ab | 100 | 2.1 c | 20.0 c | 2.1 b | 20.8 b | 11.8 a | 8.8 ab |
| VI045276 (R-Check) | 94 ab | 100 | 4.2 c | 7.5 c | 4.2 b | 8.3 b | 11.5 a | 8.5 ab |
| VI046095 (S-Check) | 99 ab | 100 | 100.0 a | 87.5 a | 100.0 a | 91.7 a | 0.0 c | 0.0 c |
| Tomato (Self-grafted) | 100 a | 100 | 50.0 b | 100.0 a | 43.8 a | 100.0 a | 7.0 b | 2.0 c |
| Tomato (non-grafted) | - | - | 72.9 b | 97.5 a | 63.3 a | 100.0 a | 5.0 b | 2.3 bc |
| Accession | SPAD Value 2018 | Marketable Fruit/Plot a 2018 | Mean Fruit Weight (g) | Mean Fruit Length (mm) | Mean Fruit Width (mm) | Fruit (L:W) | Total Yield (kg/plot) | Total Yield (kg/plot) | |
|---|---|---|---|---|---|---|---|---|---|
| Weight (kg) | Number of Fruits/Plots | 2018 | 2018 | 2018 | 2018 | 2018 | 2019 | ||
| VI041809A | 45.78 ± 2.02 | 17.62 ± 2.65 | 280 ± 42 | 62.9 ± 0.65 a | 54.4 ± 1.69 a | 47.6 ± 1.29 | 0.88 | 26.29 ± 5.37 ab | 0.71 ± 0.71 |
| VI041943 | 46.93 ± 0.63 | 17.07 ± 2.38 | 285 ± 35 | 59.9 ± 1.86 ab | 53.9 ± 1.14 a | 47.5 ± 1.76 | 0.88 | 29.36 ± 5.24 a | 1.27 ± 0.87 |
| VI041945 | 47.85 ± 2.68 | 11.42 ± 2.45 | 226 ± 28 | 50.5 ± 5.19 bc | 48.3 ± 2.64 b | 44.5 ± 2.51 | 0.92 | 17.61 ± 2.9 bc | 1.01 ± 0.48 |
| VI041979A | 45.03 ± 1.2 | 19.32 ± 3.20 | 318 ± 53 | 60.9 ± 0.71 a | 53.3 ± 1.28 ab | 47.5 ± 2.07 | 0.89 | 32.49 ± 41 a | 0.65 ± 0.33 |
| VI041984 | 45.57 ± 1.67 | 11.24 ± 0.97 | 235 ± 15 | 47.8 ± 1.08 c | 51.3 ± 3.62 ab | 45.1 ± 2.75 | 0.88 | 17.66 ± 2.43 bc | 0.92 ± 0.58 |
| VI045276 (R-Check) | 48.04 ± 1.94 | 15.80 ± 2.84 | 284 ± 40 | 55.5 ± 5.97 abc | 53.6 ± 1.65 ab | 46.7 ± 1.25 | 0.87 | 24.33 ± 4.04 abc | 0.83 ± 0.52 |
| VI046095 (S-Check) | - | 0.0 ± 0.00 | - | - | - | - | - | 0.0 ± 0.00 d | 0.29 ± 0.58 |
| Tomato (Self-grafted) | 46.28 ± 1.53 | 8.45 ± 4.35 | 136 ± 75 | 64.3 ±6.45 a | 55.1 ± 2.36 a | 48.3 ± 0.79 | 0.88 | 17.49 ± 7.77 bc | 0.00 ± 00 |
| Tomato (non-grafted) | 49.03 ± 2.55 | 8.25 ± 2.71 | 131 ± 47 | 63.6 ±6.77 a | 53.9 ± 2.88 a | 47.8 ± 2.08 | 0.89 | 15.44 ± 4.69 c | 0.07 ± 0.13 |
| Accession | pH * | SS (°Brix) | Acidity (% Citric Acid) | Color (a/b) | β-Carotene (mg/100g) | Lycopene (mg/100g) | AA (μmole TE/100g) |
|---|---|---|---|---|---|---|---|
| VI041809A | 4.14 a | 4.95 b | 0.32 b | 1.25 | 0.49 | 5.71 | 326.15 b |
| VI041943 | 4.10 ab | 5.00 b | 0.34 ab | 1.25 | 0.53 | 5.93 | 350.35 b |
| VI041945 | 4.04 b | 6.15 a | 0.36 ab | 1.25 | 0.52 | 6.57 | 437.67 a |
| VI041979A | 4.16 a | 4.90 b | 0.33 ab | 1.28 | 0.43 | 5.82 | 320.25 b |
| VI041984 | 4.11 ab | 6.10 a | 0.37 ab | 1.28 | 0.53 | 6.97 | 393.77 ab |
| VI045276 | 4.10 ab | 5.30 b | 0.35 ab | 1.26 | 0.54 | 6.01 | 389.85 ab |
| VI046095 | - | - | - | - | - | - | - |
| Self-grafted Tomato | 4.17 a | 4.80 b | 0.35 ab | 1.25 | 0.48 | 6.22 | 382.71 ab |
| Non-grafted Tomato | 4.16 a | 5.00 b | 0.40 a | 1.18 | 0.48 | 5.21 | 379.36 ab |
| WorldVeg Accession Number or Cultivar Name | Rootstock/Scion | Species | Character | Origin |
|---|---|---|---|---|
| VI041809A | Rootstock | Solanum melongena | BW resistant | India |
| VI041943 | Rootstock | S. melongena | BW resistant | India |
| VI041945 | Rootstock | S. melongena | BW resistant | India |
| VI041979A | Rootstock | S. melongena | BW resistant | India |
| VI041984 | Rootstock | S. melongena | BW resistant | India |
| VI045276 (EG203) | Rootstock | S. melongena | BW Resistant check | India |
| VI046095 (EG048) | Rootstock | S. melongena | BW Susceptible check | Denmark |
| Victoria | Scion | Solanum lycopersicum | Fresh market Tomato | Taiwan |
| TStarE | Scion | S. lycopersicum | Fresh market Tomato | Taiwan |
| Season | First Season | Second Season |
|---|---|---|
| Transplanting date | 19 October 2018 | 1 April 2019 |
| Last harvest date | 30 January 2019 | 30 June 2019 |
| Total duration days | 104 | 91 |
| Average Temperature (°C) | 21.5 ± 2.6 | 26.4 ± 2.4 |
| T Max (°C) | 27.6 ± 3.0 | 31.4 ± 3.0 |
| T Min (°C) | 17.7 ± 2.8 | 23.2 ± 2.3 |
| RH Max (%) | 82.5 ± 7.0 | 88.1 ± 5.5 |
| RH Min (%) | 54.3 ± 10.0 | 64.7 ± 12.6 |
| Precipitation (mm) | 5 ± 0.2 | 2404.8 ± 15.3 |
| Season | Winter/dry | Summer/rainy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manickam, R.; Chen, J.-R.; Sotelo-Cardona, P.; Kenyon, L.; Srinivasan, R. Evaluation of Different Bacterial Wilt Resistant Eggplant Rootstocks for Grafting Tomato. Plants 2021, 10, 75. https://doi.org/10.3390/plants10010075
Manickam R, Chen J-R, Sotelo-Cardona P, Kenyon L, Srinivasan R. Evaluation of Different Bacterial Wilt Resistant Eggplant Rootstocks for Grafting Tomato. Plants. 2021; 10(1):75. https://doi.org/10.3390/plants10010075
Chicago/Turabian StyleManickam, Ravishankar, Jaw-Rong Chen, Paola Sotelo-Cardona, Lawrence Kenyon, and Ramasamy Srinivasan. 2021. "Evaluation of Different Bacterial Wilt Resistant Eggplant Rootstocks for Grafting Tomato" Plants 10, no. 1: 75. https://doi.org/10.3390/plants10010075
APA StyleManickam, R., Chen, J.-R., Sotelo-Cardona, P., Kenyon, L., & Srinivasan, R. (2021). Evaluation of Different Bacterial Wilt Resistant Eggplant Rootstocks for Grafting Tomato. Plants, 10(1), 75. https://doi.org/10.3390/plants10010075







