Metabolic Engineering of Isoflavonoid Biosynthesis by Expressing Glycine max Isoflavone Synthase in Allium cepa L. for Genistein Production
Abstract
1. Introduction
2. Results
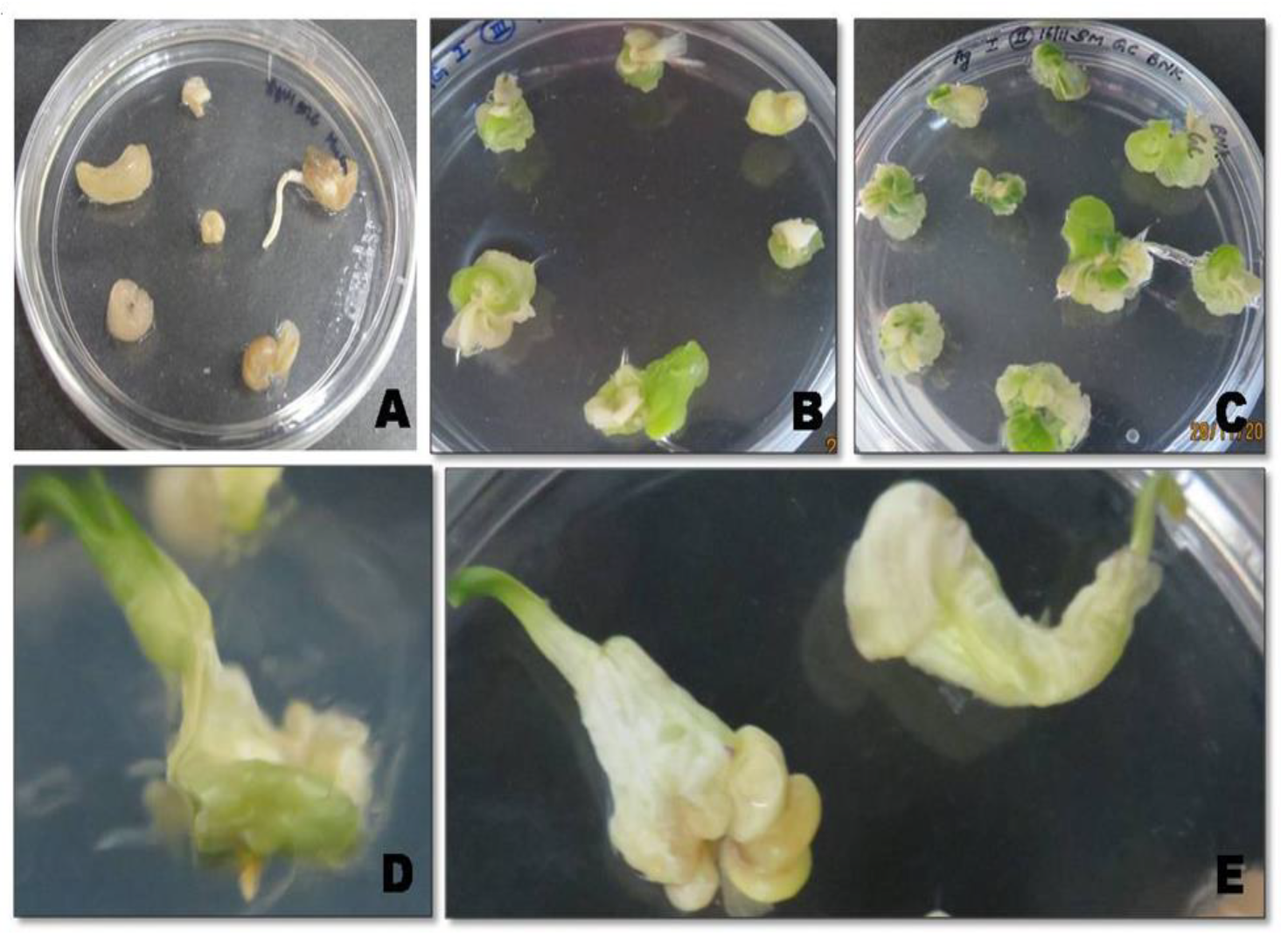
2.1. In Vitro Propagation and Genetic Transformation of Onion Calli
2.2. Molecular Characterization of Transgenic Onion Calli
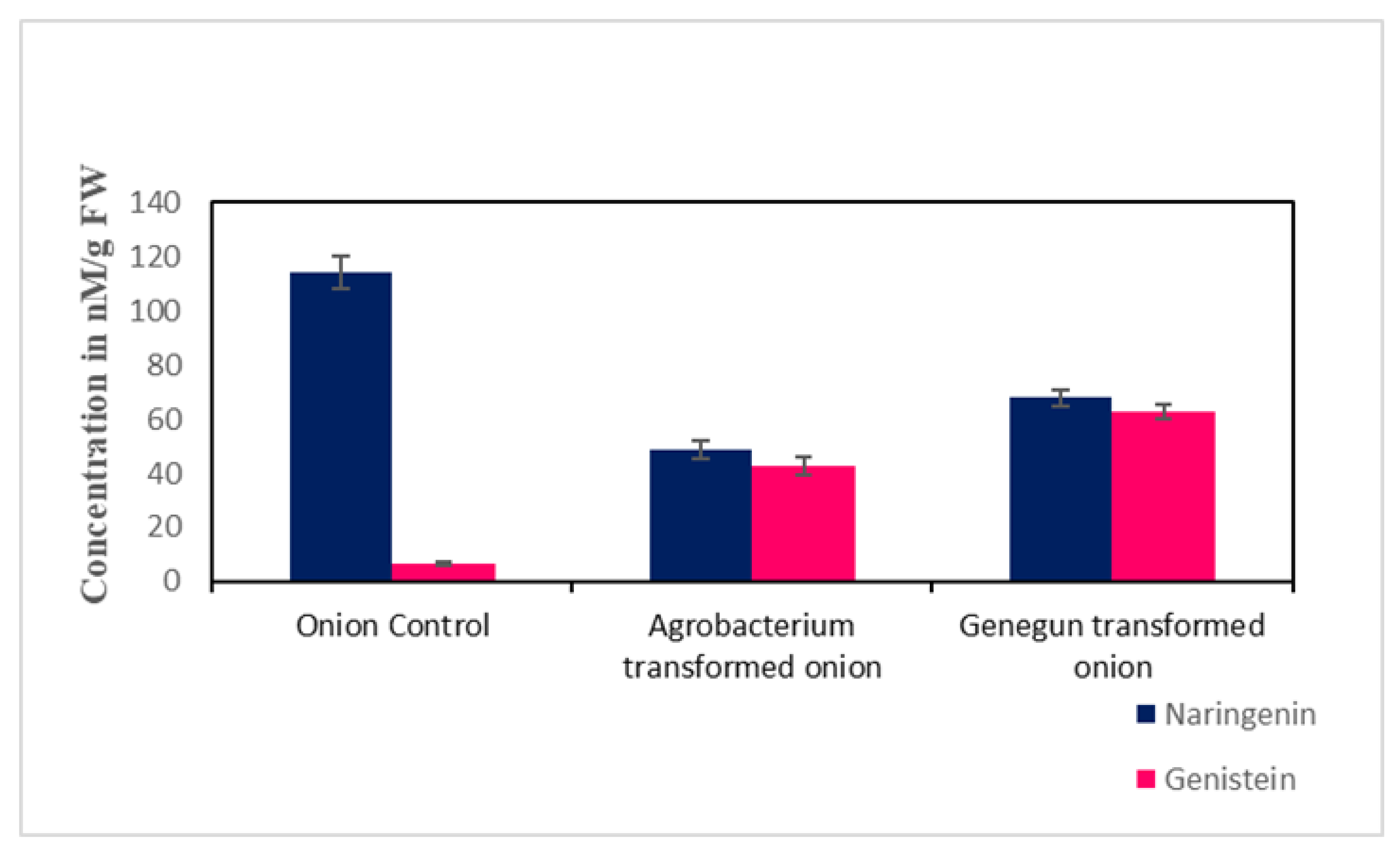
2.3. Metabolite HPLC Analysis
3. Discussion
4. Materials and Methods
4.1. Construction of Plant Expression Vector
4.2. In Vitro Culture and Agrobacterium-Mediated Genetic Transformation of Onion
4.3. Biolistic-Mediated Genetic Transformation
4.4. Screening of Transformants
4.5. Metabolite Analysis of Transgenic Onion Extracts
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAP | Benzyl Amino Purine |
| CaMV | Cauliflower Mosaic Virus |
| cDNA | Complementary Deoxyribonucleic acid |
| FW | Fresh Weight |
| GC-MS Gas | Chromatography-Mass Spectroscopy |
| GmIFS | Glycine max Isoflavone synthase |
| HPLC | High Performance Liquid Chromatography |
| IFS | Isoflavone synthase |
| KIN | Kinetin |
| LB | Luria-Bertani Broth |
| mg L-1 | Milligram per litre |
| MN | Macheray Nagel |
| MS | Murashige and Skoog medium |
| MS + B5 | MS Gamborg B5 medium |
| NAA | Naphthalene Acetic Acid |
| nm | Nanometer |
| nM | Nanomolar |
| PCR | Polymerase Chain Reaction |
| Psi | Pound per square inch |
| RNA | Ribonucleic acid |
| rpm | Revolution per min |
| YENB | Yeast Extract Nutrient Broth |
| μg | Microgram |
| μL | Microlitre |
| μM | Micromolar |
References
- Agarwal, R.K.; Dewar, H.A.; Newell, D.J.; Das, B. Controlled trial of the effect of cycloalliin on the fibrinolytic activity of venous blood. Atherosclerosis 1977, 27, 347–351. [Google Scholar] [CrossRef]
- Kalus, U.; Pindur, G.; Jung, F.; Mayer, B.; Radtke, H.; Bachmann, K.; Mrowietz, C.; Koscielny, J.; Kiesewetter, H. Influence of the Onion as an Essential Ingredient of the Mediterranean Diet on Arterial Blood Pressure and Blood Fluidity. Arzneimittelforschung 2000, 50, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Ashwini, M.; Sathishkumar, R. Onion (Allium cepa)—Ethnomedicinal and Therapeutic Properties. In Handbook of Medicinal Plants and Their Bioactive Compounds; Research Signpost: Kerala, India, 2014. [Google Scholar]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Butt, M.S.; Anjum, F.M.; Saeed, F.; Khalid, N. Onion: Nature Protection Against Physiological Threats. Crit. Rev. Food Sci. Nutr. 2015, 55, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Sreevidya, V.; Srinivasa Rao, C.; Sullia, S.; Ladha, J.K.; Reddy, P.M. Metabolic engineering of rice with soybean isoflavone synthase for promoting nodulation gene expression in rhizobia. J. Exp. Bot. 2006, 57, 1957–1969. [Google Scholar] [CrossRef]
- Jung, W.; Yu, O.; Lau, S.-M.C.; O’Keefe, D.P.; Odell, J.; Fader, G.; McGonigle, B. Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat. Biotechnol. 2000, 18, 208–212. [Google Scholar] [CrossRef]
- Yu, O.; Jung, W.; Shi, J.; Croes, R.A.; Fader, G.M.; McGonigle, B.; Odell, J.T. Production of the Isoflavones Genistein and Daidzein in Non-Legume Dicot and Monocot Tissues. Plant Physiol. 2000, 124, 781–794. [Google Scholar] [CrossRef]
- Li, X.; Qin, J.-C.; Wang, Q.-Y.; Wu, X.; Lang, C.-Y.; Pan, H.-Y.; Gruber, M.Y.; Gao, M.-J. Metabolic engineering of isoflavone genistein in Brassica napus with soybean isoflavone synthase. Plant Cell Rep. 2011, 30, 1435–1442. [Google Scholar] [CrossRef]
- Shih, C.-H.; Chen, Y.; Wang, M.; Chu, I.K.; Lo, C. Accumulation of Isoflavone Genistin in Transgenic Tomato Plants Overexpressing a Soybean Isoflavone Synthase Gene. J. Agric. Food Chem. 2008, 56, 5655–5661. [Google Scholar] [CrossRef]
- Malla, A.; Srinivasan, B.; Shanmugaraj, B.M.; Ramalingam, S. Micropropagation and DNA delivery studies in onion cultivars of Bellary, CO3. J. Crop Sci. Biotechnol. 2015, 18, 37–43. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Kokkalou, E.; Georgarakis, M.; Niopas, I. Validated high-performance liquid chromatographic method utilizing solid-phase extraction for the simultaneous determination of naringenin and hesperetin in human plasma. J. Chromatogr. B 2004, 801, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Malla, A.; Ramalingam, S. Chapter 11—Health Perspectives of an Isoflavonoid Genistein and its Quantification in Economically Important Plants. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 353–379. [Google Scholar] [CrossRef]
- Li, X.-Q.; Liu, C.-N.; Ritchie, S.W.; Peng, J.-Y.; Gelvin, S.B.; Hodges, T.K. Factors influencing Agrobacterium-mediated transient expression of gusA in rice. Plant Mol. Biol. 1992, 20, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Maiwald, F.; Lorenzen, P.; Steinbiss, H.-H. Factors Influencing T-DNA Transfer into Wheat and Barley Cells by Agrobacterium Tumefaciens. Cereal Res. Commun. 1998, 26, 15–22. [Google Scholar] [CrossRef]
- Godwin, I.; Todd, G.; Ford-Lloyd, B.; Newbury, H.J. The effects of acetosyringone and pH on Agrobacterium-mediated transformation vary according to plant species. Plant Cell Rep. 1991, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hooykaas, P.J.J. Transformation of plant cells via Agrobacterium. Plant Mol. Biol. 1989, 13, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Yokoi, S.; Toriyama, K.; Hinata, K. Transgenic plant production mediated by Agrobacterium in Indica rice. Plant Cell Rep. 1996, 15, 727–730. [Google Scholar] [CrossRef]
- Arencibia, A.D.; Carmona, E.R.; Tellez, P.; Chan, M.-T.; Yu, S.-M.; Trujillo, L.E.; Oramas, P. An efficient protocol for sugarcane (Saccharum spp. L.) transformation mediated by Agrobacterium tumefaciens. Transgenic Res. 1998, 7, 213–222. [Google Scholar] [CrossRef]
- Khanna, H.; Raina, S. Agrobacterium-mediated transformation of indica rice cultivars using binary and superbinary vectors. Funct. Plant Biol. 1999, 26, 311–324. [Google Scholar] [CrossRef]
- Cheng, M.; Lowe, B.A.; Spencer, T.M.; Ye, X.; Armstrong, C.L. Factors influencing Agrobacterium-mediated transformation of monocotyledonous species. In Vitro Cell. Dev. Biol. Plant 2004, 40, 31–45. [Google Scholar] [CrossRef]
- Hayta, S.; Smedley, M.A.; Demir, S.U.; Blundell, R.; Hinchliffe, A.; Atkinson, N.; Harwood, W.A. An efficient and reproducible Agrobacterium-mediated transformation method for hexaploid wheat (Triticum aestivum L.). Plant Methods 2019, 15, 121. [Google Scholar] [CrossRef]
- Anand, A.; Wu, E.; Li, Z.; TeRonde, S.; Arling, M.; Lenderts, B.; Mutti, J.S.; Gordon-Kamm, W.; Jones, T.J.; Chilcoat, N.D. High efficiency Agrobacterium-mediated site-specific gene integration in maize utilizing the FLP-FRT recombination system. Plant Biotechnol. J. 2019, 17, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Dönmez, B.A.; Dangol, S.; Bakhsh, A. Transformation Efficiency of Five Agrobacterium Strains in Diploid and Tetraploid Potatoes. Sarhad J. Agric. 2019, 35, 1344–1350. [Google Scholar] [CrossRef]
- Branca, F.; Lorenzetti, S. Health Effects of Phytoestrogens. Forum Nutr. 2005, 57, 100–111. [Google Scholar] [CrossRef]
- Liu, C.-J.; Blount, J.W.; Steele, C.L.; Dixon, R.A. Bottlenecks for metabolic engineering of isoflavone glycoconjugates in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 14578–14583. [Google Scholar] [CrossRef]
- Liu, R.; Hu, Y.; Li, J.; Lin, Z. Production of soybean isoflavone genistein in non-legume plants via genetically modified secondary metabolism pathway. Metab. Eng. 2007, 9, 1–7. [Google Scholar] [CrossRef]
- Graham, T.L. Flavonoid and isoflavonoid distribution in developing soybean seedling tissues and in seed and root exudates. Plant Physiol. 1991, 95, 594–603. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malla, A.; Shanmugaraj, B.; Srinivasan, B.; Sharma, A.; Ramalingam, S. Metabolic Engineering of Isoflavonoid Biosynthesis by Expressing Glycine max Isoflavone Synthase in Allium cepa L. for Genistein Production. Plants 2021, 10, 52. https://doi.org/10.3390/plants10010052
Malla A, Shanmugaraj B, Srinivasan B, Sharma A, Ramalingam S. Metabolic Engineering of Isoflavonoid Biosynthesis by Expressing Glycine max Isoflavone Synthase in Allium cepa L. for Genistein Production. Plants. 2021; 10(1):52. https://doi.org/10.3390/plants10010052
Chicago/Turabian StyleMalla, Ashwini, Balamurugan Shanmugaraj, Balamurugan Srinivasan, Ashutosh Sharma, and Sathishkumar Ramalingam. 2021. "Metabolic Engineering of Isoflavonoid Biosynthesis by Expressing Glycine max Isoflavone Synthase in Allium cepa L. for Genistein Production" Plants 10, no. 1: 52. https://doi.org/10.3390/plants10010052
APA StyleMalla, A., Shanmugaraj, B., Srinivasan, B., Sharma, A., & Ramalingam, S. (2021). Metabolic Engineering of Isoflavonoid Biosynthesis by Expressing Glycine max Isoflavone Synthase in Allium cepa L. for Genistein Production. Plants, 10(1), 52. https://doi.org/10.3390/plants10010052





