LED Light Quality of Continuous Light before Harvest Affects Growth and AsA Metabolism of Hydroponic Lettuce Grown under Increasing Doses of Nitrogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Condition
2.2. Sampling and Growth Parameter Measurements
2.3. Soluble Sugar, Nitrate and AsA Determination
2.4. Enzyme Extraction and Assay
2.5. Statistic Analysis
3. Results
3.1. Biomass Index
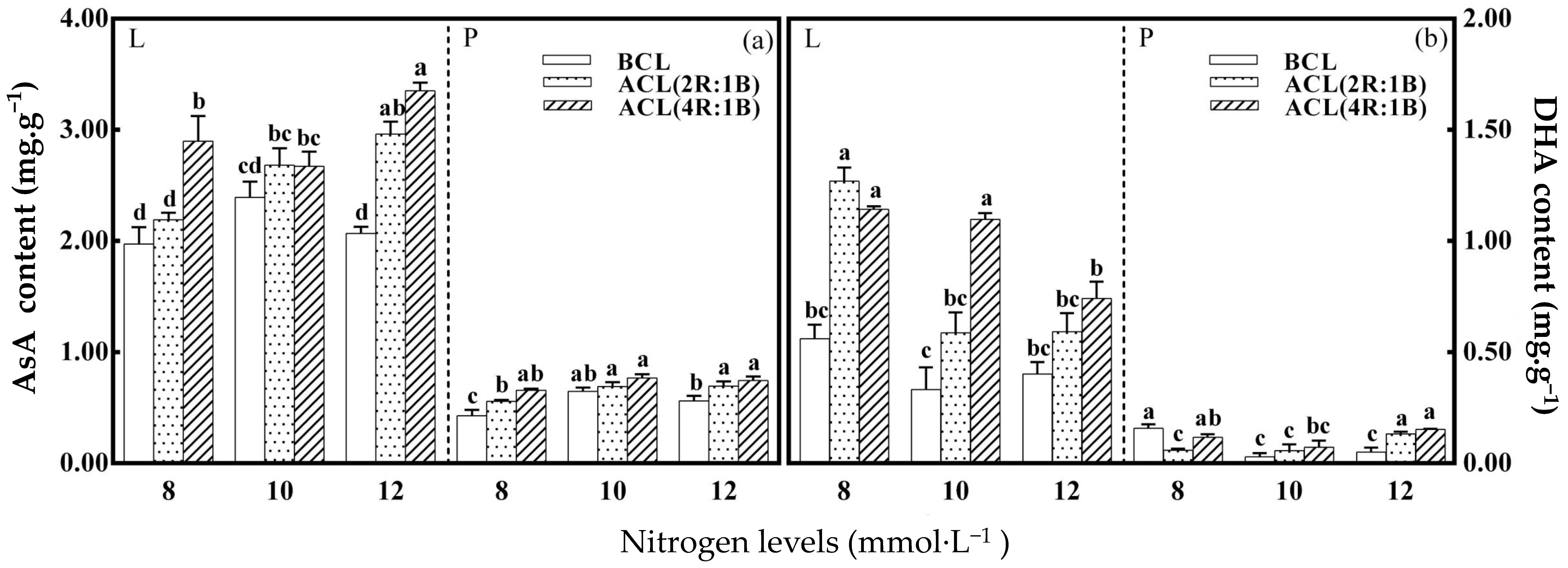
3.2. Ascorbate and Glutathione Pools
3.3. The Activity of GalLDH in The Synzyme of AsA
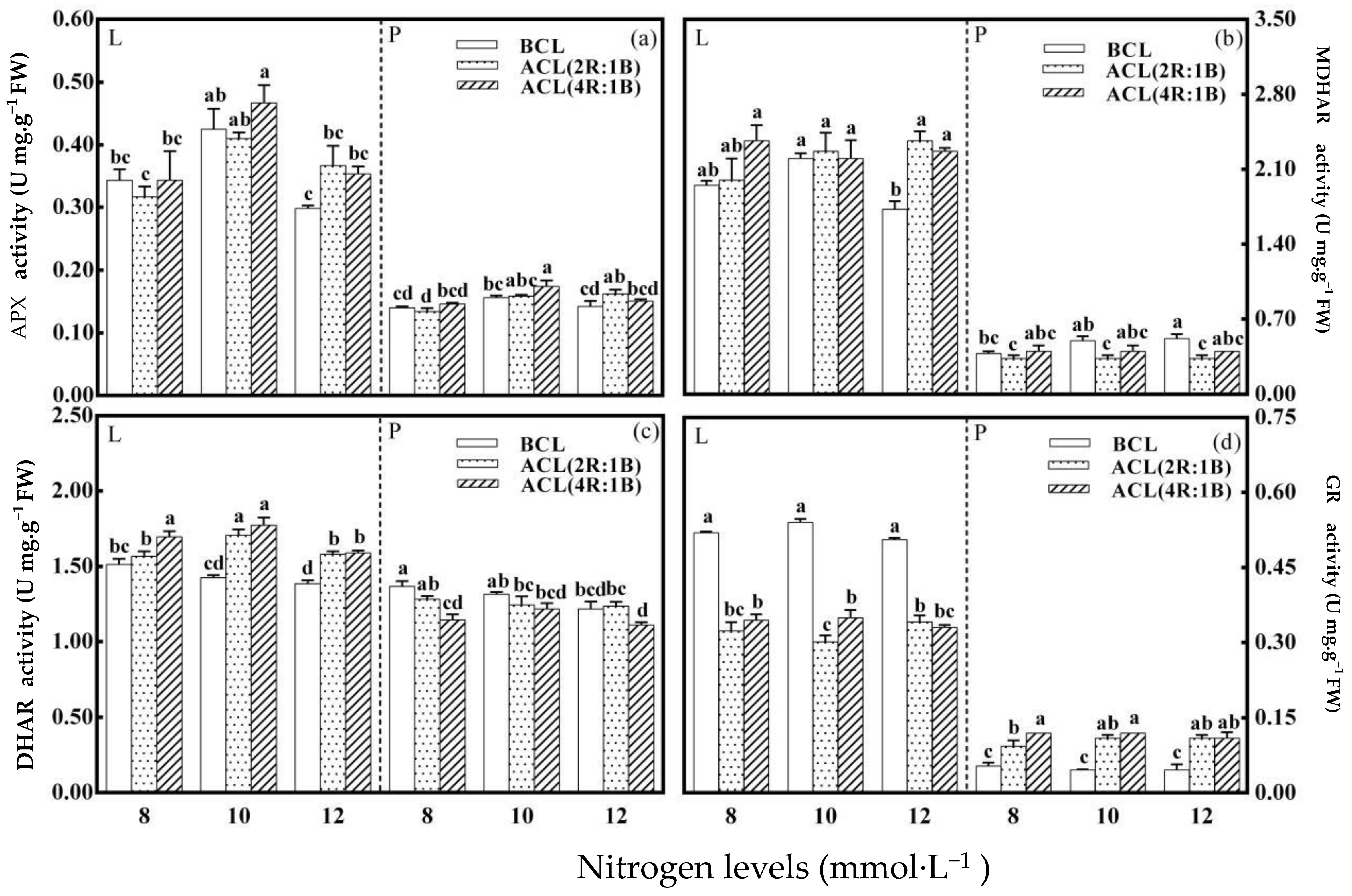
3.4. The Activity of Key Enzymes Involved in AsA Recycling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bian, Z.; Cheng, R.; Wang, Y.; Yang, Q.; Lu, C. Effect of green light on nitrate reduction and edible quality of hydroponically grown lettuce (Lactuca sativa L.) under short-term continuous light from red and blue light-emitting diodes. Environ. Exp. Bot. 2018, 153, 63–71. [Google Scholar] [CrossRef]
- Cendrero-mateo, M.P.; Carmo-silva, A.E.; Porcar-castelL, A.; Hamerlynck, E.P.; Papuga, S.A.; Moran, M.S. Dynamic response of plant chlorophyll fluorescence to light, water and nutrient availability. Funct. Plant Biol. 2015, 42, 746–757. [Google Scholar] [CrossRef]
- Zhou, W.L.; Liu, W.K.; Yang, Q.C. Reducing nitrate content in lettuce by pre-harvest continuous light delivered by red and blue light-emitting diodes. J. Plant Nutr. 2013, 36, 481–490. [Google Scholar] [CrossRef]
- Liu, W.K.; Yang, Q.C.; Wei, L.L. Light-Emitting Diodes (LEDs) and Their Applications in Protected Horticulture as Light Sources; China Agricultural Science and Technology Press: Beijing, China, 2012. (In Chinese) [Google Scholar]
- Ferrante, A.; Spinardi, A.; Maggiore, T.; Testoni, A.; Gallina, P.M. Effect of nitrogen fertilisation levels on melon fruit quality at the harvest time and during storage. Sci. Food Agric. 2008, 88, 707–713. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Xu, X.X.Y.; Lin, X.Y.; Zhang, Y.S.; Zhang, L.S.; Chen, T.T. Influence of different nitrogen levels on biomass, nitrate and oxalate accumulation in spinach. Plant Nutr. Fert. Sci. 2004, 10, 494–498. (In Chinese) [Google Scholar]
- Sorensen, J.N. Use of the Nmin-method for optimization of vegetable nitrogen nutrition. J. Acta Hortic. 1993, 339, 79–192. [Google Scholar] [CrossRef]
- Lawlor, D.W. Carbon and nitrogen assimilation in relation to yield: Mechanisms are the key to understanding production systems. J. Exp. Bot. 2002, 53, 773–787. [Google Scholar] [CrossRef]
- Sysoeva, M.; Markovskaya, E.; Shibaeva, T. Plants under continuous light: A review. Plant Stress 2010, 4, 5–17. [Google Scholar]
- Velez-ramirez, A.I.; Ieperen, W.V.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Murage, E.N.; Watashiro, N.; Masuda, M. Influence of light quality, PPFD and temperature on leaf chlorosis of eggplants grown under continuous illumination. Sci. Hortic. 1997, 68, 73–82. [Google Scholar] [CrossRef]
- Zhou, W.L.; Liu, W.K.; Yang, Q.C. Quality changes in hydroponic lettuce grown under pre-harvest short-duration continuous light of different intensities. J. Hortic. Sci. Biotechnol. 2012, 87, 429–434. [Google Scholar] [CrossRef]
- Ohyama, K.; Manabe, K.; Omura, Y.; Kozai, T.; Kubota, C. Potential use of a 24-hour photoperiod (continuous light) with alternating air temperature for production of tomato plug transplants in a closed system. Hortic. Sci. 2005, 40, 374–377. [Google Scholar] [CrossRef]
- Su, W.J. Optimal Proportion Selection of Nutrient Solution and Quality Adjustment of Hydroponic Lettuce. Master’s Thesis, Northwest AF University, Xianyang China, 2016. (In Chinese). [Google Scholar]
- Plumb, W.; Townsend, A.J.; Rasool, B.; Alomrani, S.; Razak, N.; Karpinska, B.; Ruban, A.; Foyer, C. Ascorbate-mediated regulation of growth, photoprotection and photoinhibition in arabidopsis thaliana. J. Exp. Bot. 2018, 69, 2823–2835. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Plant Sci. 2000, 19, 267–290. [Google Scholar] [CrossRef]
- Ntagkas, N.; Woltering, E.J.; Marcelis, L.F.M. Light regulates ascorbate in plants: An integrated view on physiology and biochemistry. Environ. Exp. Bot. 2018, 147, 271–280. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Zha, L.Y.; Zhang, Y.B.; Liu, W.K. Dynamic responses of ascorbate pool and metabolis m in lettuce to long-term continuous light provided by red and blue LEDs. Environ. Exp. Bot. 2019, 7, 15–23. [Google Scholar] [CrossRef]
- Zha, L.Y.; Liu, W.K.; Zhang, Y.B.; Shao, M.J. Morphological and Physiological Stress Responses of Lettuce to Different Intensities of Continuous Light. Front. Plant Sci. 2019, 10, 1440. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Yu, J.; Facundo, G.; Laura, F.; Mcintosh, L.; Foyer, A.C. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in arabidopsis thaliana leaves. J. Exp. Bot. 2006, 57, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, Y.; Mieda, T.; Rapolu, M.; Nakamura, A.; Motoki, T.; Maruta, T. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 2007, 58, 2661–2671. [Google Scholar] [CrossRef] [PubMed]
- Cope, K.R.; Snowden, M.C.; Bugbee, B. Photobiological interactions of blue light and photosynthetic photon flux: Effects of monochromatic and broad-spectrum light sources. Photochem. Photobiol. 2014, 90, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Cataldo, D.A. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Spínola, V.; Mendes, B.; Câmara, J. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal. Bioanal. Chem. 2012, 403, 1049–1058. [Google Scholar] [CrossRef]
- Cao, J.K.; Jiang, W.B.; Zhao, Y.M. Experimental Guidance on Post-Harvest Physiology and Biochemistry of Fruits and Vegetables; China Light Industry Press: Beijing, China, 2007. [Google Scholar]
- Ma, F.; Cheng, L. Exposure of the shaded side of apple fruit to full sun leads to up-regulation of both the xanthophyll cycle and the ascorbate-glutathione cycle. Plant Sci. 2004, 166, 1479–1486. [Google Scholar] [CrossRef]
- Tei, F.; Benincasa, P.; Guiducci, M. Effect of nitrogen availability on growth and nitrogen uptake in lettuce. Acta Hortic. 2000, 533, 385–392. [Google Scholar] [CrossRef]
- Wang, X.L. The Effects of Nitrogen Supply on Photosynthetic Characteristics in Leaves of Tobacco Seedlings. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. (In Chinese). [Google Scholar]
- Parisi, M.; Giordano, L.; Pentangelo, A.; D’Onofrio, B.; Villari, G. Effects of different levels of nitrogen fertilization on yield and fruit quality in processing tomato. In Proceedings of the International Symposium Towards Ecologically Sound Fertilisation Strategies for Field Vegetable Production, Perugia, Italy, 7–10 June 2004; Volume 6, pp. 129–132. [Google Scholar] [CrossRef]
- Jacques, L.B.; Camille, B.; Christophe, R.; Frédéric, B.; Stéphane, A. The ‘trade-off’ between synthesis of primary and secondary compounds in young tomato leaves is altered by nitrate nutrition: Experimental evidence and model consistency. J. Exp. Bot. 2009, 60, 4301–4314. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Zha, L.Y.; Liu, W.K.; Yang, Q.C.; Zhang, Y.B.; Shao, M.J. Regulation of Ascorbate Accumulation and Metabolism in Lettuce by the Red:Blue Ratio of Continuous Light Using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yamamoto, T.; Fujimoto, K.; Okabe, K.; Masuda, M.; Abe, T.; Maeda, K. Low-pungent sweet pepper selected under continuous fluorescent illumination. Acta Hortic. 2011, 907, 243–246. [Google Scholar] [CrossRef]
- Homma, T.; Anzai, T.; Matsuo, K.; Kanemitsu, N.; Satoh, H.; Hiramoto, H. Effects of continuous irradiation of LED light on growth of young tea plants. Acta Hortic. 2011, 900, 233–236. [Google Scholar] [CrossRef]
- Bian, Z.H.; Cheng, R.F.; Yang, Q.C.; Wang, J. Continuous light from red, blue, and green light-emitting diodes reduces nitrate content and enhances phytochemical concentrations and antioxidant capacity in lettuce. J. Am. Soc. Hortic. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef]
- Liu, W.K.; Yang, Q.C. Semiconductor Lighting for Protected Horticulture Production; China Agricultural Science and Technology Press: Beijing, China, 2016. (In Chinese) [Google Scholar]
- Mccree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Liu, T.; Lu, J.W.; Ren, T.; Wang, W.; Wang, Z.; Wang, S.H. Characteristics of photosynthetic nitrogen allocation in leaves of different positions in winter oilseed rape at seedling stage under suitable nitrogen level. Sci. Agric. Sin. 2016, 49, 3532–3541. (In Chinese) [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Li, T.; Cheng, R.F.; Barnett, Y. Study of the beneficial effects of green light on lettuce grown under short-term continuous red and blue light-emitting diodes. Physiol. Plant. 2018, 164, 226–240. [Google Scholar] [CrossRef]
- Martinoia, E.; Heck, U.; Wiemken, A. Vacuoles as storage compartments for nitrate in barley leaves. Nature 1981, 289, 292–294. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef]
- Aires, A.; Rosa, E.; Carvalho, R. Effect of nitrogen and sulfur fertilization on glucosinolates in the leaves and roots of broccoli sprouts (Brassica oleracea var italica). J. Sci. Food Agric. 2006, 86, 1512–1516. [Google Scholar] [CrossRef]
- Liu, D.; Liu, W.; Zhu, D.; Geng, M.; Zhou, W.; Yang, T. Nitrogen effects on total flavonoids, chlorogenic acid, and antioxidant activity of the medicinal plant Chrysanthemum morifolium. J. Plant. Nutr. Soil Sci. 2010, 173, 268–274. [Google Scholar] [CrossRef]
- Janda, T.; Hideg, V.; Vanková, R. Editorial: The Role of Light in Abiotic Stress Acclimation. Front. Plant Sci. 2020, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Gallie, D.R. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 2013, 64, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Haghjou, M.M.; Shariati, M.; Smirnoff, N. The effect of acute high light and low temperature stresses on the ascorbate-glutathione cycle and superoxide dismutase activity in two Dunaliella salina strains. Physiol. Plant. 2009, 135, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Srivastava, P.K.; Prasad, S.M. Differential effect of UV-B radiation on growth oxidative stress and ascorbate-glutathione cycle in two cyanobacteria under copper toxicity. Plant Physiol. Biochem. 2012, 61, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Young, T.E.; Ling, J.; Chang, S.C.; Gallie, D.R. Increasing vitamin c content of plants through enhanced ascorbate recycling. Proc. Natl. Acad. Sci. USA 2003, 100, 3525–3530. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; M A, F.W.; Zhang, M.; Pu, F. Distribution and metabolism of ascorbic acid in apple fruits (malus domestica borkh cv. gala). Plant Sci. 2008, 174, 606–612. [Google Scholar] [CrossRef]
- Loscos, J.; Matamoros, M.A.; Becana, M. Ascorbate and homoglutathione metabolism in common bean nodules under stress conditions and during natural senescence. Plant Physiol. 2008, 3, 1282–1292. [Google Scholar] [CrossRef]
- Eltayeb, A.E.; Kawano, N.; Badawi, G.H.; Kaminaka, H.; Sanekata, T.; Shibahara, T. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 2007, 225, 1255–1264. [Google Scholar] [CrossRef]
- Smirnoff, N. Vitamin C: Biosynthesis of vitamins in plants. Adv. Bot. Res. 2011, 59, 107–177. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zha, L.; Liu, W.; Zhou, C.; Shao, M.; Yang, Q. LED Light Quality of Continuous Light before Harvest Affects Growth and AsA Metabolism of Hydroponic Lettuce Grown under Increasing Doses of Nitrogen. Plants 2021, 10, 176. https://doi.org/10.3390/plants10010176
Zhang Y, Zha L, Liu W, Zhou C, Shao M, Yang Q. LED Light Quality of Continuous Light before Harvest Affects Growth and AsA Metabolism of Hydroponic Lettuce Grown under Increasing Doses of Nitrogen. Plants. 2021; 10(1):176. https://doi.org/10.3390/plants10010176
Chicago/Turabian StyleZhang, Yubin, Lingyan Zha, Wenke Liu, Chengbo Zhou, Mingjie Shao, and Qichang Yang. 2021. "LED Light Quality of Continuous Light before Harvest Affects Growth and AsA Metabolism of Hydroponic Lettuce Grown under Increasing Doses of Nitrogen" Plants 10, no. 1: 176. https://doi.org/10.3390/plants10010176
APA StyleZhang, Y., Zha, L., Liu, W., Zhou, C., Shao, M., & Yang, Q. (2021). LED Light Quality of Continuous Light before Harvest Affects Growth and AsA Metabolism of Hydroponic Lettuce Grown under Increasing Doses of Nitrogen. Plants, 10(1), 176. https://doi.org/10.3390/plants10010176





