Enhanced Abiotic Stress Tolerance of Vicia faba L. Plants Heterologously Expressing the PR10a Gene from Potato
Abstract
1. Introduction
2. Results
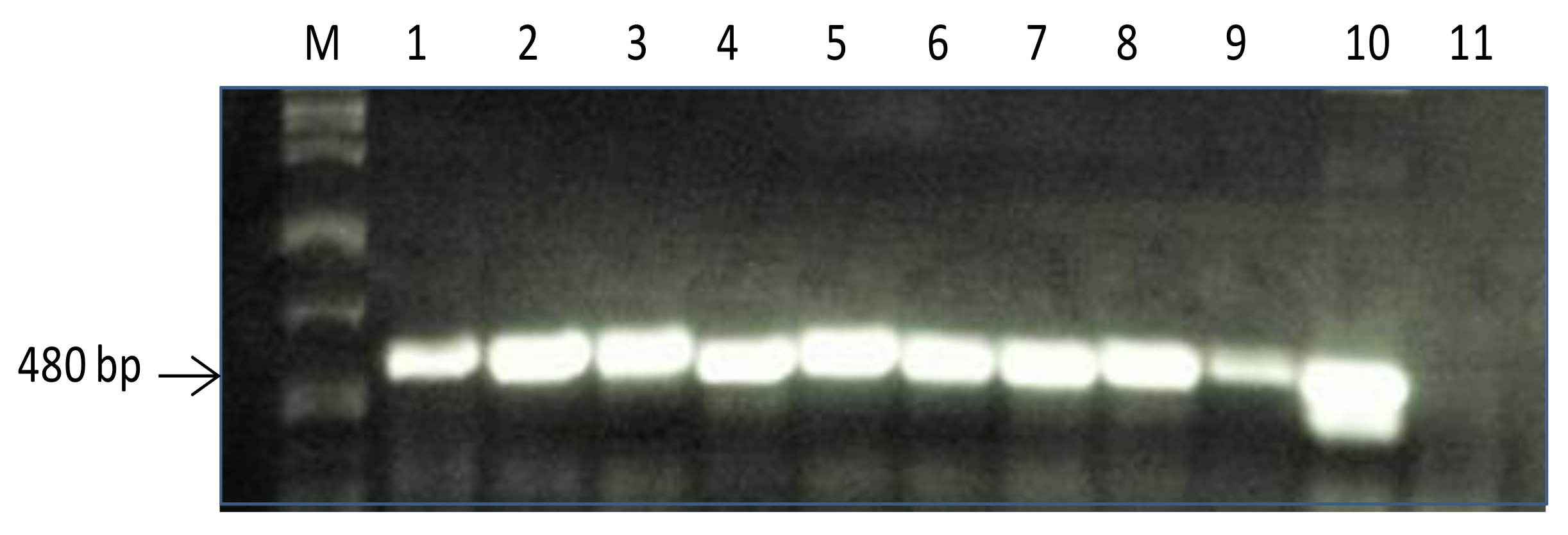
2.1. Screening of PR10a Transgenic Plants
2.2. Growth Reactions of PR10a Transgenic Faba Bean Lines to Salt Stress
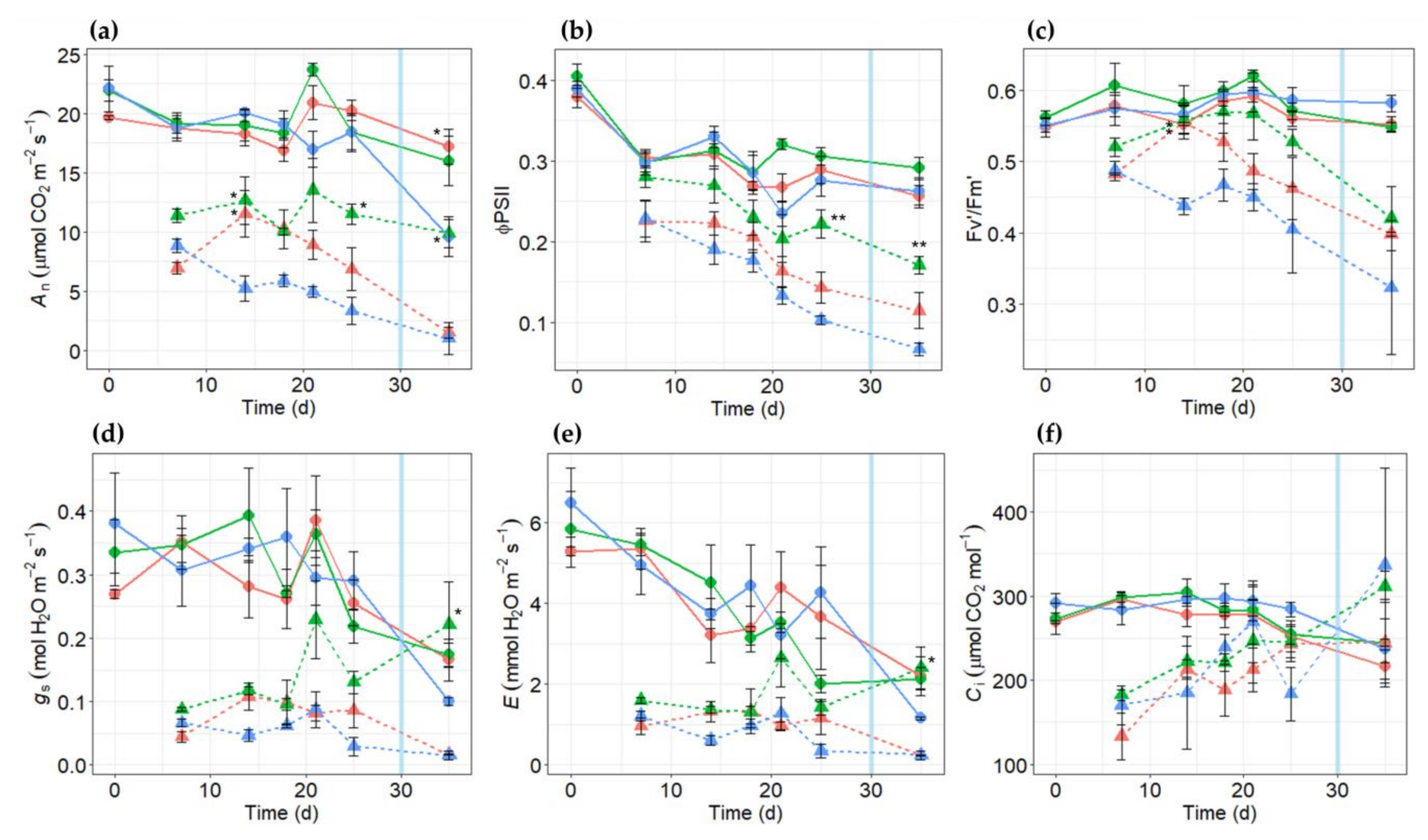
2.3. Effects of Salt Stress on Gas Exchange Parameters in PR10a Faba Bean Lines
2.4. Osmotic Potential
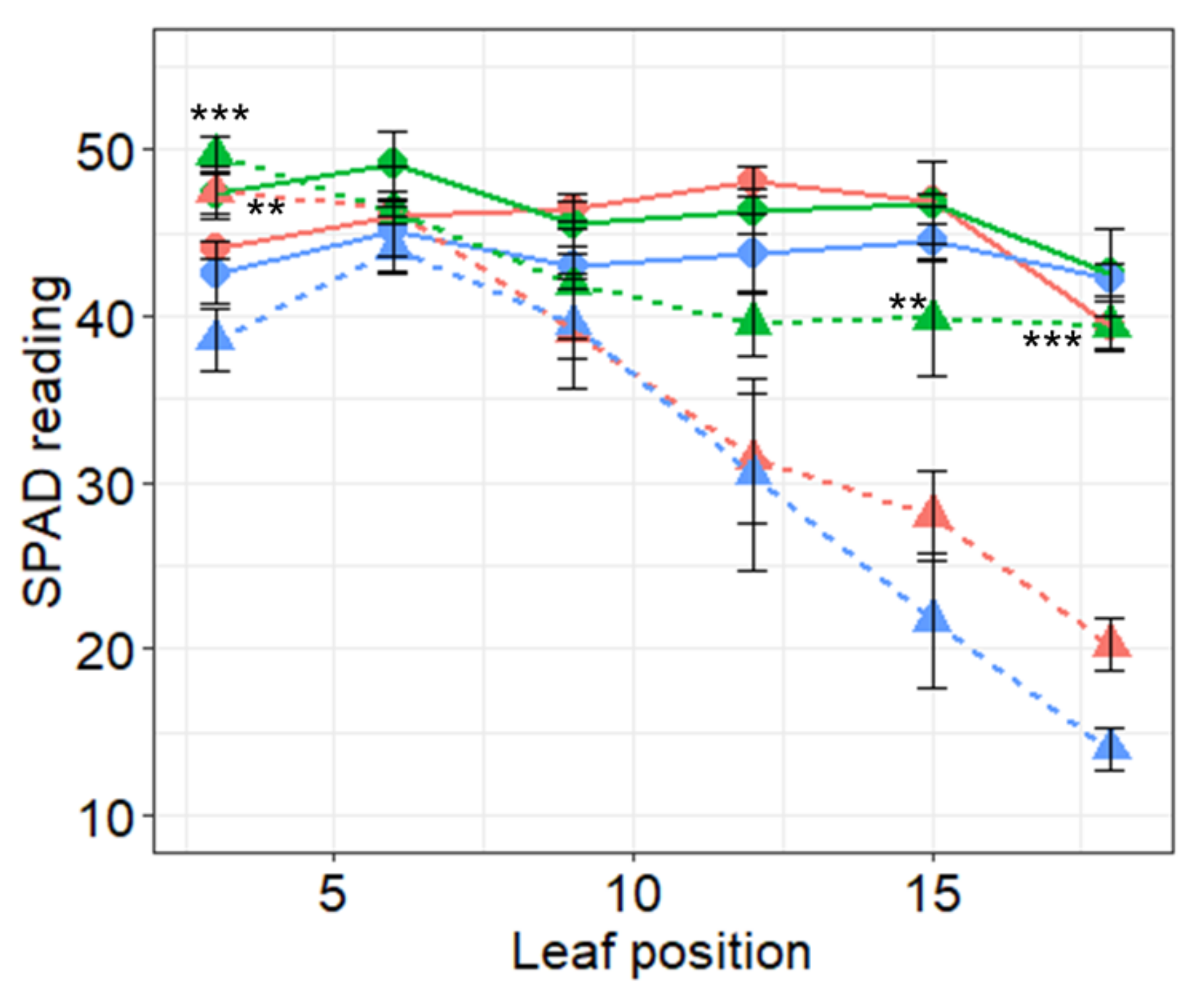
2.5. Chlorophyll Content Estimation by SPAD Chlorophyll Meter
2.6. Ion Analysis
2.7. Phenotyping of the PR10a Faba Bean Plants under Drought Stress
2.8. Measurement of Physiological Changes under Drought
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growing Conditions
4.2. PCR Analysis of Transgenic Plants
4.3. Leaf Paint Test
4.4. Stress Treatments
4.5. Phenotyping and Physiological Analysis of Transgenic Plants
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Bray, E.; Bailey-Serres, J.; Weretilnyk, E. Responses to abiotic stresses. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; American Society of Plant Biologists: Rockville, MD, USA, 2000; pp. 149–158. [Google Scholar]
- Wang, W.X.; Vinocur, B.; Shoseyov, O.; Altman, A. Biotechnology of plant osmotic stress tolerance physiological and molecular considerations. Acta Hortic. 2001, 285–292. [Google Scholar] [CrossRef]
- Tang, L.-L.; Cai, H.; Zhai, H.; Luo, X.; Wang, Z.; Cui, L.; Bai, X. Overexpression of Glycine soja WRKY20 enhances both drought and salt tolerance in transgenic alfalfa (Medicago sativa L.). Plant Cell Tissue Organ Cult. (Pctoc) 2014, 118, 77–86. [Google Scholar] [CrossRef]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef]
- Ashraf, M. Some important physiological selection criteria for salt tolerance in plants. Flora Morphol. Distrib. Funct. Ecol. Plants 2004, 199, 361–376. [Google Scholar] [CrossRef]
- Ashraf, M. Organic substances responsible for salt tolerance in Eruca sativa. Biol. Plant. 1994, 36, 255–259. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic press: Cambridge, MA, USA, 1995. [Google Scholar]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Bhatnagar-Mathur, P.; Vadez, V.; Sharma, K.K. Transgenic approaches for abiotic stress tolerance in plants: Retrospect and prospects. Plant Cell Rep. 2008, 27, 411–424. [Google Scholar] [CrossRef]
- Srivastava, S.; Fristensky, B.; Kav, N.N. Constitutive Expression of a PR10 Protein Enhances the Germination of Brassica napus under Saline Conditions. Plant Cell Physiol. 2004, 45, 1320–1324. [Google Scholar] [CrossRef]
- Liu, J.-J.; Ekramoddoullah, A.K.M.; Yu, X. Differential expression of multiple PR10 proteins in western white pine following wounding, fungal infection and cold-hardening. Physiol. Plant. 2003, 119, 544–553. [Google Scholar] [CrossRef]
- Stintzi, A.; Heitz, T.; Prasad, V.; Wiedemann-Merdinoglu, S.; Kauffmann, S.; Geoffroy, P.; Legrand, M.; Fritig, B. Plant ‘pathogenesis-related’proteins and their role in defense against pathogens. Biochimie 1993, 75, 687–706. [Google Scholar] [CrossRef]
- Hanafy, M.; El-Banna, A.; Schumacher, H.M.; Jacobsen, H.-J.; Hassan, F. Enhanced tolerance to drought and salt stresses in transgenic faba bean (Vicia faba L.) plants by heterologous expression of the PR10a gene from potato. Plant Cell Rep. 2013, 32, 663–674. [Google Scholar] [CrossRef]
- Stratilová, B.; Řehulka, P.; Garajová, S.; Řehulková, H.; Stratilová, E.; Hrmova, M.; Kozmon, S. Structural characterization of the Pet c 1.0201 PR-10 protein isolated from roots of Petroselinum crispum (Mill.) Fuss. Phytochemistry 2020, 175, 112368. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Pierpoint, W.S.; Boller, T.; Conejero, V. Recommendations for naming plant pathogenesis-related proteins. Plant Mol. Biol. Rep. 1994, 12, 245–264. [Google Scholar] [CrossRef]
- Van Loon, L.; Van Strien, E. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Somssich, I.E.; Schmelzer, E.; Bollmann, J.; Hahlbrock, K. Rapid activation by fungal elicitor of genes encoding “pathogenesis-related” proteins in cultured parsley cells. Proc. Natl. Acad. Sci. USA 1986, 83, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Pnueli, L.; Hallak-Herr, E.; Rozenberg, M.; Cohen, M.; Goloubinoff, P.; Kaplan, A.; Mittler, R. Molecular and biochemical mechanisms associated with dormancy and drought tolerance in the desert legume Retama raetam. Plant J. 2002, 31, 319–330. [Google Scholar] [CrossRef]
- Park, C.-J.; Kim, K.-J.; Shin, R.; Park, J.M.; Shin, Y.-C.; Paek, K.-H. Pathogenesis-related protein 10 isolated from hot pepper functions as a ribonuclease in an antiviral pathway. Plant J. 2003, 37, 186–198. [Google Scholar] [CrossRef]
- Jain, S.; Srivastava, S.; Sarin, N.B.; Kav, N.N. Proteomics reveals elevated levels of PR 10 proteins in saline-tolerant peanut (Arachis hypogaea) calli. Plant Physiol. Biochem. 2006, 44, 253–259. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B.; Lin, J.; Fei, J.; Chen, Z.; Pang, Y.; Sun, X.; Tang, K. A novel pathogenesis-related protein (SsPR10) from Solanum surattense with ribonucleolytic and antimicrobial activity is stress- and pathogen-inducible. J. Plant Physiol. 2006, 163, 546–556. [Google Scholar] [CrossRef]
- Liu, J.-J.; Ekramoddoullah, A.K. The family 10 of plant pathogenesis-related proteins: Their structure, regulation, and function in response to biotic and abiotic stresses. Physiol. Mol. Plant Pathol. 2006, 68, 3–13. [Google Scholar] [CrossRef]
- Kim, S.T.; Yu, S.; Kang, Y.H.; Kim, S.G.; Kim, J.-Y.; Kim, S.-H.; Kang, K.Y. The rice pathogen-related protein 10 (JIOsPR10) is induced by abiotic and biotic stresses and exhibits ribonuclease activity. Plant Cell Rep. 2008, 27, 593. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-R.; Chen, Z.-Y.; Brown, R.L.; Bhatnagar, D. Expression and functional characterization of two pathogenesis-related protein 10 genes from Zea mays. J. Plant Physiol. 2010, 167, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.U.; Park, J.-I.; Jung, H.-J.; Seo, M.-S.; Kumar, T.S.; Lee, I.-H.; Nou, I.-S. Identification and characterization of stress resistance related genes of Brassica rapa. Biotechnol. Lett. 2012, 34, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Abreu, I.A.; Farinha, A.P.; Negrão, S.; Gonçalves, N.M.; Fonseca, C.; Rodrigues, M.A.A.; Batista, R.; Saibo, N.J.; Oliveira, M.M. Coping with abiotic stress: Proteome changes for crop improvement. J. Proteom. 2013, 93, 145–168. [Google Scholar] [CrossRef]
- Agarwal, P.; Dabi, M.; More, P.; Patel, K.; Jana, K.; Agarwal, P.K. Improved shoot regeneration, salinity tolerance and reduced fungal susceptibility in transgenic tobacco constitutively expressing PR-10a gene. Front. Plant Sci. 2016, 7, 217. [Google Scholar] [CrossRef]
- Kav, N.N.; Srivastava, S.; Goonewardene, L.; Blade, S.F. Proteome-level changes in the roots of Pisum sativum in response to salinity. Ann. Appl. Biol. 2004, 145, 217–230. [Google Scholar] [CrossRef]
- Srivastava, S.; Emery, R.J.N.; Kurepin, L.V.; Reid, D.M.; Fristensky, B.; Kav, N.N. Pea PR 10.1 is a Ribonuclease and its Transgenic Expression Elevates Cytokinin Levels. Plant Growth Regul. 2006, 49, 17–25. [Google Scholar] [CrossRef]
- Verma, S.S.; Sinha, R.; Rahman, M.; Megha, S.; Deyholos, M.K.; Kav, N.N.V. miRNA-mediated posttranscriptional regulation of gene expression in ABR17-transgenic Arabidopsis thaliana under salt stress. Plant Mol. Biol. Rep. 2014, 32, 1203–1218. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kisseleva, L.; Sawa, S.; Furukawa, T.; Komatsu, S.; Koshiba, T. A Novel Rice PR10 Protein, RSOsPR10, Specifically Induced in Roots by Biotic and Abiotic Stresses, Possibly via the Jasmonic Acid Signaling Pathway. Plant Cell Physiol. 2004, 45, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.R.; Pulla, R.K.; Kim, Y.-J.; Balusamy, S.R.D.; Yang, D.-C. Expression and stress tolerance of PR10 genes from Panax ginseng CA Meyer. Mol. Biol. Rep. 2012, 39, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Marineau, C.; Matton, D.P.; Brisson, N. Differential accumulation of potato tuber mRNAs during the hypersensitive response induced by arachidonic acid elicitor. Plant Mol. Biol. 1987, 9, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Moons, A.; Prinsen, E.; Bauw, G.; Van Montagu, M. Antagonistic effects of abscisic acid and jasmonates on salt stress-inducible transcripts in rice roots. Plant Cell 1997, 9, 2243–2259. [Google Scholar]
- Agrawal, G.K.; Rakwal, R.; Yonekura, M.; Kubo, A.; Saji, H. Proteome analysis of differentially displayed proteins as a tool for investigating ozone stress in rice (Oryza sativa L.) seedlings. Proteomics 2002, 2, 947–959. [Google Scholar] [CrossRef]
- Rakwal, R.; Agrawal, G.K.; Yonekura, M. Separation of proteins from stressed rice (Oryza sativa L.) leaf tissues by two-dimensional polyacrylamide gel electrophoresis: Induction of pathogenesis-related and cellular protectant proteins by jasmonic acid, UV irradiation and copper chloride. Electrophoresis 1999, 20, 3472–3478. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Subba, P.; Pandey, A.; Bhushan, D.; Kumar, R.; Datta, A.; Chakraborty, S.; Chakraborty, N. Analysis of the grasspea proteome and identification of stress-responsive proteins upon exposure to high salinity, low temperature, and abscisic acid treatment. Phytochemistry 2011, 72, 1293–1307. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takeda, K. Proteomic Analysis of Specific Proteins in the Root of Salt-Tolerant Barley. Biosci. Biotechnol. Biochem. 2009, 73, 2762–2765. [Google Scholar] [CrossRef]
- Vincent, D.; Ergül, A.; Bohlman, M.C.; Tattersall, E.A.R.; Tillett, R.L.; Wheatley, M.D.; Woolsey, R.; Quilici, D.R.; Joets, J.; Schlauch, K.; et al. Proteomic analysis reveals differences between Vitis vinifera L. cv. Chardonnay and cv. Cabernet Sauvignon and their responses to water deficit and salinity. J. Exp. Bot. 2007, 58, 1873–1892. [Google Scholar] [CrossRef]
- Han, X.; He, X.; Qiu, W.; Lu, Z.; Zhang, Y.; Chen, S.; Liu, M.; Qiao, G.; Zhuo, R.-Y. Pathogenesis-related protein PR10 from Salix matsudana Koidz exhibits resistance to salt stress in transgenic Arabidopsis thaliana. Environ. Exp. Bot. 2017, 141, 74–82. [Google Scholar] [CrossRef]
- Duc, G. Faba bean (Vicia faba L.). Field Crop. Res. 1997, 53, 99–109. [Google Scholar] [CrossRef]
- El-Banna, A.; Hajirezaei, M.-R.; Wissing, J.; Ali, Z.; Vaas, L.; Heine-Dobbernack, E.; Jacobsen, H.-J.; Schumacher, H.M.; Kiesecker, H. Over-expression of PR-10a leads to increased salt and osmotic tolerance in potato cell cultures. J. Biotechnol. 2010, 150, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, P.; Swarcewicz, B.; Chmielewska, K.; Wojakowska, A.; Stobiecki, M. Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiol. Plant. 2014, 36, 1–19. [Google Scholar] [CrossRef]
- Dresselhaus, T.; Hückelhoven, R. Biotic and Abiotic Stress Responses in Crop Plants. Agronomy 2018, 8, 267. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Tian, S.S.; Liu, S.S.; Wang, W.Q.; Sui, N. Energy dissipation and antioxidant enzyme system protect photosystem II of sweet sorghum under drought stress. Photosynthetica 2018, 56, 861–872. [Google Scholar] [CrossRef]
- Zhu, T.; Deng, X.; Zhou, X.; Zhu, L.; Zou, L.; Li, P.; Zhang, D.; Lin, H. Ethylene and hydrogen peroxide are involved in brassinosteroid-induced salt tolerance in tomato. Sci. Rep. 2016, 6, 35392. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic Stress Tolerance in Plants: Myriad Roles of Ascorbate Peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, W.; Wang, Y.; Yong, T.; Yang, F.; Liu, W.; Wu, X.; Du, J.; Shu, K.; Liu, J.; et al. Leaf area and photosynthesis of newly emerged trifoliolate leaves are regulated by mature leaves in soybean. J. Plant Res. 2018, 131, 671–680. [Google Scholar] [CrossRef]
- Iqbal, N.; Hussain, S.; Raza, M.A.; Yang, C.-Q.; Safdar, M.E.; Brestic, M.; Aziz, A.; Hayyat, M.S.; Asghar, M.A.; Yang, W.; et al. Drought tolerance of soybean (Glycine max L. Merr.) by improved photosynthetic characteristics and an efficient antioxidant enzyme system under a split-root system. Front. Physiol. 2019, 10, 786. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Rastogi, A.; Zivcak, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lotfi, R.; Stypinski, P.; Samborska, I.A.; et al. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, A. Probing Photosynthesis: Mechanisms, Regulation and Adaptation; CRC Press: Boca Raton, FL, USA, 2000; pp. 445–483. [Google Scholar]
- Baker, N.R.; Rosenqvist, E. Corrigendum to: Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2020, 71, 1647. [Google Scholar] [CrossRef] [PubMed]
- Kocheva, K.; Lambrev, P.; Georgiev, G.; Goltsev, V.; Karabaliev, M. Evaluation of chlorophyll fluorescence and membrane injury in the leaves of barley cultivars under osmotic stress. Bioelectrochemistry 2004, 63, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Rustagi, A.; Jain, S.; Kumar, D.; Shekhar, S.; Jain, M.; Bhat, V.; Sarin, N.B. High Efficiency Transformation of Banana [Musa acuminata L. cv. Matti (AA)] for Enhanced Tolerance to Salt and Drought Stress Through Overexpression of a Peanut Salinity-Induced Pathogenesis-Related Class 10 Protein. Mol. Biotechnol. 2014, 57, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kumar, D.; Jain, M.; Chaudhary, P.; Deswal, R.; Sarin, N.B. Ectopic overexpression of a salt stress-induced pathogenesis-related class 10 protein (PR10) gene from peanut (Arachis hypogaea L.) affords broad spectrum abiotic stress tolerance in transgenic tobacco. Plant Cell Tissue Organ Cult. (Pctoc) 2012, 109, 19–31. [Google Scholar] [CrossRef]
- Takeuchi, K.; Hasegawa, H.; Gyohda, A.; Komatsu, S.; Okamoto, T.; Okada, K.; Terakawa, T.; Koshiba, T. Overexpression of RSOsPR10, a root-specific rice PR10 gene, confers tolerance against drought stress in rice and drought and salt stresses in bentgrass. Plant Cell Tissue Organ Cult. (Pctoc) 2016, 127, 35–46. [Google Scholar] [CrossRef]
- Kole, C.; Muthamilarasan, M.; Henry, R.J.; Edwards, D.; Sharma, R.; Abberton, M.T.; Batley, J.; Bentley, A.R.; Blakeney, M.; Bryant, J.; et al. Application of genomics-assisted breeding for generation of climate resilient crops: Progress and prospects. Front. Plant Sci. 2015, 6, 563. [Google Scholar] [CrossRef]
- Goyal, R.K.; Efatima, T.; Topuz, M.; Bernadec, A.; Sicher, R.; Handa, A.K.; Mattoo, A.K. Pathogenesis-Related Protein 1b1 (PR1b1) Is a Major Tomato Fruit Protein Responsive to Chilling Temperature and Upregulated in High Polyamine Transgenic Genotypes. Front. Plant Sci. 2016, 7, 901. [Google Scholar] [CrossRef]
- Wang, J.; Mao, X.; Wang, R.; Li, A.; Zhao, G.; Zhao, J.; Jing, R.-L. Identification of wheat stress-responding genes and TaPR-1-1 function by screening a cDNA yeast library prepared following abiotic stress. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Sinha, R.K.; Verma, S.S.; Rastogi, A. Role of Pathogen-Related Protein 10 (PR 10) under Abiotic and Biotic Stresses in Plants. Phyton 2020, 89, 167–182. [Google Scholar] [CrossRef]
- Dubos, C.; Plomion, C. Drought differentially affects expression of a PR-10 protein, in needles of maritime pine (Pinus pinaster Ait.) seedlings. J. Exp. Bot. 2001, 52, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Y.; Liu, G.; Li, H. Cloning and Characterization of a Pathogenesis-Related Gene (ThPR10) from Tamarix hispida. Acta Biol. Crac. S. Bot. 2010, 52, 17–25. [Google Scholar] [CrossRef]
- Percival, G.C.; Fraser, G.A.; Oxenham, G. Foliar salt tolerance of Acer genotypes using chlorophyll fluorescence. Arboric. Urban For. 2003, 29, 61. [Google Scholar]
- Gama, P.B.S.; Inanaga, S.; Tanaka, K.; Nakazawa, R. Physiological response of common bean (Phaseolus vulgaris L.) seedlings to salinity stress. Afr. J. Biotechnol. 2007, 6, 79–88. [Google Scholar]
- Ganieva, R.A.; Allahverdiyev, S.R.; Guseinova, N.B.; Kavakli, H.I.; Nafisi, S. Effect of salt stress and synthetic hormone polystimuline K on the photosynthetic activity of cotton (Gossypium hirsutum). Turk. J. Bot. 1998, 22, 217–222. [Google Scholar]
- Lal, S.; Gulyani, V.; Khurana, P. Overexpression of HVA1 gene from barley generates tolerance to salinity and water stress in transgenic mulberry (Morus indica). Transgenic Res. 2007, 17, 651–663. [Google Scholar] [CrossRef]
- Zhou, H.; Li, W. The effects of oasis ecosystem hydrological processes on soil salinization in the lower reaches of the Tarim River, China. Ecohydrology 2013, 6, 1009–1020. [Google Scholar] [CrossRef]
- Yildirim, E.; Turan, M.; Guvenc, I. Effect of Foliar Salicylic Acid Applications on Growth, Chlorophyll, and Mineral Content of Cucumber Grown Under Salt Stress. J. Plant Nutr. 2008, 31, 593–612. [Google Scholar] [CrossRef]
- Silva, E.A.; Gouveia-Neto, A.D.S.; Oliveira, R.A.; Moura, D.S.; Cunha, P.C.; Costa, E.B.; Câmara, T.J.R.; Willadino, L.G. Water Deficit and Salt Stress Diagnosis Through LED Induced Chlorophyll Fluorescence Analysis in Jatropha curcas L. J. Fluoresc. 2012, 22, 623–630. [Google Scholar] [CrossRef]
- Evans, J.R. Nitrogen and Photosynthesis in the Flag Leaf of Wheat (Triticum aestivum L.). Plant Physiol. 1983, 72, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.; Bandehagh, A.; Toorchi, M. Effects of NaCl Stress on Chlorophyll Content and Chlorophyll Fluorescence in Sunflower (Helianthus annuus L.) Lines. Yüzüncü Yıl Üniversitesi Tarım Bilimleri Derg. 2014, 24, 111–120. [Google Scholar] [CrossRef][Green Version]
- Singh, P.; Singh, N.; Sharma, K.D.; Kuhad, M.S. Plant water relations and osmotic adjustment in Brassica species under salinity stress. J. Am. Sci. 2010, 6, 1–4. [Google Scholar]
- Nawaz, K.; Hussain, K.; Majeed, A.; Khan, F.; Afghan, S.; Ali, K. Fatality of salt stress to plants: Morphological, physiological and biochemical aspects. Afr. J. Biotechnol. 2010, 9, 5475–5480. [Google Scholar]
- Desingh, R.; Kanagaraj, G. Influence of salinity stress on photosynthesis and antioxidative systems in two cotton varieties. Gen. Appl. Plant Physiol. 2007, 33, 221–234. [Google Scholar]
- Garg, N.; Noor, Z. Genotypic differences in plant growth, osmotic and antioxidative defence of Cajanus cajan (L.) Millsp. modulated by salt stress. Arch. Agron. Soil Sci. 2009, 55, 3–33. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Khan, A.A.; McNeilly, T.; Azhar, F.M. Stress tolerance in crop plants. Int. J. Agric. Biol 2001, 3, 250–255. [Google Scholar]
- Romero-Aranda, R.; Soria, T.; Cuartero, J. Tomato plant-water uptake and plant-water relationships under saline growth conditions. Plant Sci. 2001, 160, 265–272. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Ashraf, M. Growth stage-based modulation in physiological and biochemical attributes of two genetically diverse wheat (Triticum aestivum L.) cultivars grown in salinized hydroponic culture. Environ. Sci. Pollut. Res. 2016, 23, 6227–6243. [Google Scholar] [CrossRef]
- Madan, S.; Nainawatee, H.S.; Jain, R.K.; Chowdhury, J.B. Proline and proline metabolising enzymes in in-vitro selected NaCl-tolerant Brassica juncea L. under salt stress. Ann. Bot. 1995, 76, 51–57. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.; Srivastava, G. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.-H.; Gao, J.-P.; Li, L.-G.; Cai, X.-L.; Huang, W.; Chao, D.-Y.; Zhu, M.-Z.; Wang, Z.-Y.; Luan, S.; Lin, H.-X. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005, 37, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Rasel Arif, T.-U.; Hossain, M.A.; Hassan, L.; Farzana, S.; Brestic, M. Screening of Salt-Tolerant Rice Landraces by Seedling Stage Phenotyping and Dissecting Biochemical Determinants of Tolerance Mechanism. J. Plant Growth Regul. 2020, 1–16. [Google Scholar] [CrossRef]
- Bao, A.-K.; Du, B.Q.; Touil, L.; Kang, P.; Wang, Q.L.; Wang, S.-M. Co-expression of tonoplast Cation/H+ antiporter and H+-pyrophosphatase from xerophyte Zygophyllum xanthoxylum improves alfalfa plant growth under salinity, drought and field conditions. Plant Biotechnol. J. 2016, 14, 964–975. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Botía, P.; Fernández-Ballester, G.; Cerdá, A.; Lopez, V.M. Uptake, Transport, and Concentration of Chloride and Sodium in Three Citrus Rootstock Seedlings. J. Plant Nutr. 2005, 28, 1933–1945. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G. The response of barley to salinity stress differs between hydroponic and soil systems. Funct. Plant Biol. 2010, 37, 621–633. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Bhatla, S.C. Effect of sodium chloride levels on growth, water status, uptake, transport, and accumulation pattern of sodium and chloride ions in young sunflower plants. Commun. Soil Sci. Plant Anal. 2011, 42, 815–831. [Google Scholar] [CrossRef]
- Najar, R.; Aydi, S.; Sassi-Aydi, S.; Zarai, A.; Abdelly, C. Effect of salt stress on photosynthesis and chlorophyll fluorescence in Medicago truncatula. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2018, 153, 88–97. [Google Scholar] [CrossRef]
- Sul, I.-W.; Korban, S. A highly efficient method for isolating genomic DNA from plant tissues. Plant Tissue Cult. Biotechnol. 1996, 2, 113–116. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]




| Treatment | Line | Fresh Weight per Plant (g) | Branch Number per Plant | |||
|---|---|---|---|---|---|---|
| Total | Leaf | Stem | Root | |||
| Salt-stressed | TL-2 | 60.6 ± 12.6 | 23.7 ± 6.12 | 29.8 ± 6.60 ns | 7.07 ± 0.090 | 2.0 ± 0.0 ns |
| TL-3 | 107 ± 14.2 | 41.9 ± 5.08 | 50.0 ± 6.99 ns | 14.6 ± 2.27 | 3.75 ± 0.25 ** | |
| WT | 37.6 ± 10.2 | 15.3 ± 5.04 | 17.2 ± 4.76 | 5.10 ± 0.868 | 0.667 ± 0.333 | |
| Non-stressed | TL-2 | 349 ± 48.1 | 144 ± 15.3 | 150 ± 16.4 ** | 38.8 ± 10.0 | 8.67 ± 0.333 ns |
| TL-3 | 288 ± 38.9 | 103 ± 13.4 | 108 ± 5.66 ns | 77.5 ± 30.4 | 6.67 ± 0.882 ns | |
| WT | 239 ± 54.7 | 74.5 ± 38.4 | 75.0 ± 28.4 | 89.1 ± 12.1 | 7.5 ± 1.50 | |
| Two-way ANOVA | Treatment (T) | *** | *** | *** | *** | *** |
| Line (L) | * | ** | ** | ns | ** | |
| T × L | ns | ns | * | ns | * | |
| Relative value under stress | TL-2 | 0.174 ± 0.036 ns | 0.164 ± 0.043 ns | 0.199 ± 0.044 ns | 0.182 ± 0.002 * | |
| TL-3 | 0.370 ± 0.049 * | 0.408 ± 0.049ns | 0.464 ± 0.065 ns | 0.189 ± 0.029 * | ||
| WT | 0.158 ± 0.043 | 0.205 ± 0.068 | 0.230 ± 0.063 | 0.057 ± 0.010 | ||
| Treatment | Line | Dry Weight per Plant (g) | |||
|---|---|---|---|---|---|
| Total | Leaf | Stem | Root | ||
| Salt-stressed | TL-2 | 9.92 ± 3.37 | 5.73 ± 1.50 | 3.55 ± 1.66 | 0.64 ± 0.21 |
| TL-3 | 10.8 ± 1.15 | 5.76 ± 0.557 | 4.21 ± 0.618 | 0.82 ± 0.083 | |
| WT | 6.37 ± 0.632 | 4.11 ± 0.248 | 1.88 ± 0.255 | 0.37 ± 0.151 | |
| Non-stressed | TL-2 | 34.3 ± 4.28 | 14.5 ± 1.86 | 17.6 ± 1.96 | 2.25 ± 0.553 |
| TL-3 | 25.8 ± 2.32 | 9.93 ± 1.07 | 12.0 ± 1.17 | 3.85 ± 1.25 | |
| WT | 32.6 ± 5.71 | 13.2 ± 1.83 | 14.8 ± 3.60 | 4.63 ± 0.28 | |
| Two-way ANOVA | Treatment (T) | *** | *** | *** | *** |
| Line (L) | * | * | * | ns | |
| T × L | ns | ns | ns | ns | |
| Relative value under stress | TL-2 | 0.289 ± 0.098 ns | 0.394 ± 0.104 ns | 0.202 ± 0.095 ns | 0.284 ± 0.093 * |
| TL-3 | 0.418 ± 0.045 * | 0.580 ± 0.056 * | 0.351 ± 0.052 * | 0.213 ± 0.022 ns | |
| WT | 0.195 ± 0.020 | 0.313 ± 0.019 | 0.127 ± 0.017 | 0.080 ± 0.033 | |
| Treatment | Line | Osmotic Potential (MPa) | |
|---|---|---|---|
| 14 Days | 28 Days | ||
| Salt-stressed | TL-2 | −1.01 ± 0.025 * | −1.37 ± 0.060 ** |
| TL-3 | −1.18 ± 0.048 ns | −1.31 ± 0.077 *** | |
| WT | −1.20 ± 0.051 | −1.91 ± 0.249 | |
| Non-stressed | TL-2 | −0.739 ± 0.048 ns | −0.683 ± 0.044 ns |
| TL-3 | −0.702 ± 0.050 ns | −0.690 ± 0.054 ns | |
| WT | −0.708 ± 0.078 | −0.735 ± 0.050 | |
| Two-way ANOVA | Treatment (T) | *** | *** |
| Line (L) | ns | ** | |
| T × L | * | * | |
| Treatment | Line | Sodium Content (mg Na+ g−1 DW) | ||
|---|---|---|---|---|
| Leaf | Stem | Root | ||
| Salt-stressed | TL-2 | 35.4 ± 5.07 * | 38.8 ± 8.95 | 12.1 ± 0.355 ns |
| TL-3 | 30.7 ± 1.98 ** | 17.5 ± 7.57 | 28.3 ± 4.37 ** | |
| WT | 55.1 ± 8.79 | 16.5 ± 12.8 | 11.6 ± 3.62 | |
| Non-stressed | TL-2 | 2.62 ± 0.150 ns | 20.9 ± 16.8 | 7.32 ± 0.11 ns |
| TL-3 | 2.56 ± 0.282 ns | 23.2 ± 10.2 | 8.66 ± 1.17 ns | |
| WT | 2.76 ± 0.03 | 17.2 ± 13.6 | 6.54 ± 1.40 | |
| Two-way ANOVA | Treatment (T) | *** | ns | ** |
| Line (L) | ** | ns | ** | |
| T × L | * | ns | p = 0.0583 | |
| Treatment | Line | Plant Height (m) | Number of Leaves per Plant |
|---|---|---|---|
| Drought-stressed | TL-2.2 | 0.630 ± 0.010 *** | 21.0 ± 3.0 |
| TL-2.3 | 0.630 ± 0.010 *** | 20.7 ± 0.3 | |
| TL-2.4 | 0.470 ± 0.010 *** | 16.0 ± 2.0 | |
| WT | 0.340 ± 0.030 | 10.0 ± 2.0 | |
| Non-stressed | TL-2.2 | 0.645 ± 0.005 *** | 21.5 ± 2.5 |
| TL-2.3 | 0.655 ± 0.005 *** | 22.5 ± 2.5 | |
| TL-2.4 | 0.500 ± 0.010 ns | 16.0 ± 2.0 | |
| WT | 0.505 ± 0.015 | 15.0 ± 1.0 | |
| Two-way ANOVA | Treatment (T) | *** | ns |
| Line (L) | *** | ** | |
| T × L | ** | ns |
| Treatment | Line | An (µmol CO2 m−2 s−1) | ϕPSII | gs (mol H2O m−2 s−1) | E (mmol H2O m−2 s−1) | Ci (µmol CO2 mol−1) |
|---|---|---|---|---|---|---|
| Drought-stressed | TL-2.2 | 6.30 ± 0.431 ns | 0.169 ± 0.006 * | 0.065 ± 0.020 | 1.13 ± 0.149 | 209 ± 67.4 ** |
| TL-2.3 | 8.04 ± 1.90 * | 0.154 ± 0.006 ns | 0.073 ± 0.001 | 1.33 ± 0.048 | 208 ± 39.2 ** | |
| TL-2.4 | 5.44 ± 0.647 ns | 0.169 ± 0.007 * | 0.073 ± 0.023 | 1.25 ± 0.380 | 234 ± 52.1 * | |
| WT | 3.02 ± 1.11 | 0.056 ± 0.010 | 0.095 ± 0.027 | 1.60 ± 0.346 | 340 ± 8.20 | |
| Drought-stressed + 2-d recovery | TL-2.2 | 9.42 ± 0.749 * | 0.222 ± 0.031 * | 0.113 ± 0.002 | 2.12 ± 0.332 | 249 ± 12.4 ns |
| TL-2.3 | 10.7 ± 0.377 ** | 0.184 ± 0.001 ns | 0.115 ± 0.004 | 2.21 ± 0.099 | 232 ± 5.24 * | |
| TL-2.4 | 8.50 ± 0.366 ns | 0.156 ± 0.001 ns | 0.103 ± 0.005 | 2.06 ± 0.047 | 250 ± 1.38 ns | |
| WT | 3.87 ± 0.145 | 0.084 ± 0.003 | 0.136 ± 0.023 | 2.43 ± 0.339 | 338 ± 9.89 | |
| Non-stressed | TL-2.2 | 9.17 ± 0.768 ns | 0.153 ± 0.018 ns | 0.116 ± 0.008 | 1.97 ± 0.062 | 255 ± 12.9 ns |
| TL-2.3 | 9.96 ± 0.844 ns | 0.138 ± 0.025 ns | 0.152 ± 0.024 | 2.50 ± 0.411 | 269 ± 9.38 ns | |
| TL-2.4 | 9.21 ± 0.602 ns | 0.169 ± 0.013 ns | 0.130 ± 0.016 | 2.13 ± 0.181 | 265 ± 11.5 ns | |
| WT | 9.67 ± 1.26 | 0.197 ± 0.028 | 0.137 ± 0.028 | 2.28 ± 0.435 | 262 ± 15.1 | |
| Two-way ANOVA | Treatment (T) | *** | ns | ** | ** | ns |
| Line (L) | *** | ns | ns | ns | ** | |
| T × L | * | ** | ns | ns | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desouky, A.F.; Ahmed, A.H.H.; Stützel, H.; Jacobsen, H.-J.; Pao, Y.-C.; Hanafy, M.S. Enhanced Abiotic Stress Tolerance of Vicia faba L. Plants Heterologously Expressing the PR10a Gene from Potato. Plants 2021, 10, 173. https://doi.org/10.3390/plants10010173
Desouky AF, Ahmed AHH, Stützel H, Jacobsen H-J, Pao Y-C, Hanafy MS. Enhanced Abiotic Stress Tolerance of Vicia faba L. Plants Heterologously Expressing the PR10a Gene from Potato. Plants. 2021; 10(1):173. https://doi.org/10.3390/plants10010173
Chicago/Turabian StyleDesouky, Abeer F., Ahmed H. Hanafy Ahmed, Hartmut Stützel, Hans-Jörg Jacobsen, Yi-Chen Pao, and Moemen S. Hanafy. 2021. "Enhanced Abiotic Stress Tolerance of Vicia faba L. Plants Heterologously Expressing the PR10a Gene from Potato" Plants 10, no. 1: 173. https://doi.org/10.3390/plants10010173
APA StyleDesouky, A. F., Ahmed, A. H. H., Stützel, H., Jacobsen, H.-J., Pao, Y.-C., & Hanafy, M. S. (2021). Enhanced Abiotic Stress Tolerance of Vicia faba L. Plants Heterologously Expressing the PR10a Gene from Potato. Plants, 10(1), 173. https://doi.org/10.3390/plants10010173








